Abstract
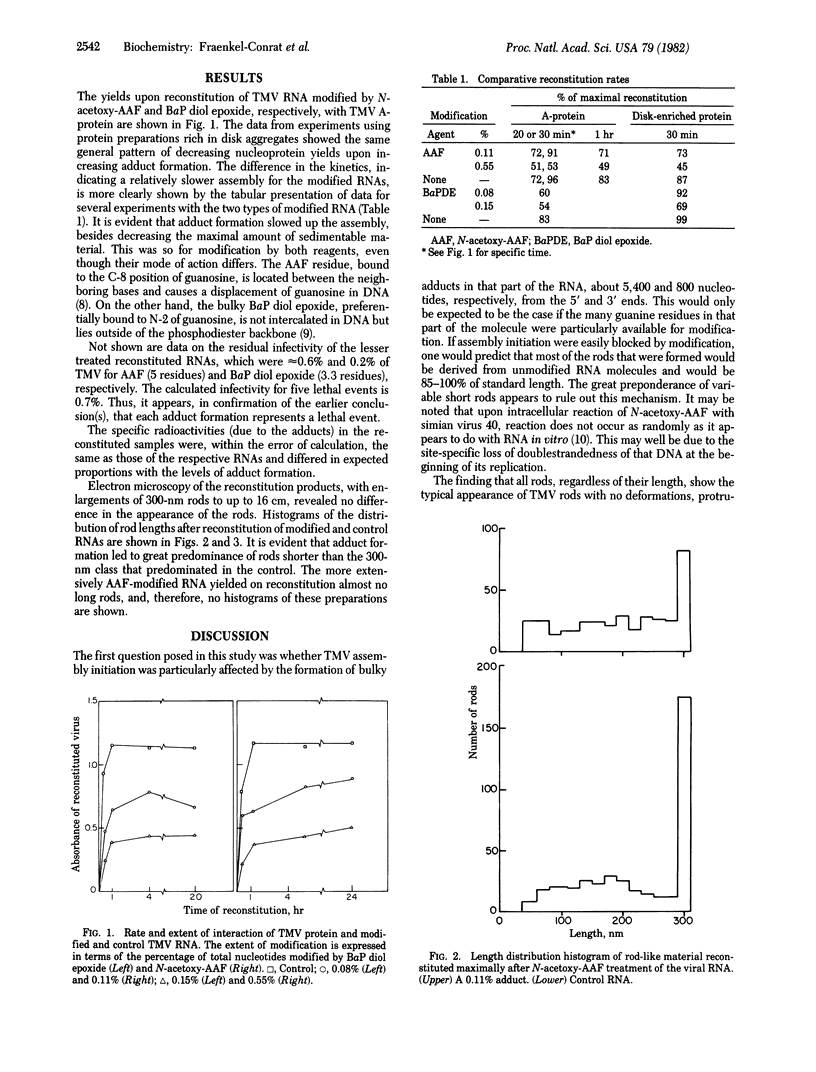
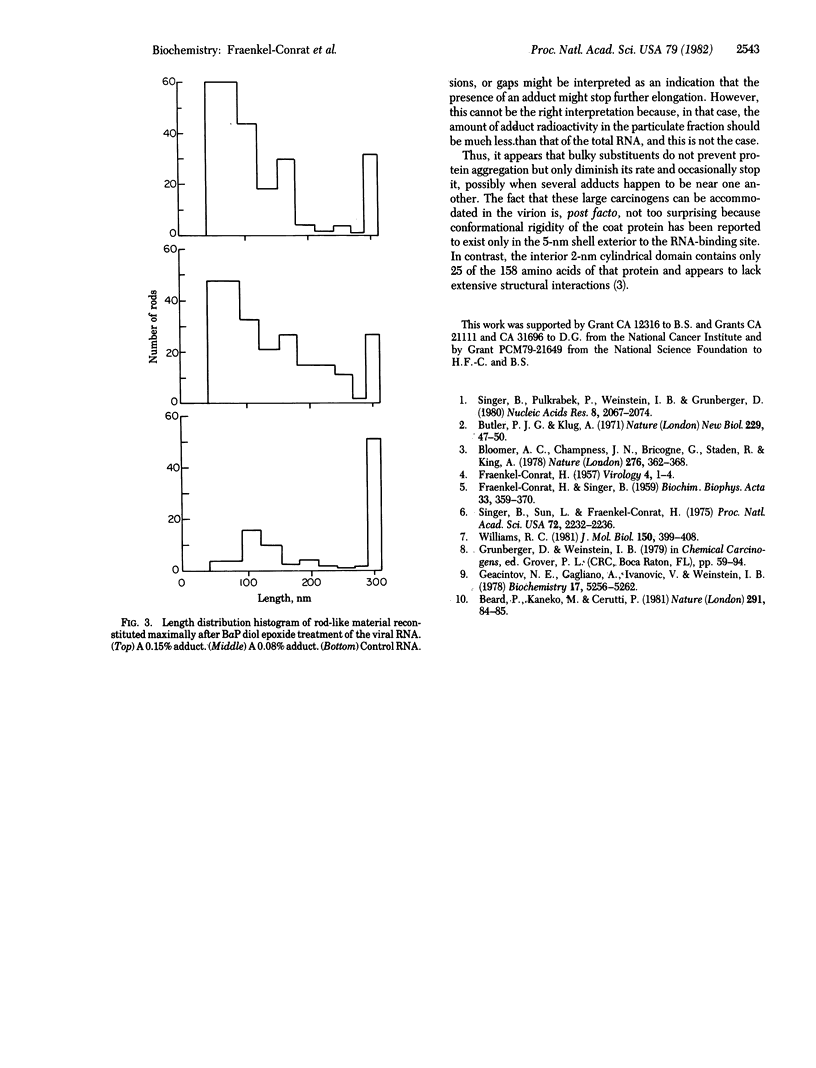
Tobacco mosaic virus (TMV) RNA was treated with radioactive N-acetoxy-2-acetylaminofluorene (N-acetoxy-AAF) and (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene (BaP diol epoxide) to obtain 3-25 adducts per molecule. Modified full length 30S RNAs and unmodified RNA were reconstituted for various time periods with TMV protein. The particulate products were separated by ultracentrifugation, and the amounts of virus-like material were quantitated by UV spectrophotometry. The length distribution and general appearance of the virus-like rods were studied by electron microscopy. Neither type of carcinogen prevented typical rod formation, but the rate of formation and the maximal yield of reconstituted particles diminished with increasing modification by both agents. The rod length distribution also showed progressively lesser numbers of full-length virus rods. The particulate material contained approximately the same number of adducts as the modified RNA. Thus, it appears that these carcinogen modifications of guanine residues at the N-2 or C-8 atoms did not prevent orderly protein assembly on the RNA but instead slowed up this process and frequently stopped it, possibly at sites where adducts happen to be clustered.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Hanei M., Garavito R. M. The Chou-Fasman secondary structure prediction method with an extended data base. FEBS Lett. 1978 Sep 1;93(1):19–24. doi: 10.1016/0014-5793(78)80795-9. [DOI] [PubMed] [Google Scholar]
- Armstrong R. N., Kaiser E. T. Sulfhydryl group reactivity of adenosine 3',5'-monophosphate dependent protein kinase from bovine heart: a probe of holoenzyme structure. Biochemistry. 1978 Jul 11;17(14):2840–2845. doi: 10.1021/bi00607a022. [DOI] [PubMed] [Google Scholar]
- Beale E. G., Dedman J. R., Means A. R. Isolation and characterization of a protein from rat testis which inhibits cyclic AMP-dependent protein kinase and phosdiesterase. J Biol Chem. 1977 Sep 25;252(18):6322–6327. [PubMed] [Google Scholar]
- Beard P., Kaneko M., Cerutti P. N-Acetoxy-acetylaminofluorene reacts preferentially with a control region of intracellular SV40 chromosome. Nature. 1981 May 7;291(5810):84–85. doi: 10.1038/291084a0. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Mechanisms of control for cAMP-dependent protein kinase from skeletal muscle. Adv Cyclic Nucleotide Res. 1975;5:241–251. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Klug A. Assembly of the particle of tobacco mosaic virus from RNA and disks of protein. Nat New Biol. 1971 Jan 13;229(2):47–50. doi: 10.1038/newbio229047a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., West L., Flockhart D. A., Lincoln T. M., McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Jun 10;253(11):3997–4003. [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Dills W. L., Goodwin C. D., Lincoln T. M., Beavo J. A., Bechtel P. J., Corbin J. D., Krebs E. G. Purification of cyclic nucleotide receptor proteins by cyclic nucleotide affinity chromatography. Adv Cyclic Nucleotide Res. 1979;10:199–217. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Rosenfeld R., Rosen O. M. Phosphorylation of a cyclic adenosine 3':5'-monophosphate-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1974 Aug 10;249(15):5000–5003. [PubMed] [Google Scholar]
- Erlichman J., Rubin C. S., Rosen O. M. Physical properties of a purified cyclic adenosine 3':5'-monophosphate-dependent protein kinase from bovine heart muscle. J Biol Chem. 1973 Nov 10;248(21):7607–7609. [PubMed] [Google Scholar]
- Erlichman J., Sarkar D., Fleischer N., Rubin C. S. Identification of two subclasses of type II cAMP-dependent protein kinases. Neural-specific and non-neural protein kinases. J Biol Chem. 1980 Sep 10;255(17):8179–8184. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Degradation of tobacco mosaic virus with acetic acid. Virology. 1957 Aug;4(1):1–4. doi: 10.1016/0042-6822(57)90038-7. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B. Reconstitution of tobacco mosaic virus. III. Improved methods and the use of mixed nucleic acids. Biochim Biophys Acta. 1959 Jun;33(2):359–370. doi: 10.1016/0006-3002(59)90126-x. [DOI] [PubMed] [Google Scholar]
- Fletterick R. J., Madsen N. B. The structures and related functions of phosphorylase a. Annu Rev Biochem. 1980;49:31–61. doi: 10.1146/annurev.bi.49.070180.000335. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Watterson D. M., Corbin J. D. Studies on functional domains of the regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1980 May 25;255(10):4435–4440. [PubMed] [Google Scholar]
- Geacintov N. E., Gagliano A., Ivanovic V., Weinstein I. B. Electric linear dichroism study on the orientation of benzo[alpha]pyrene-7,8-dihydrodiol 9,10-oxide covalently bound to DNA. Biochemistry. 1978 Nov 28;17(24):5256–5262. doi: 10.1021/bi00617a027. [DOI] [PubMed] [Google Scholar]
