Abstract
Application of chemical synthesis to gain access to high purity hPTH as well as more stable analogs was accomplished through a menu of extended NCL followed by metal free dethiylation.
Introduction
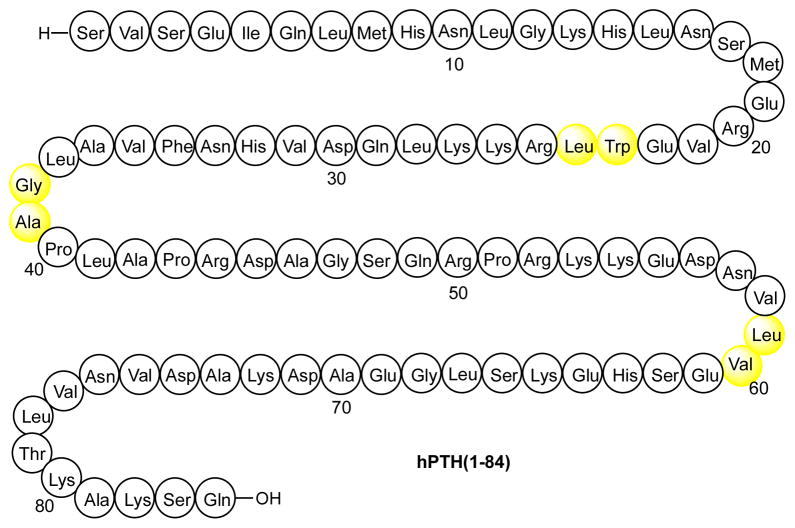
Human parathyroid hormone [hPTH(1-84), Figure 1] is an 84–amino acid polypeptide secreted by the chief cells of the parathyroid gland.1 Upon binding to PTH receptor type-1 (PTH1R, or PTHR), PTH acts to elevate the concentration of calcium ion (Ca2+) in the blood.2 As a result of its advantageous physiological activity, recombinant hPTH(1-84) (Preotact®), along with one of its synthetic fragments, hPTH(1-34) (Forteo®), is used in the treatment of hypoparathyroidism3 and osteoporosis.4 However, like many other hormonal drugs, PTH must be administered through daily subcutaneous injections – or more frequently for hypoparathyroidism – due to its short half-life in the human body.5 Thus, the development of a more stable, therapeutically viable analog of PTH would offer a major advantage for its biomedical usage. Although hPTH(1-34) has been well studied, research on full length PTH is relatively limited, presumably due to the difficulty in obtaining the 84-mer peptide in high purity, via either recombinant technology or chemical synthesis. A practical synthetic route to homogeneous hPTH(1-84) and related structures could well substantially facilitate future SAR studies on this important protein.
Figure 1.
Native sequence of human parathyroid hormone hPTH(1-84). Sites of proposed non-cysteine ligation depicted in yellow.
Some of the most effective activity-enhancing structural permutations thus far identified for PTH involve the substitution of non-encoded amino acid analogs, such as: alpha-amino-isobutyric acid (Aib) for alanine-1 and/or alanine-3;6 homoarginine for leucine-11;7 and a lactam ring between glutamate-22 and lysine-26.8 These modifications serve to enhance receptor binding affinity, signaling activity and/or bioavailability. However, the investigation of such substitutions has thus far been restricted to shorter-length N-terminal synthetic PTH fragments – such as PTH(1-14), PTH(1-31) and PTH(1-34) – as their incorporation into full-length PTH(1-84) has not been practical or even feasible through recourse to standard biosynthetic approaches. Further advances in improving the therapeutic utility of the PTH scaffold will likely depend on the incorporation of non-encoded amino acid modifications through synthetic means.
A growing focus of our laboratory9 has been the development of strategies to enable the controlled total synthesis of complex biologic targets, including peptides,9a carbohydrates, 10 and glycopeptides.11 Through major advances in de novo chemical synthesis, we have gained the capacity to assemble challenging “biologic” level target systems, with standards of homogeneity and purity not always achievable through biosynthetic or recombinant approaches. In the context of the research described below,12 we envisioned applying the synthetic advances developed in our laboratory to the preparation of a series of PTH-derived compounds for biological evaluation, with the ultimate goal of identifying therapeutically viable analogs with enhanced shelf- and bio-stability.
In designing our target analog structures, we took note of the finding that degradative oxidation of the two methionine residues (Met8 and Met18) appears to generate products with decreased adenylate cyclase activity.13 We postulated that replacement of such methionine residues with sulfur-free, closely isosteric norleucine (Nle) residues might well mitigate oxidative degradation of the biologic agent, thereby enhancing peptide stability, both under storage conditions and in in vivo settings. An analog of the hPTH fragment incorporating unnatural Nle residues at positions 8 and 18 had been previously prepared,14 although no full-length Nle-containing analogs have been reported. We thus sought to prepare, through chemical synthesis, hPTH(1-84) as well as the full-length [Nle8,18]hPTH(1-84) analog. For comparison purposes, we would also synthesize the hPTH(1-34) and [Nle8,18]hPTH(1-34) fragments. We describe herein the synthesis and preliminary biological evaluations of these PTH-derived peptides. We believe that the approaches described are of interest in terms of the PTH field itself, but also are suggestive of future directions at a more general level.
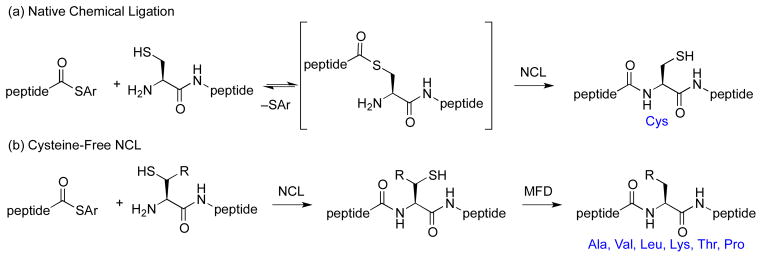
Central to our proposed program was the perception that chemical synthesis has reached the stage where it can be seen as a realistic possibility for gaining access to proteins, and even glycoproteins, previously seen as “biologics” to be synthesized by biological means (i.e. by natural or naturally inspired expression synthesis). Clearly, a major leap forward in this regard was the discovery and development of solid phase peptide synthesis (SPPS). Another major advance was the advent of Native Chemical Ligation (NCL), pioneered by Kent and associates (Figure 2a).15,16 NCL provided a means of covalently joining substantially sized unprotected synthetic peptidic segments. In this way, one could assemble polypeptides of sizes which exceed the capacity of SPPS to produce adducts of required purity.
