Abstract
Objectives
Anti-neutrophil cytoplasmic antibody (ANCA) vasculitis is a complex disease, with much debate about the utility of systems for classification and diagnosis. We compared three currently used classification systems in predicting disease prognosis.
Methods
Three classification systems were applied to 502 patients with biopsy proven ANCA vasculitis: the Chapel Hill Consensus Conference (CHCC) definition with categories for granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis), microscopic polyangiitis (MPA) and the Kidney Limited Disease (KLD); European Medicines Agency (EMA) system with categories for GPA and MPA, and classification based on ANCA specificity (PR3 versus MPO). Outcomes included treatment resistance, relapse, end stage kidney disease (ESKD) and death. Proportional hazards models were compared between systems using an information-theoretic approach to rank models by predictive fit. Hazard Ratios (HR) with 95% confidence intervals (CI) and p-values are reported.
Results
ANCA specificity was predictive of relapse, with PR3-ANCA patients almost twice as likely as those with MPO-ANCA to relapse (HR=1.89, 95% CI=1.33–2.69, p=0.0004), and ANCA specificity had the best predictive model fit (Model Rank=1) compared to CHCC and EMA. CHCC and EMA systems did not predict relapse. By ANCA specificity, categories of GPA, MPA and KLD did not distinguish differences in probability of relapse-free survival. None of the systems predicted treatment resistance, ESKD or death.
Conclusion
ANCA specificity independently predicts relapse among patients with ANCA vasculitis with renal disease. Classification and diagnostic systems that incorporate ANCA specificity, such as PR3-ANCA-MPA and MPO-ANCA-MPA, provide a more useful tool for predicting relapse than the clinic pathologic category alone.
The name of a disease should be informative about clinical and pathologic phenotypes, etiology and pathogenesis (when known), natural history and response to therapy. It should permit the differentiation of similar diseases that have different outcomes. Optimally, the name of a disease should reflect its underlying etiology. In 1994, the Chapel Hill Consensus Conference (CHCC) aimed to standardize nomenclature and definitions for vasculitis, including microscopic polyangiitis, Wegener’s granulomatosis, Churg Strauss syndrome, and polyarteritis nodosa.1 In 2007, the European Medicines Agency (EMA) classification system2 proposed the same disease names but different definitions that refined and expanded the 1990 American College of Rheumatology classification system.3 Since that time, granulomatosis with polyangiitis (GPA) has been proposed as an alternative term for Wegener’s granulomatosis, and will be used in place of Wegener’s granulomatosis for the remainder of this article.4
The Chapel Hill nomenclature was meant to provide disease definitions. Neither the CHCC nor EMA classification system provides diagnostic criteria for practicing physicians to discriminate microscopic polyangiitis (MPA) from GPA. The advent of widespread anti-neutrophil cytoplasmic antibody (ANCA) testing and accumulating evidence that ANCA may participate in the cause of small vessel vasculitis5 have spawned the terms ANCA associated vasculitis, ANCA vasculitis or ANCA disease as overarching terms for MPA, GPA and Kidney Limited Disease (KLD) that aid patients and clinicians in therapeutic decision-making. This approach has substantial value, yet may mask real differences in disease phenotype and prognosis unless the ANCA specificity is included in the diagnosis.
We sought to evaluate the utility of three classification systems in predicting the outcomes of treatment resistance, disease relapse, end stage kidney disease (ESKD) and death in a cohort of ANCA vasculitis patients. The classification systems compared for this project were a system based on the Chapel Hill Consensus Conference (CHCC) definitions,1 the European Medicines Agency (EMA) classification system,2 and classification based on ANCA serologic specificity. We hypothesized that ANCA specificity that is anti-proteinase 3 (PR3) antibodies (PR3-ANCA) versus anti-myeloperoxidase (MPO) antibodies (MPO-ANCA), would provide a more useful classification system in distinguishing both clinical phenotype and prognosis in ANCA vasculitis than the CHCC or EMA systems alone. We also studied the added value of appending the ANCA specificity to the CHCC categories.
PATIENTS AND METHODS
Cohort Description
The inception cohort included patients diagnosed between 1985 and 2007 with biopsy-proven ANCA vasculitis (including KLD) followed by the Glomerular Disease Collaborative Network as previously described.6 ANCA tests were done by indirect immunofluorescence microscopy or antigen-specific enzyme-linked immunosorbent assays (ELISA). Patients were categorized as having cytoplasmic-ANCA (C-ANCA), PR3-ANCA, or both and referred to collectively as PR3-ANCA, or perinuclear-ANCA (P-ANCA), MPO-ANCA, or both and referred to as MPO-ANCA. Patients having only P-ANCA were required to have a negative antinuclear antibody test. Patients included were diagnosed between January 1985 and December 2007. Histological confirmation was based on a kidney, lung or upper respiratory tract biopsy, consistent with pauci-immune small vessel vasculitis or glomerulonephritis, with or without granulomatous inflammation. All renal biopsies were evaluated by the University of North Carolina Nephropathology Laboratory. A small cohort of additional patients in our population who were persistently ANCA negative were excluded from the ANCA positive cohort and statistical modeling, but are described as a group with respect to the three classification systems. Patients were excluded if they had specificity for both PR3- and MPO-ANCA, were diagnosed with Churg-Strauss Syndrome, or had overlap with any other autoimmune disorder.
Our proposed system of describing ANCA vasculitis based on PR3 and MPO specificity was compared to two established classification systems: the CHCC1 and the EMA.2 Each patient was assigned a clinical phenotype for each system as well as ANCA specificity, disease clinical manifestations, and outcomes all which were recorded from review of medical records. The EMA system describes clinical phenotypes as GPA or MPA, with a priority inclusion of any ENT involvement as GPA,1,2 while the CHCC classification includes these GPA and MPA with an additional category for KLD. All patients were classified by CHCC and EMA through discussion and a consensus decision among three investigators (RJ Falk, S Lionaki and SL Hogan). Detailed information regarding organ involvement was recorded by criteria described previously.6–8
Outcomes of interest included treatment resistance, time to relapse, ESKD and death, as previously defined.6–8 In brief, treatment resistance was defined as persistence or new appearance of any extra-renal manifestation of vasculitis despite immunosuppressive therapy, and/or progressive decline in renal function in the setting of active urine sediment. Relapse was defined as the reactivation of vasculitis in any system organ. Disease relapse required initial response to treatment, so those who were treatment resistant were not eligible to have a relapse. ESKD was defined as the chronic need for renal replacement therapy. Death from any cause was noted. The frequency of ANCA type, ranked from lowest to highest frequency for PR3-ANCA across clinical phenotype categories was plotted by various organ system combinations, which were not mutually exclusive.
