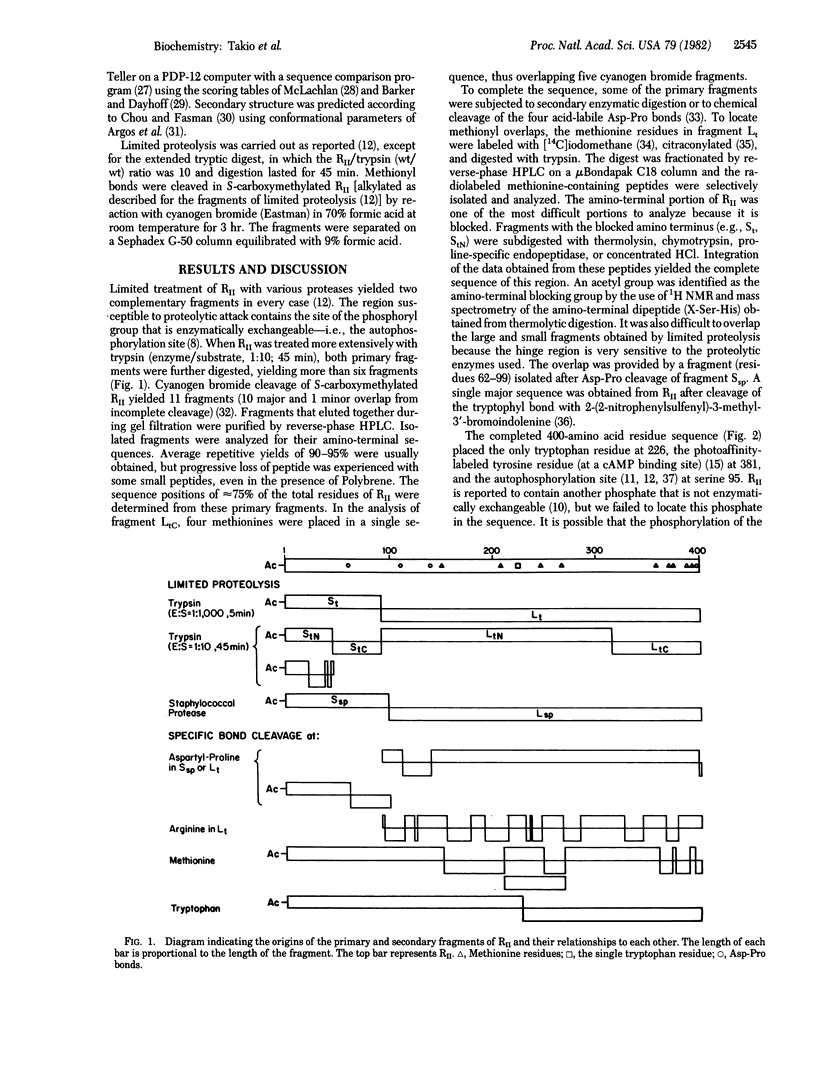
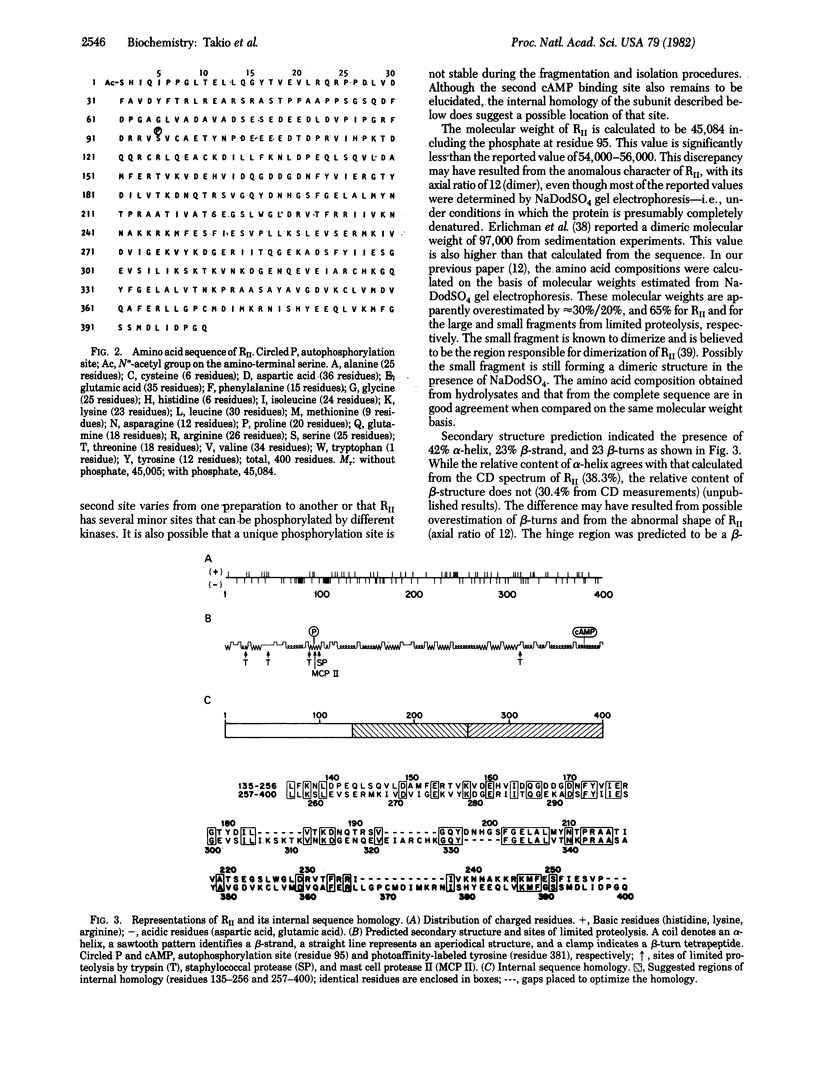
Abstract
The complete amino acid sequence of the regulatory subunit of type II cAMP-dependent protein kinase from bovine cardiac muscle is presented. Primary fragments for the sequence determination were obtained by limited proteolysis with various proteases or by cleavage with cyanogen bromide. The sequence of the 400 amino acid residues has two homologous regions, strongly suggesting tandem gene duplication. The predicted secondary structure suggests the presence of 42% alpha-helix, 23% beta-strand, and 23 beta-turns. The molecular weight of the subunit, as derived from the sequence, is 45,084 including a phosphate group at residue 95. This is significantly less than earlier estimates based on NaDodSO4 gel electrophoresis and sedimentation experiments. The structure is discussed in terms of putative sites of interaction with cAMP and with the catalytic subunit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Hanei M., Garavito R. M. The Chou-Fasman secondary structure prediction method with an extended data base. FEBS Lett. 1978 Sep 1;93(1):19–24. doi: 10.1016/0014-5793(78)80795-9. [DOI] [PubMed] [Google Scholar]
- Armstrong R. N., Kaiser E. T. Sulfhydryl group reactivity of adenosine 3',5'-monophosphate dependent protein kinase from bovine heart: a probe of holoenzyme structure. Biochemistry. 1978 Jul 11;17(14):2840–2845. doi: 10.1021/bi00607a022. [DOI] [PubMed] [Google Scholar]
- Beale E. G., Dedman J. R., Means A. R. Isolation and characterization of a protein from rat testis which inhibits cyclic AMP-dependent protein kinase and phosdiesterase. J Biol Chem. 1977 Sep 25;252(18):6322–6327. [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Mechanisms of control for cAMP-dependent protein kinase from skeletal muscle. Adv Cyclic Nucleotide Res. 1975;5:241–251. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., West L., Flockhart D. A., Lincoln T. M., McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Jun 10;253(11):3997–4003. [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Dills W. L., Goodwin C. D., Lincoln T. M., Beavo J. A., Bechtel P. J., Corbin J. D., Krebs E. G. Purification of cyclic nucleotide receptor proteins by cyclic nucleotide affinity chromatography. Adv Cyclic Nucleotide Res. 1979;10:199–217. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Rosenfeld R., Rosen O. M. Phosphorylation of a cyclic adenosine 3':5'-monophosphate-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1974 Aug 10;249(15):5000–5003. [PubMed] [Google Scholar]