Figure 2.
Cysteine-Based and Cysteine-Free Native Chemical Ligation.
While NCL has indeed proven to be a powerful tool, in its initial form it carried with it a significant limitation; i.e., that there had to emerge a cysteine at the N-terminus of the proposed ligation site. The possibility of using NCL to achieve ligation at what would become an alanine residue was well recognized by Dawson and was reduced to practice through post-ligation Raney Ni reduction of the erstwhile cysteine thiol function.17a While providing a very clever insight, this method lacked broad generality for two main reasons. First, the protocol was not well translated to the aqueous type conditions in which the NCL had been conducted. Moreover, the metal induced technology was not shown to be compatible with common glycopeptide functionalities, such as thiazolidine (Thz) moieties or carbohydrate domains.
The next large advance was occasioned by the discovery of metal free dethiylation (MFD).18 The method employed involved application of the Walling–Rabinowitz dethiylation logic,19 discovered in a totally different context as a curiosity in physical organic chemistry. Applicable in aqueous medium, the Walling-Rabinowitz reaction suggested that an alanine residue could be crafted at a specific point in the primary structure, even in the presence of protected cysteines destined to eventually emerge as cysteine residues (Figure 2b). More recently, our laboratory and then others have expanded the logic of NCL to encompass a menu of non-cysteine amino acids, including: phenylalanine,17b,20 leucine,21 lysine,22 threonine,23 valine,24 and proline25 residues, using MFD. As will be shown, application of this powerful new technology was to prove crucial to the successful total synthesis of PTH and its analogs.
Results and Discussion
Synthetic Design
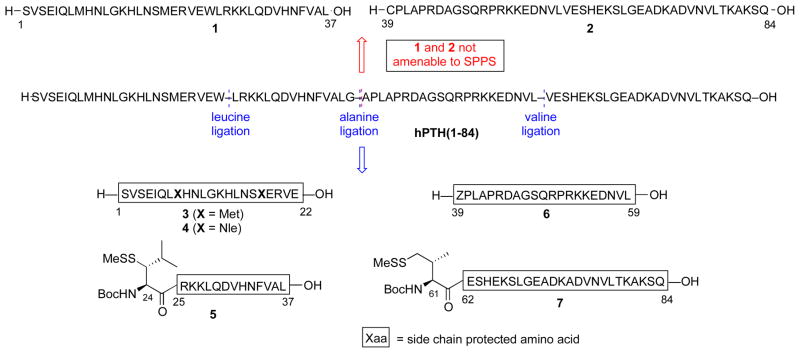
Previous approaches to full-length hPTH have required either coupling of fully protected peptide segments26 or linear assembly through solid phase peptide synthesis (SPPS).27 However, these strategies suffer from significant practical limitations arising from synthetic inefficiency and difficulties in peptide purification. In contrast, we envisioned a convergent, ligation-based approach to hPTH and our selected analogs. We first attempted to prepare hPTH(1-84) from two segments, Ser1–Gly38 and Ala39–Gln84 (Figure 3, in red). However, our efforts to synthesize the requisite peptides, 1 and 2, through standard Fmoc-based methods resulted in significant quantities of byproducts, arising from amino acid deletions and aspartimide formation. Thus, even at this writing, the power of SPPS can be quite sequence sensitive.
Figure 3.
PTH(1-84) target amino acid sequence and synthetic strategy.
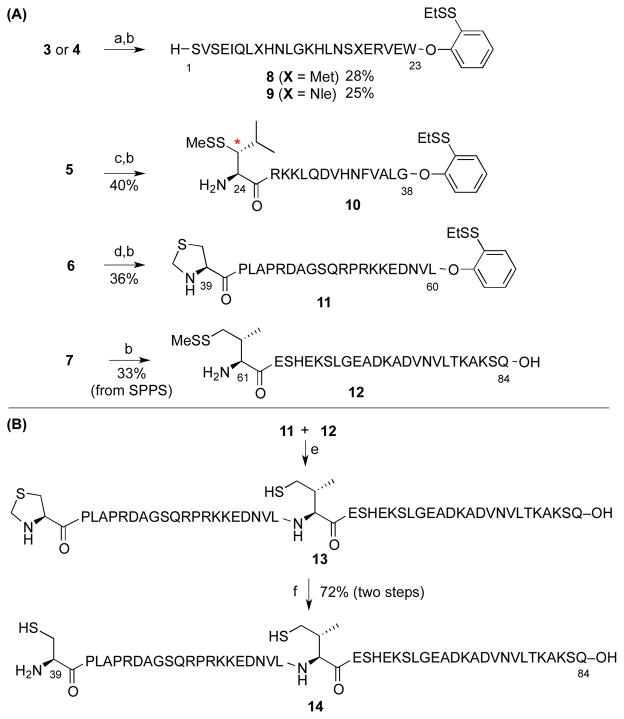
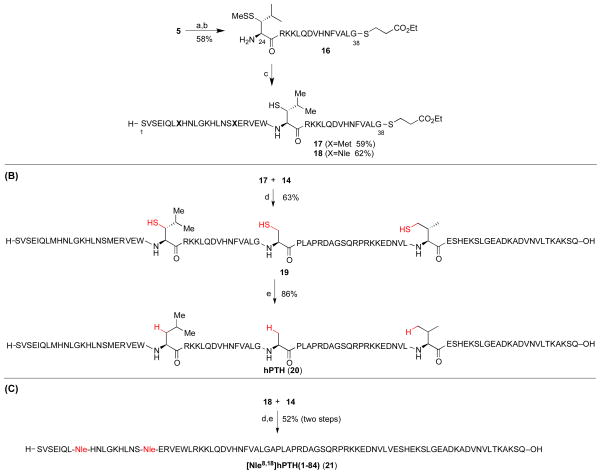
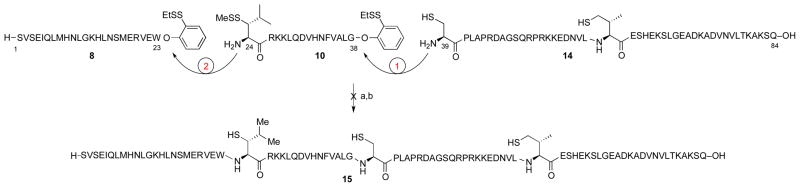
In order to maximize product purity and synthetic efficiency, we next devised a modified route, wherein the peptide would be dissected into four fragments (Figure 3, in blue). Through iterative leucine, alanine, and valine ligations, the individual peptides would be merged to generate the full PTH sequence. Notably, the oxidation-prone Met8 and Met18 residues are both contained within fragment 3. Thus, this proposed disconnection approach offers high modularity for the assembly of our proposed Nle-containing analogs (see peptide 4). In the event, peptide fragments 3–7 were synthesized through standard Fmoc-based SPPS protocols in good yield and purity. Interestingly, segment 6 was found to be prone to formation of aspartimide byproducts during SPPS. This problem could be eliminated through the use of Fmoc-Asp(ODie)-OH in place of the Fmoc-Asp(OtBu)-OH amino acid.28 As outlined in Scheme 1a, the ligation substrates (8–12) were prepared from the SPPS precursors through established protocols. We note that, in the case of the thio-leucine amino acid surrogate (cf. 10), we had previously observed a significant rate differential, dependent on the stereochemical configuration of the β-thiol functionality (see asterisk). Thus, the thio-leucine isomer depicted in structure 10 is significantly more reactive than is its β-thio epimer. 