Statistical Methods
Univariate comparisons of the prevalence of each outcome (treatment resistance, relapse, ESKD and death) across classification systems were first evaluated with Chi-square tests. Kaplan-Meier estimates were used to plot the probability of relapse-free survival over time, with log-rank tests to evaluate univariate differences in relapse-free survival by subgroups.
Receiver operating characteristic (ROC) curve analysis was used to assess prediction ability of each model by individual classification systems, with a concordance index (C-index) reported for each model. Classification systems with a C-index greater than 0.65, indicating adequate model fit, where then directly compared for each outcome using an information-theoretic approach, which is a system for comparing models using multi-model inferences that allows direct comparison of the models of the three classification systems9,10.
This approach uses the delta of Akaike’s information criteria (AIC), Akaike weights (or model probabilities), and evidence ratios to assess which model (and corresponding system) best predicted each outcome.9,10 An additional bias correction term (AICc) was used instead of AIC.10 The evidence ratio of interest was between the estimated best model (delta AICc=0) and each compared model.10
Model averaging, which includes all information from the three classification system models together, weighted by model fit, was used to obtain point estimates, 95% confidence intervals (CI) and p-values for specific predictors of interest.9,10 Logistic regression models were used to assess factors associated with treatment resistance, with odds ratios (OR) and 95% CI reported. Proportional hazard models were used to evaluate the risk in time to relapse, ESKD and death, with Hazard Ratios (HR) and 95% CI provided.
Models were evaluated first with only the classification systems of interest, then controlling for potential confounding factors, with the latter reported in the results. Predictors found in previous analyses to be predictors of each outcome in a subgroup of this cohort6,11 were evaluated in the multivariable model averaging. In addition to the classification systems, control variables within each model-system included: for relapse, disease involvement in the lungs and upper respiratory airways; for treatment resistance, age, race and initial treatment (with or without cyclophosphamide); for ESKD: age, race, peak entry creatinine initial treatment (with or without cyclophosphamide), and vasculitis of the skin; and for death: age, peak entry creatinine and initial treatment (with or without cyclophosphamide). Treatment with cyclophosphamide as a first therapy regimen after diagnosis typically included intravenous pulse (0.5 to 1 g/m2 per month) or oral (1 to 2 mg/kg per day) doses in conjunction with oral corticosteroids and frequently methylprednisolone also. Treatment without cyclophosphamide included initial treatment with corticosteroids alone or in conjunction with other immunosuppressive regimens including azathioprine, mycophenolate mofetil, and cyclosporine. Therapy was not randomly assigned. Peak serum creatinine (reported as both µmol/L and mg/dL) was noted as the highest measure at the time of diagnosis and prior to beginning treatment. Racial groups compared were White versus non-White, since only 14% of the cohort was non-White. The majority of the factors have been well described in the literature, but the control of skin involvement at onset has only been seen in our prior evaluation of predictors where it was associated with a protective effect against ESKD (HR=0.20, 95% CI=0.06–0.75, p= 0.02)6. ENT involvement was included in the multivariable model as a potential predictor of relapse. As a sensitivity analysis, all models re-evaluated MPA and KLD patients using the CHCC system grouped together.
A proportional hazards model was used to evaluate the impact of categorical subgroups defined by ANCA specificity and CHCC definitions, controlling for lung and upper respiratory involvement of the disease. Analyses were done using SAS v.9.2 statistical software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Study Cohort
Initially, 523 patients were considered for study inclusion. All patients were ANCA positive with a biopsy proven pauci-immune small vessel vasculitis. Of those, 502 were finally included in the study (of the 21 patients who were excluded: 9 had been reported as ANCA positive but the actual specificity result by IFF and/or ELISA was not available, 1 for positivity for both MPO- and PR3-ANCA specificity, 4 with Churg Strauss Syndrome, and 7 because long-term disease outcome was not available).
In addition to the 523 ANCA positive cases, we identified 12 additional patients who were consistently ANCA negative. Those 12 cases were not considered for inclusion by study design, as they could not be analyzed within the ANCA specificity system. However, understanding the particular interest surrounding the clinical entity of ANCA negative pauci-immune vasculitis, we provide a thorough description of this group included in this manuscript’s discussion.
A detailed description of our study population (N=502) is displayed in Table 1. Kidney involvement was present in 488 patients, with 466 confirmed by renal biopsy and 22 with active urine sediment with or without renal insufficiency. A diagnostic kidney biopsy was performed in 466 of 502 patients (93%), while the remaining 36 (7%) had a diagnostic biopsy in other tissue (with 22 of 36 having an active urine sediment). Among the 466 with a kidney biopsy, 103 (22%) also had diagnostic biopsy in one or more additional organs or tissues. The most common sites for non-renal biopsies (N=139) included the lung (N=61), skin (N=42), and sinuses (N=17, with an additional 17 sinus biopsies that were non-diagnostic).
Table 1.
Description of the study cohort (N=502)
| Characteristic | Mean (±SD) or Absolute # (%) |
|---|---|
| Follow-up time post diagnosis of ANCA-SVV(months) | 40.5±44.9 |
| Age at diagnosis, (years) | 56.5±18.7 |
| Gender, Female | 234(47%) |
| Race, Caucasian | 433(86%) |
| Lung involvement | 252(50%) |
| Skin involvement | 115(23%) |
| ENT involvement | 185(37%) |
| Kidney involvement | 488(97%) |
| Gastrointestinal involvement | 59(12%) |
| Nerve involvement | 60(12%) |
| Peak entry creatinine, (mg/dl) | 4.6±3.6 |
| Acute dialysis at onset | 94(19%) |
| Initial Induction Therapy | |
| No treatment | 19(4%) |
| Corticosteroids alone | 53(11%) |
| Cyclophosphamide (intravenous) and prednisone | 238(47%) |
| Cyclophosphamide (oral) and prednisone | 166(33%) |
| Other immunosuppressive therapy | 26(5%) |
| Response to Initial Induction Therapy (n=483; 19 not treated were excluded) | |
| Treatment resistance | 109 (23%) |
| Complete remission (all therapy discontinued) | 217 (45%) |
| Remission on therapy | 157 (32%) |
| Mean months to outcome (±SD) | |
| Relapse (N=147, among 374 who had a response)) | 31.3±38.4 |
| ESKD (N=161) | 39.2±40.2 |
| Death (total, N=139) | 50.6±53.9 |
| Death (censored by ESKD, N=80) | 52.7±62.5 |
Analysis of clinical phenotype and response to therapy
Although the CHCC and EMA use identical names for disease categories, there were substantial discrepancies in the allocation of patients into specific clinic pathologic categories between the two systems. Sixty-four percent of cases classified as MPA using the CHCC system were considered GPA by the EMA algorithm. As a consequence, the EMA system diagnosed the majority of patients (324/502=65%) as having GPA. In contrast, this diagnosis was far less frequent within the CHCC system (117/502=23%, p<0.0001). The difference between classification systems in GPA was predominantly due to the priority inclusion of ENT involvement as GPA by the EMA system. Specifically, we found 185 (37%) patients having ENT involvement, with 100% classified as GPA by EMA, by definition, and 87(47%) classified as GPA by the CHCC. Distribution of ANCA specificity was also different between these two systems. Using the CHCC, 74% of PR3-ANCA positive patients were diagnosed with GPA, whereas only 54% of PR3-ANCA positive patients were diagnosed with GPA using the EMA algorithm. Using the CHCC definitions, 55% of MPO-ANCA patients were diagnosed with MPA, in contrast to only 34% of MPO-ANCA patients who were diagnosed as MPA in the EMA system. MPO-ANCA was the dominant serotype (81%) among patients with KLD, a category that is grouped with MPA within the EMA system.
