Abstract
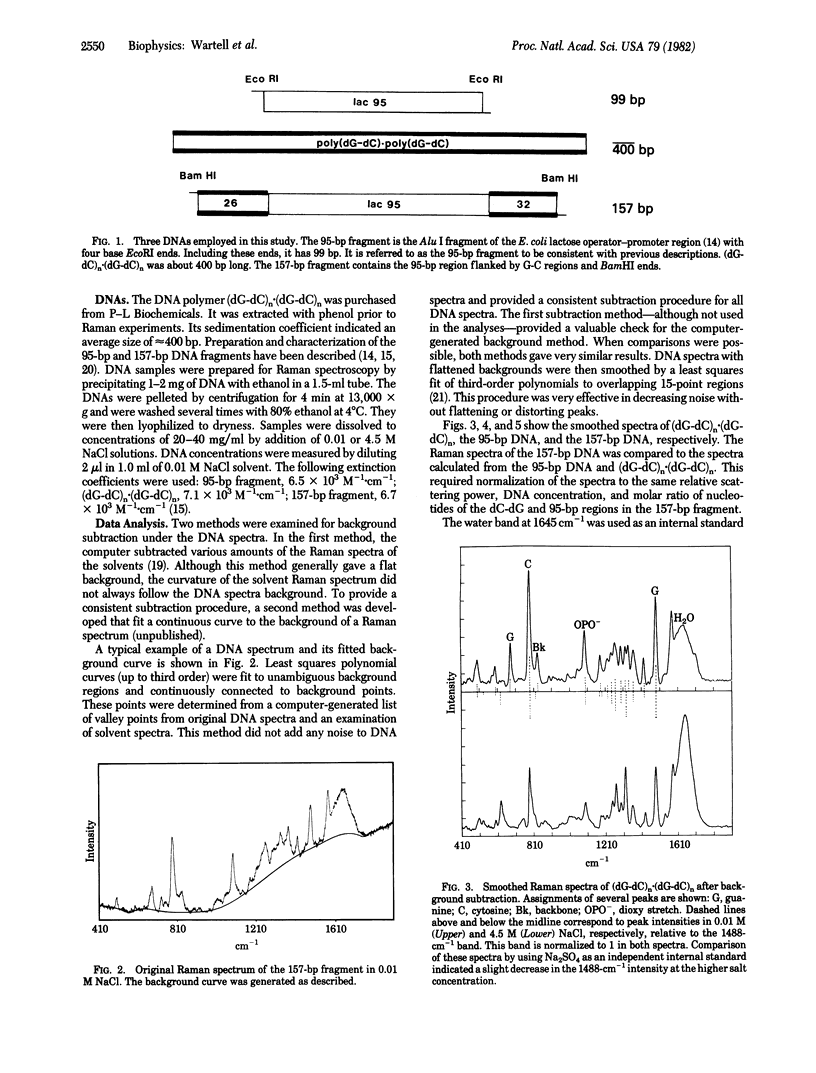
Raman vibrational spectra were obtained from two DNA restriction fragments and the DNA polymer (dG-dC)n . (dG-dC)n in 0.01 and 4.5 M NaCl. One fragment contained 95 base pairs (bp) of the Escherichia coli lactose operator-promoter region (95-bp fragment). The other fragment consisted of the 95-bp region flanked by 26 and 32 bp of dC-dG sequences and BamHI ends (157-bp fragment). In 0.01 M NaCl all three DNAs have Raman spectra characteristic of a right-handed B conformation. The high salt spectrum of the 95-bp fragment is also characteristic of a B conformation. However, the spectrum of the 157-bp fragment in 4.5 M NaCl shows major intensity changes from the 0.01 M NaCl spectrum. These changes are also observed in the high salt spectra of (dG-dC)n . (dG-dC)n and are correlated with the presence of a left-handed Z conformation. Comparisons between the high salt Raman spectra of the 157-bp fragment and spectra calculated from (dG-dC)n . (dG-dC)n and the 95-bp fragment indicated that essentially all of the dC-dG regions in the 157-bp fragment are in the Z conformation and a large part (approximately 80%) of the 95-bp region no longer has a B-type backbone vibration. However, this non-B-DNA-like character of the central region is not indicated by base vibrations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Burd J. F., Larson J. E., Wells R. D. Further studies on telestability in DNA. The synthesis and characterization of the duplex block polymers d(C20A10) - d(T10G20) and d(C20A15) - d(T15G20). J Biol Chem. 1975 Aug 10;250(15):6002–6007. [PubMed] [Google Scholar]
- Burd J. F., Wartell R. M., Dodgson J. B., Wells R. D. Transmission of stability (telestability) in deoxyribonucleic acid. Physical and enzymatic studies on the duplex block polymer d(C15A15) - d(T15G15). J Biol Chem. 1975 Jul 10;250(13):5109–5113. [PubMed] [Google Scholar]
- Chan H. W., Wells R. D. Structural uniqueness of lactose operator. Nature. 1974 Nov 15;252(5480):205–209. doi: 10.1038/252205a0. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Kolpak F. J., Wang A. H., Quigley G. J., van Boom J. H., van der Marel G., Rich A. The tetramer d(CpGpCpG) crystallizes as a left-handed double helix. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4016–4020. doi: 10.1073/pnas.77.7.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Bond P. J., Peticolas W. L. Characterization of the A in equilibrium B transition of DNA in fibers and gels by laser Raman spectroscopy. Biopolymers. 1975 Jun;14(6):1245–1257. doi: 10.1002/bip.1975.360140613. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Kiser E. J., Peticolas W. L. Determination of the backbone structure of nucleic acids and nucleic acid oligomers by laser Raman scattering. Proc Natl Acad Sci U S A. 1972 Apr;69(4):938–941. doi: 10.1073/pnas.69.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfurth S. C., Peticolas W. L. Melting and premelting phenomenon in DNA by laser Raman scattering. Biopolymers. 1975 Feb;14(2):247–264. doi: 10.1002/bip.1975.360140202. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Smith H. O. Restriction and modification enzymes detect no allosteric changes in DNA with bound lac repressor or RNA polymerase. J Mol Biol. 1981 Mar 15;146(4):611–619. doi: 10.1016/0022-2836(81)90049-8. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Patient R. K., Klein R. D., Ho F., Reznikoff W. S., Wells R. D. Construction and mapping of recombinant plasmids used for the preparation of DNA fragments containing the Escherichia coli lactose operator and promoter. J Biol Chem. 1979 Jun 25;254(12):5527–5534. [PubMed] [Google Scholar]