- Geahlen R. L., Krebs E. G. Regulatory subunit of the type I cAMP-dependent protein kinase as an inhibitor and substrate of the cGMP-dependent protein kinase. J Biol Chem. 1980 Feb 10;255(3):1164–1169. [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Evaluation of several amino group reversible blocking reagents. Biochemistry. 1970 Dec 8;9(25):4939–4944. doi: 10.1021/bi00827a016. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Takio K., Krebs E. G. Studies on the site in the regulatory subunit of type I cAMP-dependent protein kinase phosphorylated by cGMP-dependent protein kinase. J Biol Chem. 1981 Jun 10;256(11):5604–5607. [PubMed] [Google Scholar]
- Kerlavage A. R., Taylor S. S. Covalent modification of an adenosine 3':5'-monophosphate binding site of the regulatory subunit of cAMP-dependent protein kinase II with 8-azidoadenosine 3':5'-monophosphate. Identification of a single modified tyrosine residue. J Biol Chem. 1980 Sep 25;255(18):8483–8488. [PubMed] [Google Scholar]
- Kobayashi K., Katunuma N. Selective cleavage of peptide bonds by a serine protease from the muscle layer of rat small intestine. J Biochem. 1978 Jul;84(1):65–74. doi: 10.1093/oxfordjournals.jbchem.a132120. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Landon Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Radloff D., Schweppe J. S., Jungmann R. A. Testicular protein kinases. Characterization of multiple forms and ontogeny. J Biol Chem. 1976 Feb 25;251(4):914–921. [PubMed] [Google Scholar]
- Link T. P., Stark G. R. S-methylmethionine-29 ribonuclease A. I. Preparation and proof of structure. J Biol Chem. 1968 Mar 25;243(6):1082–1088. [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto E., Petzold G. L., Kuo J. F., Greengard P. Dissociation and activation of adenosine 3',5'-monophosphate-dependent and guanosine 3',5'-monophosphate-dependent protein kinases by cyclic nucleotides and by substrate proteins. J Biol Chem. 1973 Jan 10;248(1):179–189. [PubMed] [Google Scholar]
- Omenn G. S., Fontana A., Anfinsen C. B. Modification of the single tryptophan residue of staphylococcal nuclease by a new mild oxidizing agent. J Biol Chem. 1970 Apr 25;245(8):1895–1902. [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. Correlation of the cAMP binding domain with a site of autophosphorylation on the regulatory subunit of cAMP-dependent protein kinase II from porcine skeletal muscle. J Biol Chem. 1979 Sep 25;254(18):9000–9005. [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. Relationships between