Treatment resistance was evaluated in 483 of 502 patients, excluding the 19 patients who received no treatment. Relapse was evaluated in 374 patients as patients were not considered eligible to relapse if they received no treatment (N=19) or were resistant to treatment (N=109). ESKD was reached in 161 of the 502 patients (32%), and 139 of 502 died (27%).
A number of univariate differences in outcomes amongst the various classification systems are displayed in Table 2. Treatment resistance was not markedly different across the CHCC and EMA classification systems, but was more common among those with MPO-ANCA (27%) than among those with PR3-ANCA (17%, p=0.016). Relapse was different across CHCC classifications with those identified as GPA most likely to relapse (60%), with MPA in the middle in terms of risk for relapse (37%), and KLD least likely to relapse (19%, p<0.0001). Using the EMA system those with GPA were also more likely to relapse than those with MPA, but the risk of relapse was closer; 44% versus 30%, respectively (p=0.0071). PR3-ANCA specificity also had more relapse (51%) compared to MPO-ANCA (29%). There were also variations in both ESKD and death within each of the three classification systems (Table 2).
Table 2.
Frequency of short and long term outcomes across classification systems.
| Outcomes: | Treatment Resistance |
Relapse | ESKD | Death |
|---|---|---|---|---|
| Classification Systems | (N=109/483) | (N=147/374) | (N=161/502) | (N=139/502) |
| Chapel Hill Consensus Conference | ||||
| Granulomatosis with Polyangiitis (N=117) | 20/117(17%) | 58/97(60%) | 24/117(21%) | 20/117(17%) |
| Microscopic Polyangiitis (N=264) | 56/255(22%) | 74/199(37%) | 80/264(30%) | 79/264(30%) |
| Renal limited disease (N=121) | 33/111(30%) | 15/78(19%) | 57/121(47%) | 40/121(33%) |
| P value | 0.0711 | <0.0001 | <0.0001 | 0.0091 |
| European Medicines Agency | ||||
| Granulomatosis with Polyangiitis (N=324) | 68/317(22%) | 110/249(44%) | 92/324(28%) | 84/324(26%) |
| Microscopic Polyangiitis (N=178) | 41/166(25%) | 37/125(30%) | 69/178(39%) | 55/178(31%) |
| P value | 0.4244 | 0.0071 | 0.0214 | 0.2520 |
| ANCA specificity | ||||
| MPO ANCA (N=283) | 72/270(27%) | 57/198(29%) | 105/283(37%) | 89/283(31%) |
| PR3 ANCA (N=219) | 37/213(17%) | 90/176(51%) | 56/219(26%) | 50/219(23%) |
| P value | 0.0160 | <0.0001 | 0.0069 | 0.0349 |
Among the twelve patients who were ANCA-negative, 9 of 12 were documented as negative by both immunofluorescence and antigen-specific ELISA, and the other 3 by immunofluorescence only with antigen-specificity not evaluated. According to the CHCC definitions, 2 were classified as MPA, 7 as GPA, and 3 as KLD. By the EMA system, 4 were categorized as MPA, and 8 with GPA. Although not formally compared, these distributions were generally similar to those seen in the overall ANCA-positive cohort. Of note, 7 of the 12 ANCA-negative patients had disease involvement restricted to a single organ or system, including the 3 with KLD.