- Hillen W., Klein R. D., Wells R. D. Preparation of milligram amounts of 21 deoxyribonucleic acid restriction fragments. Biochemistry. 1981 Jun 23;20(13):3748–3756. doi: 10.1021/bi00516a013. [DOI] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transmission of allosteric effects in DNA. Nature. 1979 Apr 5;278(5704):521–524. doi: 10.1038/278521a0. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lafleur L., Rice J., Thomas G. J., Jr Raman studies of nucleic acids. VII. Poly A-poly U and poly G-poly C. Biopolymers. 1972;11(12):2423–2437. doi: 10.1002/bip.1972.360111205. [DOI] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Lu K. C., Prohofsky E. W., Van Zandt L. L. Vibrational modes of A-DNA, B-DNA, and A-RNA backbones: an application of a green-function refinement procedure. Biopolymers. 1977 Nov;16(11):2491–2506. doi: 10.1002/bip.1977.360161112. [DOI] [PubMed] [Google Scholar]
- Martin J. C., Wartell R. M., O'Shea D. C. Conformational features of distamycin-DNA and netropsin-DNA complexes by Raman spectroscopy. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5483–5487. doi: 10.1073/pnas.75.11.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Netropsin-poly(dA-dT) complex in solution: structure and dynamics of antibiotic-free base pair regions and those centered on bound netropsin. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5207–5211. doi: 10.1073/pnas.74.12.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Sequence dependence of the helical repeat of DNA in solution. Nature. 1981 Jul 23;292(5821):375–378. doi: 10.1038/292375a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Klug A. Sequence-dependent helical periodicity of DNA. Nature. 1981 Jul 23;292(5821):378–380. doi: 10.1038/292378a0. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Tecklenburg M., Betz J. L. Plasmids containing many tandem copies of a synthetic lactose operator. Gene. 1980 Feb;8(3):279–300. doi: 10.1016/0378-1119(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Selsing E., Wells R. D., Alden C. J., Arnott S. Bent DNA: visualization of a base-paired and stacked A-B conformational junction. J Biol Chem. 1979 Jun 25;254(12):5417–5422. [PubMed] [Google Scholar]
- Selsing E., Wells R. D., Early T. A., Kearns D. R. Two contiguous conformations in a nucleic acid duplex. Nature. 1978 Sep 21;275(5677):249–250. doi: 10.1038/275249a0. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Shindo H. Conformation of 145 base pair length poly (dG-dC) . poly (dG-dC) in solution and in association with histones. Nucleic Acids Res. 1980 May 10;8(9):2093–2103. doi: 10.1093/nar/8.9.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Wartell R. M. Evidence for long-range interactions in DNA. Analysis of melting curves of block polymers d(C15A15)-d(T15G15),d(C20A15)-d(T15G20), and d(T15G20). Biopolymers. 1976 Aug;15(8):1461–1479. doi: 10.1002/bip.1976.360150803. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Goodman T. C., Hillen W., Horn G. T., Klein R. D., Larson J. E., Müller U. R., Neuendorf S. K., Panayotatos N., Stirdivant S. M. DNA structure and gene regulation. Prog Nucleic Acid Res Mol Biol. 1980;24:167–267. doi: 10.1016/s0079-6603(08)60674-1. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Winter R. B., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 2. The Escherichia coli repressor--operator interaction: equilibrium measurements. Biochemistry. 1981 Nov 24;20(24):6948–6960. doi: 10.1021/bi00527a029. [DOI] [PubMed] [Google Scholar]