21b Notably, (2-ethyldithiolphenyl)ester, developed in our laboratory, was installed at the C-termini of segments 8–11.29 We were now prepared to attempt three NCL-inspired ligations followed by appropriate MFD, to co-assemble the four peptide components en route to full-length hPTH. As shown in Scheme 1b, segments 11 and 12 readily underwent “thio-valine ligation”,24b followed by thiazolidine ring opening,30 to afford peptide 14, encompassing the PTH(39-84) region. The newly unveiled N-terminal cysteine residue was envisioned to serve as an implement for subsequent formal alanine ligation.
Scheme 1.
Preparation of peptide segments for ligation.a
aReaction conditions: (a) HCl·H-Trp-O(EtSS)Ph, EDC, HOOBt, TFE:CHCl3 (1:3), 4 h; (b) TFA:TIS:H2O (95:2.5:2.5), 45 min; (c) HCl·H-Gly-O(EtSS)Ph, EDC, HOOBt, TFE:CHCl3 (1:3), 4 h; (d) HCl·H-Leu-O(EtSS)Ph, EDC, HOOBt, TFE:CHCl3 (1:3), 4 h; (e) 6 M Gn·HCl, 100 mM Na2HPO4, and 50 mM TCEP, pH 7.5, 15 h; (f) MeO-NH2·HCl, pH 4, 3 h. EDC = N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide; HOOBt = Hydroxy-3,4-dihydro-4-oxo-1,2,3-benzotriazine; TFE = Trifluoroethanol; TIS = Triisopropylsilane; Gn = Guanidine; TCEP = Tris(2-carboxyethyl)phosphine.
We next explored the feasibility of a one-flask iterative ligation protocol. We had previously observed a significant rate differential between cysteine- and thio-leucine based NCL.21b It was hoped to exploit this reactivity ordering by first exposing 10 and 14 to ligation conditions and then adding peptide 8 to the reaction flask, without isolation of the ligated peptide intermediate. The thought was that 10 and 14 would first undergo the more facile cysteine ligation and the resultant peptide would then merge with 8 in a subsequent leucine ligation, to afford full length PTH bearing three extraneous thiol groups. However, in practice, no coupled product was obtained from the first ligation of segments 10 and 14. Rather, LC-MS analysis indicated formation of a cyclic peptide, presumably arising from cyclization of 10. This result suggests that the reduced steric demands of intramolecular thioester formation significantly override the enhanced ligation reactivity of cysteine compared with thio-leucine.
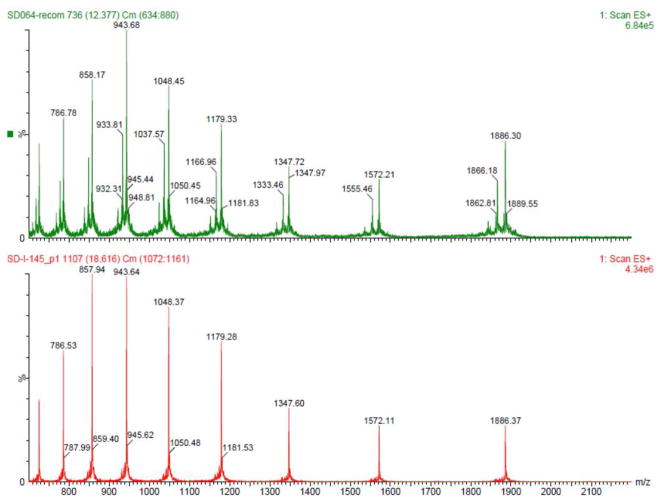
We next investigated the merger of the PTH(24-38) fragment with peptide 8 through kinetically controlled ligation (KCL).31 Thus, peptide 5 was converted to 16, bearing the less reactive alkyl thioester at the C-terminus, in place of (2-ethyldithiolphenyl)ester (see 16 vs. 10). As shown in Scheme 3a, peptide 16 underwent the hoped-for leucine ligation with peptides 8 [PTH(1-23)]or 9 [[Nle8,18]PTH(1-23)]. Notably, the alkyl thioester remained inert under these conditions, and only small amounts of cyclic peptide side product (ca. 10%) were observed. Adduct 17 was subsequently merged with peptide 14 under NCL conditions in the presence of MPAA, to afford full length hPTH(1-84) (19), bearing three extraneous thiol groups. Under standard metal-free desulfurization conditions, all three thiol groups were smoothly removed to reveal full-length hPTH protein in good overall yield (Scheme 3b). Comparative HPLC-MS analysis of recombinant sample (purchased from Bachem) and our synthetic material (20) revealed the latter to possess significantly higher purity (Figure 4).