Prognostic ability of classification systems for clinical outcomes
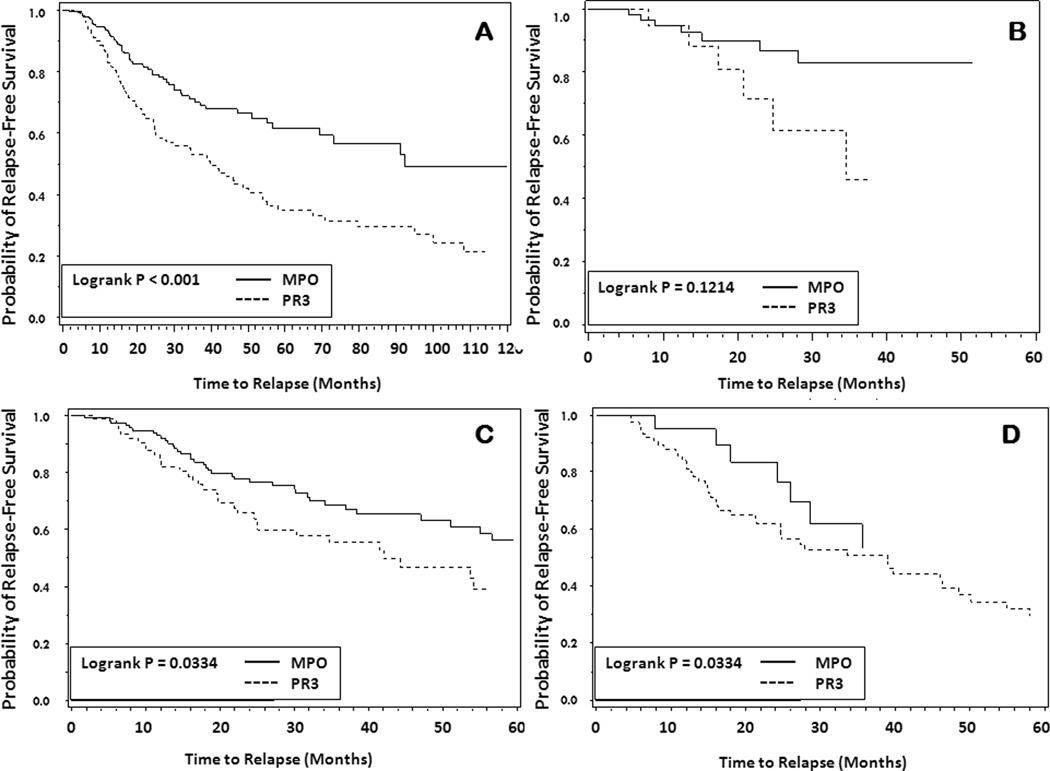
Each of the three candidate classification system models for relapse had adequate fit, with C-statistics ranging from 0.86 to 0.89. The information-theoretic modeling approach for the outcome of time to relapse, revealed that classification using ANCA specificity alone (PR3 versus MPO) had the best predictive model fit (Model Rank=1) among the three classification models, with the lowest delta AICc of 0.00 and highest weight of 0.987 (Table 3.A). The other systems had weights at or approaching zero (Table 3.A) indicating they did not provide predictive value for time to relapse. ANCA specificity was strongly predictive of relapse, with PR3-ANCA positive patients almost twice as likely as those with MPO-ANCA to relapse (HR=1.89, 95% CI=1.33–2.69, p=0.0004) (Table 3.A). The probability of relapse-free survival over time by ANCA specificity displays these results graphically in Figure 1.A. The only other predictor of relapse in the multivariable model was pulmonary involvement of the disease (HR=1.7, 95% CI=1.2–2.4, p=0.004). Upper respiratory disease involvement, which has been an inconsistent predictor of relapse across cohorts6,11, did not predict relapse in this analysis (HR=1.07, 95% CI=0.75–1.51, p=0.71). In the sensitivity analysis, the same model averaging results were seen when MPA and KLD were grouped together in the CHCC system, with ANCA specificity remaining the best predictive model and PR3-ANCA and lung involvement remaining the significant predictors with similar point estimates and confidence intervals (PR3 HR=1.88, 95% CI=1.33–2.68, p=0.0004, lung involvement HR=1.68, 95% CI=1.18–2.40, p=0.004).
Table 3.
Comparison of Classification System Modeling and Estimates for Classification Systems using Model Averaging for the Disease Relapse, Treatment Resistance, ESKD, Death
| System Modeling for Disease Relapse | Model Averaging: | |||||||
| Classification System |
Classification Category | Δ AICc per system model |
Model Weight |
Model Rank |
P values | Hazard Ratio | Low (95%CI) |
High (95%CI) |
| PR3/MPO | 0.000 | 0.989 | 1 | |||||
| MPO | Reference | |||||||
| PR3 | 0.0004 | 1.888 | 1.328 | 2.686 | ||||
| CHCC | 11.651 | 0.003 | 3 | |||||
| Microscopic polyangiitis | Reference | |||||||
| Kidney Limited Disease | 0.978 | 0.999 | 0.961 | 1.034 | ||||
| Granulomatosis with polyangiitis | 0.963 | 1.001 | 0.962 | 1.042 | ||||
| EMA | 9.516 | 0.003 | 2 | |||||
| Microscopic polyangiitis | Reference | |||||||
| Granulomatosis with polyangiitis | 0.935 | 0.995 | 0.879 | 1.126 | ||||
| System Modeling for Treatment Resistance | Model Averaging | |||||||
| Classification System |
Classification Category | Δ AICc per system model |
Weight(model or variables) |
Model Rank |
P value | OR | Low (95%CI) |
High (95%CI) |
| PR3/MPO | 1.93 | 0.256 | 2 | |||||
| MPO | Reference | |||||||
| PR3 | 0.744 | 0.952 | 0.709 | 1.278 | ||||
| CHCC | 4.47 | 0.072 | 3 | |||||
| Microscopic Polyangiitis | Reference | |||||||
| Kidney Limited Disease | 0.966 | 0.997 | 0.850 | 1.168 | ||||
| 0.961 | 1.004 | 0.841 | 1.120 | |||||
| EMA | 0 | 0.672 | 1 | |||||
| Microscopic Polyangiitis | ||||||||
| Granulomatosis with Polyangiitis | 0.257 | 1.312 | 0.817 | 2.132 | ||||
| System Modeling for ESKD | Model Averaging | |||||||
| Classification System |
Classification Category | Δ AICc per system model |
Weight(model or variables) |
Model Rank |
P value | HR | Low (95%CI) |
High (95%CI) |
| PR3/MPO | 2.849 | 0.154 | 3 | |||||
| MPO | Reference | |||||||
| PR3 | 0.907 | 1.009 | 0.871 | 1.168 | ||||
| CHCC | 0 | 0.641 | 1 | |||||
| Microscopic Polyangiitis | Reference | |||||||
| Kidney Limited Disease | 0.186 | 1.302 | 0.880 | 1.926 | ||||
| Granulomatosis with Polyangiitis | 0.693 | 0.923 | 0.619 | 1.375 | ||||
| EMA | 2.288 | 0.204 | 2 | |||||
| Microscopic Polyangiitis | Reference | |||||||
| Granulomatosis with Polyangiitis | 0.759 | 1.029 | 0.855 | 1.239 | ||||
| System Modeling for Death | Model Averaging | |||||||
| Classification System |
Classification Category | Δ AICc per system model |
Weight(model or variables) |
Model Rank |
P value |
HR |
Low (95%CI) |
High (95%CI) |
| PR3/MPO | 0 | 0.495 | 1 | |||||
| MPO | Reference | |||||||
| PR3 | 0.417 | 1.141 | 0.830 | 1.568 | ||||
| CHCC | 3.952 | 0.069 | 3 | |||||
| Microscopic Polyangiitis | Reference | |||||||
| Kidney Limited Disease | 0.987 | 1.001 | 0.900 | 1.113 | ||||
| Granulomatosis with Polyangiitis | 0.951 | 0.996 | 0.863 | 1.149 | ||||
| EMA | 0.251 | 0.437 | 2 | |||||
| Microscopic Polyangiitis | Reference | |||||||
| Granulomatosis with Polyangiitis | 0.488 | 1.113 | 0.822 | 1.508 | ||||
Figure 1.