- Erlichman J., Rubin C. S., Rosen O. M. Physical properties of a purified cyclic adenosine 3':5'-monophosphate-dependent protein kinase from bovine heart muscle. J Biol Chem. 1973 Nov 10;248(21):7607–7609. [PubMed] [Google Scholar]
- Erlichman J., Sarkar D., Fleischer N., Rubin C. S. Identification of two subclasses of type II cAMP-dependent protein kinases. Neural-specific and non-neural protein kinases. J Biol Chem. 1980 Sep 10;255(17):8179–8184. [PubMed] [Google Scholar]
- Fletterick R. J., Madsen N. B. The structures and related functions of phosphorylase a. Annu Rev Biochem. 1980;49:31–61. doi: 10.1146/annurev.bi.49.070180.000335. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Watterson D. M., Corbin J. D. Studies on functional domains of the regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1980 May 25;255(10):4435–4440. [PubMed] [Google Scholar]
- Geahlen R. L., Krebs E. G. Regulatory subunit of the type I cAMP-dependent protein kinase as an inhibitor and substrate of the cGMP-dependent protein kinase. J Biol Chem. 1980 Feb 10;255(3):1164–1169. [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Evaluation of several amino group reversible blocking reagents. Biochemistry. 1970 Dec 8;9(25):4939–4944. doi: 10.1021/bi00827a016. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Takio K., Krebs E. G. Studies on the site in the regulatory subunit of type I cAMP-dependent protein kinase phosphorylated by cGMP-dependent protein kinase. J Biol Chem. 1981 Jun 10;256(11):5604–5607. [PubMed] [Google Scholar]
- Kerlavage A. R., Taylor S. S. Covalent modification of an adenosine 3':5'-monophosphate binding site of the regulatory subunit of cAMP-dependent protein kinase II with 8-azidoadenosine 3':5'-monophosphate. Identification of a single modified tyrosine residue. J Biol Chem. 1980 Sep 25;255(18):8483–8488. [PubMed] [Google Scholar]
- Kobayashi K., Katunuma N. Selective cleavage of peptide bonds by a serine protease from the muscle layer of rat small intestine. J Biochem. 1978 Jul;84(1):65–74. doi: 10.1093/oxfordjournals.jbchem.a132120. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Landon Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Radloff D., Schweppe J. S., Jungmann R. A. Testicular protein kinases. Characterization of multiple forms and ontogeny. J Biol Chem. 1976 Feb 25;251(4):914–921. [PubMed] [Google Scholar]