Scheme 3.
Synthesis of hPTH(1-84) and [Nle8,18]hPTH(1-84) using cysteine-free NCL and metal-free desulfurization.a
aKey: (a) HCl·H-Gly-SCH2CH2COOEt, EDC, HOOBt, TFE:CHCl3 (1:3), 4 h; (b) TFA:TIS:H2O (95:2.5:2.5), 45 min; (c) 8 or 9, 6 M Gn·HCl, 100 mM Na2HPO4, and 50 mM TCEP, pH 7.2, 3 h; (d) 6 M Gn·HCl, 300 mM Na2HPO4, 200 mM MPAA, and 20 mM TCEP, pH 7.3, 2 h; (e) VA-044, tBu-SH, TCEP, H2O, MeCN, 37°C, 2 h. VA-044 = 2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride.
Figure 4.
Mass-Spec data of commercial hPTH(1-84) and synthetic hPTH(1-84). Top: Commercial sample; Bottom: Synthetic polypeptide 20.
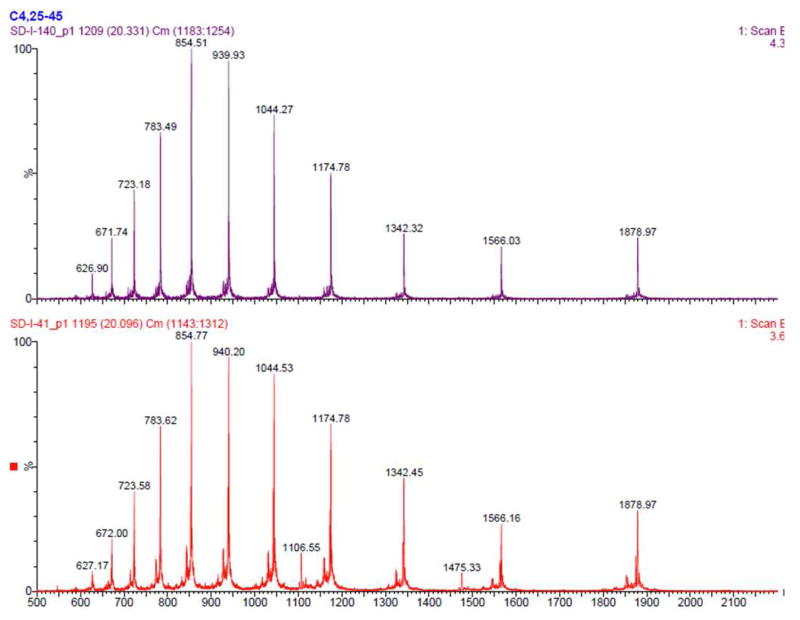
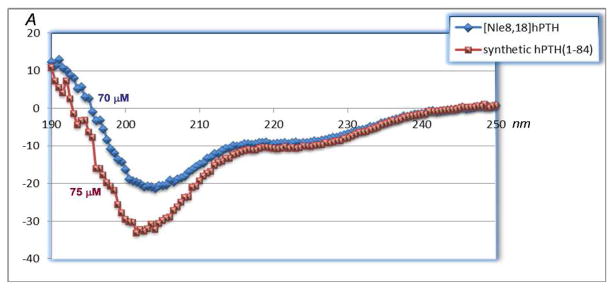
The [Nle8,18]hPTH(1-84) analog (21) was prepared from 18 and 14 in an analogous manner (Scheme 3c). HPLC-MS analysis reveals that the product was obtained in high purity (Figure 5), particularly in comparison to a synthetic sample obtained through a different route (see Supporting Information), and CD spectroscopy data strongly indicate that the secondary structure of the bis-norleucine analog, 21, is similar to that of the native sequence (Figure 6).
Figure 5.
Mass-Spec data of [Nle8,18]hPTH(1-84) prepared from two routes. Top: four segments (9, 16, 11, and 12) synthesis; Bottom: three segments (9, 16, and 2) synthesis.
Figure 6.
CD spectra of hPTH(1-84) and [Nle8,18]hPTH(1-84).
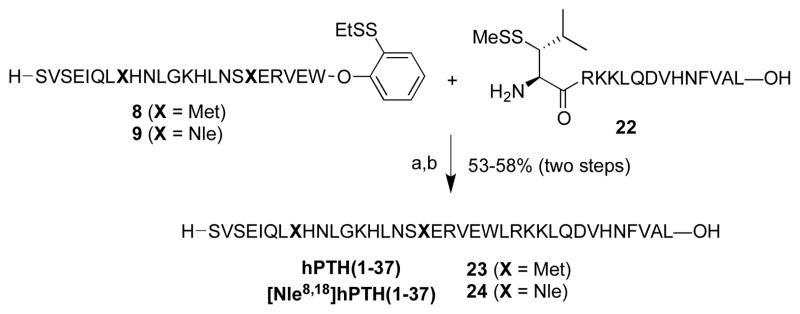
Finally, the truncated fragments, PTH(1-37) (23) and [Nle8,18]PTH(1-37) (24), were prepared through ligation of peptides 8 and 9 with peptide 22, bearing an N-terminal thio-leucine amino acid surrogate (Scheme 4). Ligation, followed by metal-free dethiylation, delivered 23 and 24 in 53% and 58% yields, respectively.