Probability of relapse-free survival by ANCA specificity (A), and by MPO-ANCA and PR3-ANCA positivity within classification groups of the Chapel Hill Consensus Conference definitions: KLD = Kidney Limited Disease (B), MPA = Microscopic Polyangiitis (C), and GPA = Granulomatosis with Polyangiitis (D).
The probability of relapse-free survival over time by MPO-ANCA and PR3-ANCA within each CHCC disease group (KLD, MPA and GPA) are shown in Figures 1.B., 1.C and 1.D. Within each disease subgroup, there is a trend for those with PR3-ANCA to be more likely to relapse. The statistical power of multivariable modeling was limited, but revealed a clear trend for all three CHCC categories among PR3-ANCA patients to have similar and consistently higher risks (HRs ranging from 2.52–3.25) than the categories among the MPO-ANCA patients (HRs ranging from 1.0–1.62) (Table 4). Results were similar using the EMA categories by ANCA specificity (data not shown).
Table 4.
Multivariable Hazard Ratios for Time to Relapse by CHCC Disease Categories for MPO- and PR3- ANCA Specificity
| ANCA specificity and Disease Definition Categories (n) |
Hazard Ratio (95% CI)* | P-value |
|---|---|---|
| MPO-ANCA, KLD (56) | Reference 1.0 | NA |
| MPO-ANCA, MPA (121) | 1.56 (0.70, 3.48) | 0.276 |
| MPO-ANCA, GPA (21) | 1.36 (0.47, 3.90) | 0.568 |
| PR3-ANCA, KLD (20) | 2.49 (0.89, 6.95) | 0.081 |
| PR3-ANCA, MPA (79) | 2.45 (1.07, 5.63) | 0.035 |
| PR3-ANCA, GPA (77) | 3.15 (1.34, 7.40) | 0.009 |
The proportional hazards model for these results controlled for disease involvement of the lung and upper airways.
MPA = Microscopic Polyangiitis, GPA = Granulomatosis with Polyangiitis, KLD=Kidney Limited Disease
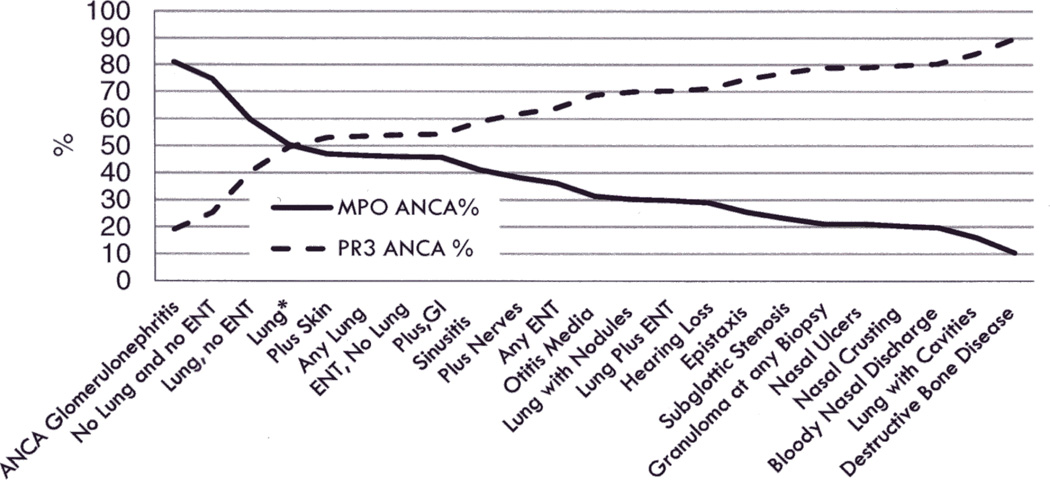
Given the consistent results for ANCA specificity as a predictor of relapse, we sought to better understand organ involvement of the disease by ANCA specificity. Categories of organ system involvement were ranked by their prevalence of PR3-ANCA, and inversely by MPO-ANCA (Figure 2). Patients with KLD or any form of vasculitis without radiological or histological proof of granulomatous inflammation were more likely to have MPO-ANCA and those with the most compelling evidence for necrotizing granulomatous inflammation were most likely to have PR3-ANCA. For instance, the majority of patients with KLD had MPO-ANCA (81%), while almost all patients with bone destruction or saddle nose deformity had PR3-ANCA (94%).
Figure 2.
Frequency of PR3- and MPO-ANCA specificity by a variety of clinical phenotypes (organ groupings are not mutually exclusive)
No Lung and No ENT: Vasculitis in any organ except the lungs and the ENT system.
Lung, no ENT: Vasculitis localized in the lungs but not in the ENT system.
Lung*: Vasculitis localized in the lungs without indicative markers (nodules, or cavities) or histological proof (granulomas) of granulomatous inflammation.
Plus Gastrointestinal (GI): Vasculitis localized at any organ plus involvement of the gastrointestinal tract.
Plus Skin: Vasculitis localized at any organ plus dermal involvement.
Plus Nerves: Vasculitis localized at any organ plus involvement of the nerves.
Any Lung: Any type of pulmonary vasculitis such as pulmonary hemorrhage, infiltrates, nodules, cavities, granulomas, or respiratory arrest.
ENT, no Lung: Vasculitis localized at the ENT system but not in the lungs.
Any ENT: Any type of vasculitic manifestation of the ENT system.
Lung with nodules: Vasculitis localized at the lungs with radiographic proof of nodules.
Lung plus ENT: Any type of pulmonary vasculitis plus any type of vasculitic manifestation of the ENT system.
Each of the three classification system models for treatment resistance, ESKD and death had adequate fit, with C-statistics for treatment resistance 0.70 for all three models, 0.78 to 0.79 for ESKD and death, respectively. In comparing models, there were slight differences between classification systems in the multivariable modeling for each outcome, however none of the three classification systems were found to be better independent predictors of these outcomes (Table 3.B). Results were similar for each of these outcomes for the sensitivity analyses when MPA and KLD were grouped together in the CHCC system. For the outcome of treatment resistance, treatment category and race were the only significant predictors.
Those not treated with cyclophosphamide at initial diagnosis were twice as likely to be resistant to therapy as those who received cyclophosphamide (HR=2.1, 95% CI=1.2–3.6, p=0.011). Those of non-White race were also twice as likely to be treatment resistant (HR= 2.0, 95% CI=1.1–3.7, p=0.016).
For ESKD, controlling for classification systems, age, race, skin involvement, peak entry creatinine, and treatment category, statistically significant predictors included peak entry serum creatinine (HR=1.15 per unit increase of mg/dL, 95% CI=1.11–1.19, p<0.0001) and non-White race (versus Whites, HR=1.7, 95% CI 1.1–2.6, p=0.018).