structural domains and function in the regulatory subunit of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979 Apr 10;254(7):2413–2418. [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. The structural domains of cAMP-dependent protein kinase I. Characterization of two sites of proteolytic cleavage and homologies to cAMP-dependent protein kinase II. J Biol Chem. 1980 Oct 25;255(20):9706–9712. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Pulkrabek P., Weinstein I. B., Grunberger D. Infectivity and reconstitution of TMV RNA modified with N-acetoxy-2-acetylaminofluorene or benzol [a] pyrene 7,8-dihydrodiol 9,10 oxide. Nucleic Acids Res. 1980 May 10;8(9):2067–2074. doi: 10.1093/nar/8.9.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Sun L., Fraenkel-Conrat H. Effects of alkylation of phosphodiesters and of bases of infectivity and stability of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2232–2236. doi: 10.1073/pnas.72.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmigielski A., Guidotti A., Costa E. Endogenous protein kinase inhibitors. Purification, characterization, and distribution in different tissues. J Biol Chem. 1977 Jun 10;252(11):3848–3853. [PubMed] [Google Scholar]
- Takio K., Walsh K. A., Neurath H., Smith S. B., Krebs E. G., Titani K. The amino acid sequence of a hinge region in the regulatory subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase II. FEBS Lett. 1980 May 19;114(1):83–88. doi: 10.1016/0014-5793(80)80865-9. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Weber W., Hilz H. Stoichiometry of cAMP binding and limited proteolysis of protein kinase regulatory subunits R I and R II. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1074–1081. doi: 10.1016/0006-291x(79)91935-1. [DOI] [PubMed] [Google Scholar]
- Williams R. C. Morphology of bovine fibrinogen monomers and fibrin oligomers. J Mol Biol. 1981 Aug 15;150(3):399–408. doi: 10.1016/0022-2836(81)90555-6. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Structure, specificity and localization of the serine proteases of connective tissue. FEBS Lett. 1980 Jun 2;114(2):189–196. doi: 10.1016/0014-5793(80)81112-4. [DOI] [PubMed] [Google Scholar]
- de Haën C., Swanson E., Teller D. C. The evolutionary origin of proinsulin. Amino acid sequence homology with the trypsin-related serine proteases detected and evaluated by new statistical methods. J Mol Biol. 1976 Sep 25;106(3):639–661. doi: 10.1016/0022-2836(76)90256-4. [DOI] [PubMed] [Google Scholar]