Scheme 4.
Synthesis of analogs PTH(1-37) and [Nle8,18]PTH(1-37).
Key: (a) 6 M Gn·HCl, 100 mM Na2HPO4, and 50 mM TCEP, pH 7.2, 2 h; (b) VA-044, tBu-SH, TCEP, H2O, MeCN, 37°C, 2 h.
Evaluation of Storage Stability of hPTH and Analogs
Oxygen or light-induced methionine oxidation is known to be one of the major degradation pathways of a number of pharmaceutically used proteins, including hPTH,32 interleukin-2,33 and human growth hormone.34 A more stable variant would offer significant advantages for the synthesis, purification, formulation, manufacturing, and storage of these therapeutically important biomedicines.35 With this consideration in mind, we first evaluated the shelf-life of our synthetic PTH analogs.
For synthetic hPTH (20) and analogs 21, 23 and 24, degradation of the pure samples was monitored using high performance liquid chromatography-mass spectroscopy (HPLC-MS) over a period of time (see Supporting Information). Under ambient conditions (room temperature, air, water solution, and neutral pH), the analytical results suggested that natural PTH(1-84) decomposed significantly over the time, and after 7 days, greater than 90% (estimated based on UV signal) of PTH degraded to fragments or other byproducts. In contrast, analog [Nle8,18]hPTH(1- 84) showed far greater stability under the same conditions, with less than 10% degradation observed after 7 days. By contrast, the truncated analogs, hPTH(1-37) and [Nle8,18]hPTH(1-37), showed similar levels of shelf stability, and the analytical results suggested approximately 70% decomposition after 7 days in both cases.
Interestingly, in the case of natural 84-mer, the major decomposition products observed were not simple oxidized products, but fragments with significant mass loss in comparison to the original peptide. It may be tentatively surmised that oxidation of the Met residues could have permutated the secondary and tertiary structures of thus protein, thus initiating or accelerating further degradation pathways.36 However, it could also be possible that the introduction of Nle residues on [Nle8,18]PTH(1-84) may alter the physiochemical characteristics of the peptide, such as folding and subunit association, leading to better sustainability under light- or oxygen-induced oxidative conditions. It should also be taken into account that other amino acid residues, including His, and Trp, are also susceptible to oxidation. Such oxidative mechanisms may be responsible for the serious shelf-life instability of the two 37-mer analogs, [PTH(1-37) and [Nle8,18]PTH(1-37)]. Presumably, in these more vulnerable systems, other common instabilities tend to mask the incremental benefits of the synthetically accomplished methionine→Nle mutations.37
Biological Evaluation of Synthetic hPTH(1-84) and analog
Parathyroid hormone (PTH), via its receptor, the PTHR (or PTHR1), plays a critical role in maintaining normal blood concentrations of ionized calcium (Ca++) and inorganic phosphate (Pi). Thus, in rapid response to a decrease in the blood Ca++ concentration, PTH is secreted from the parathyroid glands and acts on bone to promote resorption of the mineralized matrix, and on kidney to promote reabsorption of Ca++ from the glomerular filtrate. These coordinated actions in bone and kidney serve to maintain blood and fluid Ca++ levels within a narrow range (~1.2 mM ±10%). The PTHR is a class B G protein-coupled receptor that signals mainly via the Gαs/cAMP/PKA second messenger pathways. Recent studies show that the PTHR can form two, pharmacologically distinct, conformations: a G protein-dependent conformation, called RG, and G protein-independent conformation, called R0, and a high relative capacity of a ligand to bind to R0 is associated with a prolonged signaling response induced by that ligand in vitro and in vivo.38,39
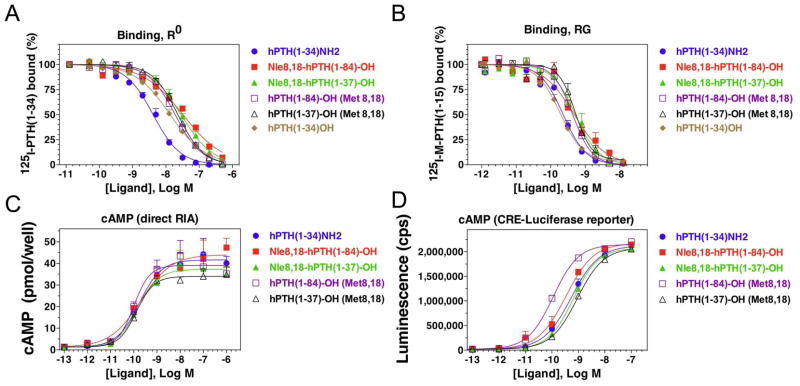
The capacities of the analogs to bind to the PTHR in a G protein-independent conformation, R0, and a G proteindependent conformation, RG, were assessed in membrane-based competition assays.38 Assays for R0 were performed using 125I-PTH(1-34) tracer radioligand and in the presence of excess GTPγS. Under these R0 conditions, each analog bound with an affinity in the low- to mid-nanomolar range (IC50s = 4 nM to 30 nM; Figure 7, A). Assays for RG binding were performed using 125I-MPTH(1-15) tracer radioligand and membranes from cells expressing a high affinity, Gαs mutant. Under these RG conditions, each analog bound with an affinity in the sub-nanomolar range (IC50s = 0.13 nM to 0.27 nM; Figure 7, B, Table 1).
Figure 7.
In vitro biological data. The capacities of the PTH analogs to bind to the PTHR in a G protein-independent conformation, R0, and a G protein-dependent conformation, RG, were assessed in membrane-based competition assays. Assays for R0 were performed using 125I-PTH(1-34) tracer radioligand and in the presence of excess GTPγS (A), and assays for RG binding were performed using 125I-M-PTH(1-15) tracer radioligand and membranes from cells expressing a high affinity, Gαs mutant. The cAMP signaling properties of the analogs were assessed using intact HEK-293 cells transfected to express the human PTHR; PTH-stimulated cAMP formation was assessed by direct radioimmuno assay performed 30 minutes after ligand application in the presence of IBMX (B); as well as by using cells stably transfected to express the PTHR together with a luciferase reporter gene under transcriptional control of a cAMP-response element-containing promoter, and measuring luciferase activity, as luminescence, four hours after ligand application (B).