Evaluation of predictors for death (N=139 deaths) revealed that when controlling for classification systems, age, ANCA specificity, peak entry creatinine, and treatment category predictor variables included age (HR=1.05, 95% CI=1.04–1.07, p<0.00001) and peak entry creatinine (HR=1.11 per mg/dL, 95% CI=1.06–1.16, p=0.00002). When death was censored by the date of ESKD (N=80 deaths), there was still no predictive value from the classification systems. Other predictors for death had equivalent risk estimates (age and peak entry creatinine).
DISCUSSION
We envision a classification system for ANCA vasculitis to be not only terms to label and describe the disease, but a functional tool for the clinician to provide assistance in disease recognition, treatment and prognosis. We focused on issues we consider critical for patient management—the clinical phenotype and prediction of disease course.
The question of prognosis in ANCA vasculitis is critically important for clinicians. Because the vast majority of the patients respond to standard induction treatment,12,13 the question is how to weigh the risk of relapse against the risk of long term maintenance therapy to prevent relapse. In this study, disease relapse was found to be independently predicted by PR3-ANCA specificity, a finding consistent with previous studies,6,14 but not by other classification systems. These PR3-ANCA positive patients may be the ones in whom prolongation of immunosuppressive therapy after achievement of remission is reasonable, although there is little direct data to support the contention that relapse can be prevented by the use of current therapies.6,8,15–17
A wide range of vasculitic manifestations involving multiple anatomic sites and tissues was observed, and was found to correlate strongly with ANCA specificity. The association between PR3- or MPO-ANCA and the anatomic site of vasculitic involvement and/or the presence of granulomatous inflammation was particularly interesting. The majority of patients with KLD had MPO-ANCA (81%) and those with destructive lesions of the upper airways had PR3-ANCA (94%). When vasculitis was expanded from the kidney-limited variant to involve the gastrointestinal or respiratory tract, MPO-ANCA was less frequent, and PR3-ANCA increased. Moreover, among 52 patients with histological proof of granulomatous inflammation at any site in our cohort, 79% had PR3-ANCA and 21% had MPO-ANCA. The principle that disease associated with MPO- and PR3-ANCA are clinically distinct is essential to the categorization of ANCA small vessel vasculitis based on antibody specificity.
Several hypotheses could explain the correlation of disease expression with ANCA serotype. Among them, the entrance-portal and/or nature of any potential environmental “pathogen,” including its ability to be distributed in the body could predict ANCA type. In addition, the availability of the target antigen in the tissue or organ18 or the genetic background of any particular individual may explain ANCA type. In this regard, it is interesting that PR3-ANCA is predominant in northern Europe, whereas MPO-ANCA is the predominant in Japan and China.19,20 ANCA disease associated with environmental factors, such as silica,21 microbes,22 medications,23 or concomitant disease24, usually demonstrate a predominance of ANCA type. Various genes have been closely associated with ANCA vasculitis.25,26 If the disease is genetically driven in a specific phenotypic direction, this might be influenced by the ANCA specificity. However, the extent to which any gene might influence the clinical phenotype of vasculitis, in what proportion of patients, and in any particular patient is not known. Different pathogenic effects of autoantibodies against PR3 versus MPO could confer different manifestations of disease.27,28 This could be explained by differences in external or internal triggering events, differences in activation of effector cells, or differences in innate or adaptive immune responses that are activated by PR3-ANCA versus MPO-ANCA21,29–34.
The CHCC and EMA classification systems failed to provide a consistent ability to predict ANCA vasculitis outcomes. This results in part from differences in disease “definitions.” For instance, the EMA system over-represents the diagnostic category of GPA2 because essentially any upper respiratory involvement is considered to be in this category according to the criteria of the American College of Rheumatology.3 Seventy eight percent of cases classified as MPA by the CHCC system were instead classified as GPA using the EMA system. Interestingly, distribution of the ANCA types differs significantly between the CHCC classification and EMA systems. PR3-ANCA is predominant in patients deemed as having GPA by the CHCC definitions, yet there is almost a 1:1 ratio for PR3- and MPO-ANCA by the EMA model in patients with GPA. Long-standing clinical experience35–37 has shown that most patients with ongoing active GPA have PR3-ANCA,38–40 although sensitivity differs across studies.38–40
Patients with pauci-immune small vessel vasculitis who were ANCA negative were not included in the statistical analyses for this study, but were described. Only 2.2% of patients in our registry were known to be persistently negative in clinical ANCA assays. Various proportions of patients with pauci-immune small vessel vasculitis fail to demonstrate ANCA positivity across study-cohorts, with studies reporting 10 to 30% across all types of pauci-immune small vessel vasculitis41, but this phenomenon is rarer in our population. Ideally, patients should be tested for ANCA when the disease is active and prior to initiation of therapy, to avoid the effect of immunomodulation. This becomes even more critical with the use of interventions that eliminate antibodies, such as plasmapheresis and B cell depletion. ANCA-negative patients in our cohort demonstrated symptoms of ANCA vasculitis indistinguishable from those in ANCA-positive patients, as also reported by others.42
The strong predictive value of PR3-ANCA and lung involvement for relapse in this study is consistent with previous studies.6,11,43 ENT involvement was not predictive of relapse in this analysis. Prior analyses have shown only a weak or non-significant association with prediction of relapse6,11.
With respect to treatment resistance, the predictors of being non-White and having induction therapy without cyclophosphamide were in accord with the evaluation in our previous cohort5. Other potential predictors of treatment resistance previously evaluated include gender and age, which were not significant predictors in this evaluation, have shown inconsistent results in previous cohorts.6,11 Older age and higher baseline creatinine as predictors of death are consistent with a recent report that found older age and higher organ damage, as measured by the Vasculitis Damage Index, to be predictors of death in a cohort of 50 patients with GPA.44 Higher serum creatinine and the need for acute or chronic dialysis have also been seen as a specific form of damage that contributes to mortality in small vessel vasculitis.45 Evaluation of predictors of death found in other studies such as pulmonary hemorrhage7 or adverse treatment events and level of disease activity46 were beyond the scope of this paper.