- Link T. P., Stark G. R. S-methylmethionine-29 ribonuclease A. I. Preparation and proof of structure. J Biol Chem. 1968 Mar 25;243(6):1082–1088. [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto E., Petzold G. L., Kuo J. F., Greengard P. Dissociation and activation of adenosine 3',5'-monophosphate-dependent and guanosine 3',5'-monophosphate-dependent protein kinases by cyclic nucleotides and by substrate proteins. J Biol Chem. 1973 Jan 10;248(1):179–189. [PubMed] [Google Scholar]
- Omenn G. S., Fontana A., Anfinsen C. B. Modification of the single tryptophan residue of staphylococcal nuclease by a new mild oxidizing agent. J Biol Chem. 1970 Apr 25;245(8):1895–1902. [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. Correlation of the cAMP binding domain with a site of autophosphorylation on the regulatory subunit of cAMP-dependent protein kinase II from porcine skeletal muscle. J Biol Chem. 1979 Sep 25;254(18):9000–9005. [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. Relationships between structural domains and function in the regulatory subunit of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979 Apr 10;254(7):2413–2418. [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. The structural domains of cAMP-dependent protein kinase I. Characterization of two sites of proteolytic cleavage and homologies to cAMP-dependent protein kinase II. J Biol Chem. 1980 Oct 25;255(20):9706–9712. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmigielski A., Guidotti A., Costa E. Endogenous protein kinase inhibitors. Purification, characterization, and distribution in different tissues. J Biol Chem. 1977 Jun 10;252(11):3848–3853. [PubMed] [Google Scholar]
- Takio K., Walsh K. A., Neurath H., Smith S. B., Krebs E. G., Titani K. The amino acid sequence of a hinge region in the regulatory subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase II. FEBS Lett. 1980 May 19;114(1):83–88. doi: 10.1016/0014-5793(80)80865-9. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Weber W., Hilz H. Stoichiometry of cAMP binding and limited proteolysis of protein kinase regulatory subunits R I and R II. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1074–1081. doi: 10.1016/0006-291x(79)91935-1. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Structure, specificity and localization of the serine proteases of connective tissue. FEBS Lett. 1980 Jun 2;114(2):189–196. doi: 10.1016/0014-5793(80)81112-4. [DOI] [PubMed] [Google Scholar]
- de Haën C., Swanson E., Teller D. C. The evolutionary origin of proinsulin. Amino acid sequence homology with the trypsin-related serine proteases detected and evaluated by new statistical methods. J Mol Biol. 1976 Sep 25;106(3):639–661. doi: 10.1016/0022-2836(76)90256-4. [DOI] [PubMed] [Google Scholar]