Table 1.
Ligand binding and cAMP-signaling properties on the hPTHR1.
| Peptide | Binding | cAMP | ||
|---|---|---|---|---|
| COS-7/hPTHR1 cell membranes | HEK-293/hPTHR1 cells | |||
| pIC50 | pEC50 | |||
| nM | nM | |||
| R0 | RG | direct RIA | Cre-Luc | |
| hPTH(1-34)NH2 | 8.38 ± 0.11 | 9.70 ± 0.05 | 9.74 ± 0.14 | 9.27 ± 0.18 |
| 4.14 | 0.20 | 0.18 | 0.54 | |
| Nle8,18-hPTH(1-84)-OH | 7.55 ± 0.14 | 9.08 ± 0.30 | 9.62 ± 0.16 | 9.57 ± 0.03 |
| 28.3 | 0.84 | 0.24 | 0.27 | |
| Nle8,18-hPTH(1-37)-OH | 7.56 ± 0.09 | 9.29 ± 0.17 | 9.68 ± 0.16 | 9.17 ± 0.09 |
| 27.7 | 0.51 | 0.21 | 0.68 | |
| hPTH(1-84)-OH (Met 8,18) | 7.73 ± 0.11 | 9.35 ± 0.05 | 9.88 ± 0.32 | 9.90 ± 0.06 |
| 18.7 | 0.44 | 0.13 | 0.13 | |
| hPTH(1-37)-OH (Met 8,18) | 7.64 ± 0.04 | 9.31 ± 0.09 | 9.58 ± 0.22 | 9.03 ± 0.09 |
| 22.7 | 0.49 | 0.27 | 0.93 | |
| hPTH(1-34)OH | 7.92 ± 0.14 | 9.74 ± 0.12 | N.D. | N.D. |
| 11.9 | 0.18 | |||
Data are means±SEM of three experiments, each peformed in duplicate;
values are shown as the negative log concentration (with SEM), and as the corresponding nM value (italic).
N.D. not done
cAMP assays
The signaling properties of the analogs were assessed using intact HEK-293 cells transfected to express the human PTHR. Cells transiently transfected with the PTHR were treated with ligand for 30 minutes in the presence of IBMX and the intracellular cAMP levels were measured directly by radioimmuno assay (RIA).36 The analogs were also assayed using cells stably transfected to express the PTHR together with a luciferase reporter gene under transcriptional control of a cAMP-response element-containing promoter. In these assays, the analogs exhibited potencies in the low-nanomolar range (EC50s =~ 0.1 nM to 0.9 nM; Figure 7 C and D, Table 1).
Effects of PTH Analogs on blood Ca++ levels in mice
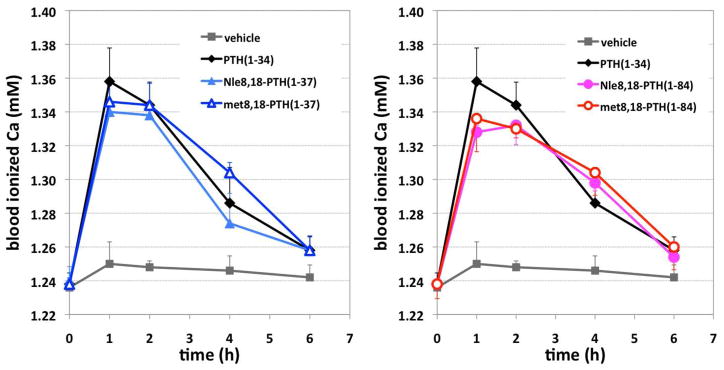
The capacities of the analogs to stimulate increases in blood Ca++ were assessed in normal 9 week-old, male, C57BL/6 mice. Prior to injection, the blood Ca++ concentrations in the wild-type mice were ~1.24 mM (Figure 8). Following injection of the PTH analogs, blood Ca++ levels increased robustly and reached a peak of ~ 1.36 mM by one hour post-injection. Blood Ca++ levels then returned to vehicle-control levels by six hours with each analog tested. Experiment results indicated that all four synthetic peptides increased the Ca2+ concentration in mice. However, no significant difference was observed in comparison to PTH(1-34), with respect to peak [Ca2+] and half-life.
Figure 8.
Effects of PTH analogs on blood Ca++ levels in mice. Nine-week-old, male mice, strain C57BL/6, were injected subcutaneously with vehicle or vehicle containing a PTH analog at a dose of 20 nanomole/Kg body weight; and blood was withdrawn at times thereafter, as well as just prior to injection (t=0), and assessed for ionized calcium.
Conclusion
Utilizing three non-cysteine-based native chemical ligations, with subsequent MFD, developed in our laboratory, we have synthesized the desired full length parathyroid hormone and a number of analogs with high levels of purity. That the four new PTH analogs presented here showed in vitro and in vivo potencies comparable to those of the PTH(1-34) control peptide confirms that a chemical synthesis-driven approach offers a plausible means for the molecular editing of therapeutic level proteins. In the case at hand, the methods and overall logic have been demonstrated to be effective, not only for the generation of full length PTH(1-84), but also for incorporating chemical modifications, such as non-coding amino acids,6,7 into the peptide chain for the purposes of improving action on the receptor, conformational stability, and ultimately in vivo activity. PTH analogs with improved activity could offer new therapeutic approaches for the diseases of osteoporosis40 and hypoparathyroidism. 41 In the former case, analogs that can stimulate new bone formation without inducing hypercalcemia, as can occur with native PTH peptides,42 would offer great advantage to current therapies. For hypoparathyroidism, long-acting analogs that can maintain normal blood Ca++ levels for prolonged periods after administration would offer clear benefit over native PTH, and the more conventional treatment of vitamin-D and Ca supplementation.43 Possibilities for integrating more closely the capacities of chemical synthesis and the needs of the biology–medicine interface are likely to expand.