Limitations for this study include that this is a cohort derived from a registry recruited by nephrologists and therefore the vast majority of patients had ANCA glomerulonephritis at presentation, alone, or in combination with other organ involvement. However, prevalence of kidney involvement in ANCA vasculitis is reported to be as high as 75 to 90%47 and therefore our population likely represents the majority of patients with this disease48. Furthermore, kidney involvement renders a significant impact on patients’ quality of life, as morbidity and mortality are dreadful, particularly for those with ESKD. Thus, studying patients with kidney involvement is useful for any specialist confronting patients with small vessel vasculitis. Other limitations, inherent to community-based cohort studies conducted over a long period of time detail a lack of uniform treatment protocols and differences in general clinical practice. Additionally, tests for ANCA have evolved with time and were not standardized across clinics; however, there is acceptable consistency between earlier ANCA test methods and the commercial kits that are currently used.49
Classification systems are designed to provide a standard method of describing groups of patients, not only to facilitate categorizing patients for clinical trials and comparing results among trials in the medical literature, but above all to assist in patient care. Etiology, the constellation of symptoms, and other predictors of outcome should be included in classification systems if they facilitate patient care. Because ANCA specificity not only is implicated in pathogenesis and correlates with clinical symptoms, but more importantly helps predict the outcome of disease, it is appropriate to include ANCA specificity in the diagnostic classification. This approach is feasible now that ANCA testing is widely available and reliable. We recommend that PR3-ANCA and MPO-ANCA be used in conjunction with the terms microscopic polyangiitis, granulomatosis with polyangiitis and pauci-immune glomerulonephritis in categorizing patients within the spectrum of ANCA vasculitis.
Acknowledgments
This study was funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases grant ‘ANCA Glomerulonephritis: from Molecules to Man’ P01DK058335 (PI Falk, RJ).
Reference List
- 1.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 2.Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, Mahr A, Segelmark M, Cohen-Tervaert JW, Scott D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fries JF, Hunder GG, Bloch DA, Michel BA, Arend WP, Calabrese LH, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW., Jr The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary. Arthritis Rheum. 1990;33:1135–1136. doi: 10.1002/art.1780330812. [DOI] [PubMed] [Google Scholar]
- 4.Falk RJ, Gross WL, Guillevin L, Hoffman GS, Jayne DR, Jennette JC, Kallenberg CG, Luqmani R, Mahr AD, Matteson EL, Merkel PA, Specks U, Watts RA. Granulomatosis with Polyangiitis (Wegener's): An alternative name for Wegener's Granulomatosis. Arthritis Rheum. 2011;63:863–864. doi: 10.1002/art.30286. [DOI] [PubMed] [Google Scholar]
- 5.Kallenberg CG. Pathophysiology of ANCA-associated small vessel vasculitis. Curr Rheumatol Rep. 2010;12:399–405. doi: 10.1007/s11926-010-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, Nachman PH. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–631. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 8.Nachman PH, Hogan SL, Jennette JC, Falk RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–39. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 9.Burnham KP, Anderson DR. Model Selection and Multimodal Inference: A Practical Information-Theoretic Approach. 2nd ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 10.Anderson DR. Model Base Inference in the Life Sciences: A Primer on Evidence. New York: Springer; 2008. [Google Scholar]
- 11.Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, Nachman PH. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: Comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–2918. doi: 10.1002/art.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauci AS, Haynes B, Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med. 1978;89:660–676. doi: 10.7326/0003-4819-89-5-660. [DOI] [PubMed] [Google Scholar]
- 13.de Groot K, Adu D, Savage CO. The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant. 2001;16:2018–2027. doi: 10.1093/ndt/16.10.2018. [DOI] [PubMed] [Google Scholar]
- 14.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, Plaisance M, Pusey CD, Jayne DR. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–784. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 15.Savage CO, Winearls CG, Evans DJ, Rees AJ, Lockwood CM. Microscopic polyarteritis: presentation, pathology and prognosis. Q J Med. 1985;56:467–483. [PubMed] [Google Scholar]
- 16.Reinhold-Keller E, Fink CO, Herlyn K, Gross WL, de Groot K. High rate of renal relapse in 71 patients with Wegener's granulomatosis under maintenance of remission with low-dose methotrexate. Arthritis Rheum. 2002;47:326–332. doi: 10.1002/art.10459. [DOI] [PubMed] [Google Scholar]
- 17.Bacon PA. The spectrum of Wegener's granulomatosis and disease relapse. N Engl J Med. 2005;352:330–332. doi: 10.1056/NEJMp048338. [DOI] [PubMed] [Google Scholar]
- 18.Brockmann H, Schwarting A, Kriegsmann J, Petrow P, Gaumann A, Muller KM, Galle PR, Mayet W. Proteinase-3 as the major autoantigen of c-ANCA is strongly expressed in lung tissue of patients with Wegener's granulomatosis. Arthritis Res. 2002;4:220–225. doi: 10.1186/ar410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts RA, Scott DG, Jayne DR, Ito-Ihara T, Muso E, Fujimoto S, Harabuchi Y, Kobayashi S, Suzuki K, Hashimoto H. Renal vasculitis in Japan and the UK--are there differences in epidemiology and clinical phenotype? Nephrol Dial Transplant. 2008;23:3928–3931. doi: 10.1093/ndt/gfn354. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Cui Z, Zhao MH. ANCA-associated vasculitis and anti-GBM disease: the experience in China. Nephrol Dial Transplant. 2010;25:2062–2065. doi: 10.1093/ndt/gfq134. [DOI] [PubMed] [Google Scholar]