Supplementary Material
Scheme 2.
Attempted one-pot sequential ligations.a
aKey: (a) 10, 14, 6 M Gn·HCl, 100 mM Na2HPO4, 50 mM TCEP, pH 7.2, 2 h; (b) 8, 6 M Gn·HCl, 100 mM Na2HPO4, 50 mM TCEP, pH 7.2.
Acknowledgments
The authors are grateful for support from the NIH (CA103823), spectroscopic assistance from Dr. George Sukenick, Hui Fang, and Sylvi Rusli of SKI’s NMR core facility for spectroscopic assistance, to Rebecca Wilson for preparation of the manuscript, and to Dr. Steven Town-send for helpful discussions.
Footnotes
General experimental procedures, including spectroscopic and analytical data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Potts JT. J Endocrinol. 2005;187:311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]; (b) Potts JT, Gardella TJ. Ann NY Acad Sci. 2007;1117:195–208. doi: 10.1196/annals.1402.088. [DOI] [PubMed] [Google Scholar]
- 2.Talmage RV, Mobley HT. Gen Comp Endocrinol. 2008;156:1–8. doi: 10.1016/j.ygcen.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Fraser WD. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez LJ, Scalisi R, Barbagallo M. Acta Biomed. 2010;81(Suppl 1):55–65. [PubMed] [Google Scholar]
- 5.(a) Bieglmayer C, Prager G, Niederle B. Clin Chem. 2002;48:1731–1738. [PubMed] [Google Scholar]; (b) Abraham AK, Mager DE, Gao X, Li M, Healy DR, Maurer TS. J Pharmacol Exp Ther. 2009;330:169–178. doi: 10.1124/jpet.109.152033. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu N, Guo J, Gardella TJ. J Biol Chem. 2001;276:49003. doi: 10.1074/jbc.M106827200. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Carter P, Khatri A, Potts J, Gardella TJ. Endocrinology. 2001;142:3068. doi: 10.1210/endo.142.7.8253. [DOI] [PubMed] [Google Scholar]
- 8.Barbier J, Neugebauer W, Morley P, Ross V, Soska M, Whitfield J, Willick G. J Med Chem. 1997;40:1373. doi: 10.1021/jm960743o. [DOI] [PubMed] [Google Scholar]
- 9.For recent reviews from our laboratory, see: Kan C, Danishefsky SJ. Tetrahedron. 2009;65:9047. doi: 10.1016/j.tet.2009.09.032.Wilson RM, Stockdill JL, Wu X, Li X, Vadola PA, Park PK, Wang P, Danishefsky S. J Angew Chem Int Ed. 2012;51:2834. doi: 10.1002/anie.201106628.
- 10.Danishefsky SJ, Bilodeau MT. Angew Chem Int Ed. 1996;35:1380. [Google Scholar]
- 11.(a) Nagorny P, Sane N, Fasching B, Aussedat B, Danishefsky SJ. Angew Chem Int Ed. 2012;51:975. doi: 10.1002/anie.201107482. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Aussedat B, Fasching B, Johnston E, Sane N, Nagorny P, Danishefsky SJ. J Am Chem Soc. 2012;134:3532. doi: 10.1021/ja2111459. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kan C, Trzupek JD, Wu B, Wan Q, Chen G, Tan Z, Yuan Y, Danishefsky SJ. J Am Chem Soc. 2009;131:5438. doi: 10.1021/ja808707w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yuan Y, Chen J, Wan Q, Tan Z, Chen G, Kan C, Danishefsky SJ. J Am Chem Soc. 2009;131:5432. doi: 10.1021/ja808705v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tan Z, Shang S, Halkina T, Yuan Y, Danishefsky SJ. J Am Chem Soc. 2009;131:5424. doi: 10.1021/ja808704m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Brailsford JA, Danishefsky SJ. Proc Natl Acad Sci USA. 2012;109:719. doi: 10.1073/pnas.1202762109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For a preliminary account of a segment of this work, see: Shang S, Tan Z, Danishefsky SJ. Proc Natl Acad Sci USA. 2011;108:4297. doi: 10.1073/pnas.1100195108.
- 13.Nabuchi Y, Fujiwara E, Ueno K, Kuboniwa H, Asoh Y, Ushio H. Pharm Res. 1995;12:2049–2052. doi: 10.1023/a:1016281031373. [DOI] [PubMed] [Google Scholar]
- 14.Rosenblatt M, Goltzman D, Keutmann HT, Tregear GW, Potts JT., Jr J Biol Chem. 1976;251:159. [PubMed] [Google Scholar]
- 15.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 16.For a recent review on NCL, see: Kent SBH. Chem Soc Rev. 2009;38:338. doi: 10.1039/b700141j.
- 17.(a) Yan LZ, Dawson PE. J Am Chem Soc. 2001;123:526–533. doi: 10.1021/ja003265m. [DOI] [PubMed] [Google Scholar]; (b) Crich D, Banerjee A. J Am Chem Soc. 2007;129:10064–10065. doi: 10.1021/ja072804l. [DOI] [PubMed] [Google Scholar]; (c) Pentelute BL, Kent SB. Org Lett. 2007;9:687–690. doi: 10.1021/ol0630144. [DOI] [PubMed] [Google Scholar]
- 18.Wan Q, Danishefsky SJ. Angew Chem Int Ed. 2007;46:9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- 19.Walling C, Rabinowitz R. J Am Chem Soc. 1957;79:5326. [Google Scholar]
- 20.Tchertchian S, Hartley O, Botti P. J Org Chem. 2004;69:9208. doi: 10.1021/jo049471k. [DOI] [PubMed] [Google Scholar]
- 21.(a) Harpaz Z, Siman P, Kumar KS, Brick A. Chem Bio Chem. 2010;11:1232–1235. doi: 10.1002/cbic.201000168. [DOI] [PubMed] [Google Scholar]; (b) Tan Z, Shang S, Danishefsky SJ. Angew Chem Int Ed. 2010;49:9500–9503. doi: 10.1002/anie.201005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Yang R, Pasunooti KK, Li F, Liu XW, Liu CF. J Am Chem Soc. 2009;131:13592–13593. doi: 10.1021/ja905491p. [DOI] [PubMed] [Google Scholar]; (b) Kumar KSA, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Angew Chem Int Ed. 2009;48:8090–8094. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Wang P, Zhu J, Wan Q, Danishefsky SJ. Tetrahedron. 2010;66:2277–2283. doi: 10.1016/j.tet.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Haase C, Rohde H, Seitz O. Angew Chem Int Ed. 2008;47:6807–6810. doi: 10.1002/anie.200801590. [DOI] [PubMed] [Google Scholar]; (b) Chen J, Wan Q, Yuan Y, Zhu JL, Danishefsky SJ. Angew Chem Int Ed. 2008;47:8521–8524. doi: 10.1002/anie.200803523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang S, Tan Z, Dong S, Danishefsky SJ. J Am Chem Soc. 2011;133:10784–10786. doi: 10.1021/ja204277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Morikawa T, Takai M, Sakakibara S. Biopolymers. 1981;20:1823. doi: 10.1002/bip.1981.360200907. [DOI] [PubMed] [Google Scholar]
- 27.(a) Fairwell T, Hospattankar AV, Ronan R, Brewer HB, Jr, Chang JK, Shimizu M, Zitzner L, Arnaud CD. Biochemistry. 1983;22:2691. doi: 10.1021/bi00280a016. [DOI] [PubMed] [Google Scholar]; (b) Goud NA, McKee RL, Sardana MK, DeHaven PA, Huelar E, Syed MM, Goud RA, Gibbons SW, Fisher JE, Levy JJ, Rodkey JA, Bennett C, Ramjit HG, Caporale LH, Caulfield MP, Bosenblatt M. J Bone Miner Res. 1991;6:781. doi: 10.1002/jbmr.5650060802. [DOI] [PubMed] [Google Scholar]
- 28.Mergler M, Dick F. J Peptide Sci. 2005;11:650. doi: 10.1002/psc.668. [DOI] [PubMed] [Google Scholar]
- 29.Warren JD, Miller JS, Keding SJ, Danishefsky SJ. J Am Chem Soc. 2004;126:6576. doi: 10.1021/ja0491836. [DOI] [PubMed] [Google Scholar]
- 30.(a) Villain M, Vizzanova J, Rose K. Chem Biol. 2001;8:673. doi: 10.1016/s1074-5521(01)00044-8. [DOI] [PubMed] [Google Scholar]; (b) Bang D, Kent SBH. Angew Chem Int Ed. 2004;116:2588. [Google Scholar]
- 31.(a) Bang D, Pentelute BL, Kent SBH. Angew Chem Int Ed. 2006;45:3985–3988. doi: 10.1002/anie.200600702. [DOI] [PubMed] [Google Scholar]; (b) Torbeev VY, Kent SBH. Angew Chem Int Ed. 2007;46:1667–1670. doi: 10.1002/anie.200604087. [DOI] [PubMed] [Google Scholar]; (c) Durek T, Torbeev VY, Kent SBH. Proc Natl Acad Sci USA. 2007;104:4846– 4851. doi: 10.1073/pnas.0610630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nabuchi Y, Fujiwara E, Ueno K, Kuboniwa H, Asoh Y, Ushio H. Pharm Res. 1995;12:2049–2052. doi: 10.1023/a:1016281031373. [DOI] [PubMed] [Google Scholar]
- 33.Sasaoki K, Hiroshima T, Kusumoto S, Nishi K. Chem Pharm Bull. 1989;37:2160–2164. doi: 10.1248/cpb.37.2160. [DOI] [PubMed] [Google Scholar]
- 34.Teh LC, Murphy LJ, Huq NL, Surus AS, Friesen HG, Lazarus L, Chaoman GE. J Biol Chem. 1987;262:6472–6477. [PubMed] [Google Scholar]
- 35.Pearlman R, Nguyen TH. J Pharm Pharmacol. 1992;44:178–185.For a recent review, see: Patel J, Kothari R, Tunga R, Ritter NM, Tunga BS. BioProcess Int. 2011;20–31
- 36.Li S, Schoneich C, Borchardt RT. Biotechnol Bioeng. 1995;48:490. doi: 10.1002/bit.260480511. [DOI] [PubMed] [Google Scholar]
- 37.It is conceivable that the observed degradation products arise via other pathways, such as acidolysis by residual acid, following lyophilization. Nevertheless, the norleucine analogs demonstrated much improved stability under such conditions.
- 38.Dean T, Linglart A, Mahon MJ, Bastepe M, Juppner H, Potts JT, Jr, Gardella TJ. Mol Endocrinol. 2006;20:931. doi: 10.1210/me.2005-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okazaki M, Ferrandon S, Vilardaga JP, Bouxsein ML, Potts JT, Jr, Gardella TJ. Proc Natl Acad Sci USA. 2008;105:16525–16530. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron R, Hesse E. J Clin Endocrinol Metab. 2012;97:311. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikjaer T, Rejnmark L, Mosekilde L. Curr Drug Saf. 2011;6:89. doi: 10.2174/157488611795684631. [DOI] [PubMed] [Google Scholar]
- 42.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mellström D, Oefjord ES, Marcinowska-Suchowierska E, Salmi J, Mulder H, Halse J, Sawicki AZ, Mitlak BH. New Engl J Med. 2001;344:1434. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 43.Winer KK, Sinaii N, Peterson D, Sainz B, Jr, Cutler GB., Jr J Clin Endocrinol Metab. 2008;93:3389. doi: 10.1210/jc.2007-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.