- 21.Hogan SL, Cooper GS, Savitz DA, Nylander-French LA, Parks CG, Chin H, Jennette CE, Lionaki S, Jennette JC, Falk RJ. Association of silica exposure with anti-neutrophil cytoplasmic autoantibody small-vessel vasculitis: a population-based, case-control study. Clin J Am Soc Nephrol. 2007;2:290–299. doi: 10.2215/CJN.03501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Staphylococcal superantigens and T cell expansions in Wegener's granulomatosis. Clin Exp Immunol. 2003;132:496–504. doi: 10.1046/j.1365-2249.2003.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130–147. doi: 10.1345/aph.1A124. [DOI] [PubMed] [Google Scholar]
- 24.Lionaki S, Hogan SL, Falk RJ, Joy MS, Chin H, Jennette CE, Jennette JC, Nachman PH. Association between thyroid disease and its treatment with ANCA small-vessel vasculitis: a case-control study. Nephrol Dial Transplant. 2007;22:3508–3515. doi: 10.1093/ndt/gfm493. [DOI] [PubMed] [Google Scholar]
- 25.Alcorta DA, Barnes DA, Dooley MA, Sullivan P, Jonas B, Liu Y, Lionaki S, Reddy CB, Chin H, Dempsey AA, Jennette JC, Falk RJ. Leukocyte gene expression signatures in antineutrophil cytoplasmic autoantibody and lupus glomerulonephritis. Kidney Int. 2007;72:853–864. doi: 10.1038/sj.ki.5002371. [DOI] [PubMed] [Google Scholar]
- 26.Lionaki S, Falk RJ. Removing antibody and preserving glomeruli in ANCA small-vessel vasculitis. J Am Soc Nephrol. 2007;18:1987–1989. doi: 10.1681/ASN.2007050575. [DOI] [PubMed] [Google Scholar]
- 27.Bautz DJ, Preston GA, Lionaki S, Hewins P, Wolberg AS, Yang JJ, Hogan SL, Chin H, Moll S, Jennette JC, Falk RJ. Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3-ANCA vasculitis. J Am Soc Nephrol. 2008;19:2421–2429. doi: 10.1681/ASN.2008030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Bautz DJ, Lionaki S, Hogan SL, Chin H, Tisch RM, Schmitz JL, Pressler BM, Jennette JC, Falk RJ, Preston GA. ANCA patients have T cells responsive to complementary PR-3 antigen. Kidney Int. 2008;74:1159–1169. doi: 10.1038/ki.2008.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi K, Shibata T, Sugisaki T. Aggravation of rat nephrotoxic serum nephritis by anti-myeloperoxidase antibodies. Kidney Int. 1995;47:454–463. doi: 10.1038/ki.1995.58. [DOI] [PubMed] [Google Scholar]
- 30.Heeringa P, Brouwer E, Klok PA, Huitema MG, van den BJ, Weening JJ, Kallenberg CG. Autoantibodies to myeloperoxidase aggravate mild anti-glomerular-basement-membrane-mediated glomerular injury in the rat. Am J Pathol. 1996;149:1695–1706. [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth CL, Smith J, Cook HT, Haskard DO, Pusey CC. Anti-myeloperoxidase associated pauci-immune focal segmental glomerulonephritis in rats [Abstract] Cleve Clin J Med. 2002;69 SII-156. [Google Scholar]
- 33.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk RJ, Jennette JC. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huugen D, Cohen Tervaert JW, Heeringa P. TNF-alpha bioactivity-inhibiting therapy in ANCA-associated vasculitis: clinical and experimental considerations. Clin J Am Soc Nephrol. 2006;1:1100–1107. doi: 10.2215/CJN.02181205. [DOI] [PubMed] [Google Scholar]
- 35.Sinico RA, Gregorini G, Radice A, Campanini M, Pozzi C, Quarenghi M, Napodano P, Giordano A, Ferrario F. Clinical significance of autoantibodies to myeloperoxidase in vasculitic syndromes. Contrib Nephrol. 1991;94:31–37. doi: 10.1159/000420608. [DOI] [PubMed] [Google Scholar]
- 36.Geffriaud-Ricouard C, Noel LH, Chauveau D, Houhou S, Grunfeld JP, Lesavre P. Clinical spectrum associated with ANCA of defined antigen specificities in 98 selected patients. Clin Nephrol. 1993;39:125–136. [PubMed] [Google Scholar]
- 37.Guillevin L, Visser H, Noel LH, Pourrat J, Vernier I, Gayraud M, Oksman F, Lesavre P. Antineutrophil cytoplasm antibodies in systemic polyarteritis nodosa with and without hepatitis B virus infection and Churg-Strauss syndrome-62 patients [see comments] J Rheumatol. 1993;20:1345–1349. [PubMed] [Google Scholar]
- 38.Cohen Tervaert JW, Van Der Woude FJ, Fauci AS, Ambrus JL, Velosa J, Keane WF, Meijer S, van der GM, van der Hem GK, The TH. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–2465. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 39.Nolle B, Specks U, Ludemann J, Rohrbach MS, DeRemee RA, Gross WL. Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener granulomatosis. Ann Intern Med. 1989;111:28–40. doi: 10.7326/0003-4819-111-1-28. [DOI] [PubMed] [Google Scholar]
- 40.Weber MF, Andrassy K, Pullig O, Koderisch J, Netzer K. Antineutrophil-cytoplasmic antibodies and antiglomerular basement membrane antibodies in Goodpasture's syndrome and in Wegener's granulomatosis. J Am Soc Nephrol. 1992;2:1227–1234. doi: 10.1681/ASN.V271227. [DOI] [PubMed] [Google Scholar]
- 41.Chen M, Kallenberg CG, Zhao MH. ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol. 2009;5:313–318. doi: 10.1038/nrneph.2009.67. [DOI] [PubMed] [Google Scholar]
- 42.Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, Lesavre P, Noel LH. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant. 2005;20:1392–1399. doi: 10.1093/ndt/gfh830. [DOI] [PubMed] [Google Scholar]
- 43.Kyndt X, Reumaux D, Bridoux F, Tribout B, Bataille P, Hachulla E, Hatron PY, Duthilleul P, Vanhille P. Serial measurements of antineutrophil cytoplasmic autoantibodies in patients with systemic vasculitis. Am J Med. 1999;106:527–533. doi: 10.1016/s0002-9343(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 44.Kamali S, Erer B, Artim-Esen B, Gul A, Ocal L, Konice M, Aral O, Inanc M. Predictors of damage and survival in patients with Wegener's granulomatosis: analysis of 50 patients. J Rheumatol. 2010;37:374–378. doi: 10.3899/jrheum.090387. [DOI] [PubMed] [Google Scholar]
- 45.Weidner S, Geuss S, Hafezi-Rachti S, Wonka A, Rupprecht HD. ANCA-associated vasculitis with renal involvement: an outcome analysis. Nephrol Dial Transplant. 2004;19:1403–1411. doi: 10.1093/ndt/gfh161. [DOI] [PubMed] [Google Scholar]
- 46.Little MA, Nightingale P, Verburgh CA, Hauser T, de GK, Savage C, Jayne D, Harper L. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69:1036–1043. doi: 10.1136/ard.2009.109389. [DOI] [PubMed] [Google Scholar]
- 47.Franssen CF, Stegeman CA, Kallenberg CG, Gans RO, De Jong PE, Hoorntje SJ, Tervaert JW. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–2206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 49.Lim LC, Taylor JG, III, Schmitz JL, Folds JD, Wilkman AS, Falk RJ, Jennette JC. Diagnostic usefulness of antineutrophil cytoplasmic autoantibody serology. Comparative evaluation of commercial indirect fluorescent antibody kits and enzyme immunoassay kits. Am J Clin Pathol. 1999;111:363–369. doi: 10.1093/ajcp/111.3.363. [DOI] [PubMed] [Google Scholar]