Abstract
The diversity of animal and plant forms is shaped by nested evolutionary innovations. Understanding the genetic and molecular changes responsible for these innovations is therefore one of the key goals of evolutionary biology. From the genetic point of view, the origin of novel traits implies the origin of new regulatory pathways to control their development. To understand how these new pathways are assembled in the course of evolution, we need model systems that combine relatively recent innovations with a powerful set of genetic and molecular tools. One such model is provided by the Drosophila sex comb – a male-specific morphological structure that evolved in a relatively small lineage related to the model species D. melanogaster. Our extensive knowledge of sex comb development in D. melanogaster provides the basis for investigating the genetic changes responsible for sex comb origin and diversification. At the same time, sex combs can change on microevolutionary timescales and differ spectacularly among closely related species, providing opportunities for direct genetic analysis and for integrating developmental and population-genetic approaches. Sex comb evolution is associated with the origin of novel interactions between HOX and sex determination genes. Activity of the sex determination pathway was brought under the control of the HOX code to become segment-specific, while HOX gene expression became sexually dimorphic. At the same time, both HOX and sex determination genes were integrated into the intrasegmental spatial patterning network, and acquired new joint downstream targets. Phylogenetic analysis shows that similar sex comb morphologies evolved independently in different lineages. Convergent evolution at the phenotypic level reflects convergent changes in the expression of HOX and sex determination genes, involving both independent gains and losses of regulatory interactions. However, the downstream cell differentiation programs have diverged between species, and in some lineages similar adult morphologies are produced by different morphogenetic mechanisms. These features make the sex comb an excellent model for examining not only the genetic changes responsible for its evolution, but also the cellular processes that translate DNA sequence changes into morphological diversity. The origin and diversification of sex combs provides insights into the roles of modularity, cooption, and regulatory changes in evolutionary innovations, and can serve as a model for understanding the origin of the more drastic novelties that define higher-order taxa.
Keywords: Evolution of development, evolutionary innovations, sexual dimorphism, convergent evolution
The origin of new morphological traits implies the origin of new regulatory pathways to control their development. In many cases, evolutionary innovations reflect the emergence of novel regulatory interactions among previously unconnected genes (Wilkins 2002; Carroll 2005; Carroll 2008). Identifying these changes remains a significant challenge. A fundamental constraint is that innovations are not very common, and rarely occur in convenient model systems. As a result, most developmental-genetic studies of morphological evolution have focused on secondary losses of traits (Swalla and Jeffery 1996; Sucena and Stern 2000; Abouheif and Wray 2002; Cresko et al. 2004; Shapiro et al. 2004; Yamamoto et al. 2004; Protas et al. 2006), while studies addressing the gain of new characters have been less common (Keys et al. 1999; Kopp et al. 2000; Wang and Chamberlin 2004; Moczek et al. 2006; Prud’homme et al. 2006). Intuitively, however, it seems that the genetic basis of innovation should be considerably more complex than the genetic basis of evolutionary loss.
A truly comprehensive understanding of evolutionary innovations must also include the cellular processes that link the evolution of genes to the evolution of phenotypes. What are the downstream consequences of changes in gene expression or protein activity? How do changes in DNA sequences affect cell differentiation and morphogenesis to produce novel adult structures? The sculpting of three-dimensional body parts involves precisely coordinated patterns of cell behavior, so that the origin and diversification of new structures requires a variety of cellular processes to be modified and brought under the control of new regulators.
The Drosophila sex comb, a recently evolved male-specific morphological structure, provides an excellent model for understanding evolutionary innovations. In this review, I describe the genetic control of sex comb development in D. melanogaster, and examine changes in the regulatory pathway that correlate with sex comb diversity. I then focus on the cellular mechanisms of sex comb formation to consider potential proximate causes of phenotypic divergence, and examine the microevolution on sex combs in an effort to understand the intraspecific origin of developmental variation and the evolutionary forces that maintain this variation in nature. Finally, I consider the general lessons that Drosophila sex combs can teach us about the processes responsible for evolutionary innovations.
Sex comb ontogeny and function
The sex comb of D. melanogaster is a male-specific array of modified bristles that develops at a precise position on the prothoracic (T1) leg from a set of precursor bristles present in both sexes (Hannah-Alavah 1958; Tokunaga 1962) (Figure 1). Bristles on the ventral-anterior surface of the distal tibia and the most proximal tarsal segment of the T1 leg are arranged in tightly packed rows perpendicular to the proximo-distal axis of the leg. These transverse bristle rows (TBRs) form a brush that the flies use to clean their head and eyes (Szebenyi 1969), and are present in many Dipterans (McAlpine 1981). In D. melanogaster, the sex comb develops from the most distal TBR on the first tarsal segment (ta1). In males, this TBR rotates 90° clockwise (as viewed from the dorsal side), so that the bristles become oriented from anterior/dorsal to posterior/ventral and point away from the leg (Tokunaga 1962; Held et al. 2004; Atallah et al. 2009a) (Figure 1 B, D, F). In females, all TBRs retain normal, proximal to distal orientation (Figure 1 A, C, E). In addition, the bristles (“teeth”) recruited into the sex comb undergo a number of morphological modifications. Sex comb teeth are curved and blunt rather than straight and pointed like other mechanosensory bristles, heavily melanized, and thicker than regular tarsal bristles (Figure 1 B, D).
Figure 1.
Sex comb structure and ontogeny in D. melanogaster. A. Female T1 leg showing TBRs (arrows). B. Male T1 leg showing the sex comb. C. SEM of a female leg showing the most distal TBR on t1. D. SEM of a male leg showing the sex comb and the area it came from. White arrow points to the “central bristle”, which develops from the same TBR as the sex comb (see also F). E. Schematic drawing of the female t1 showing the arrangement of TBRs. F. Schematic drawing of the male t1 showing sex comb migration and final position. Sex comb teeth are shown in solid grey, and the locations of their precursors in lighter grey. The central bristle develops from the ventral-most bristle precursor of the sex comb-forming TBR (compare with D).
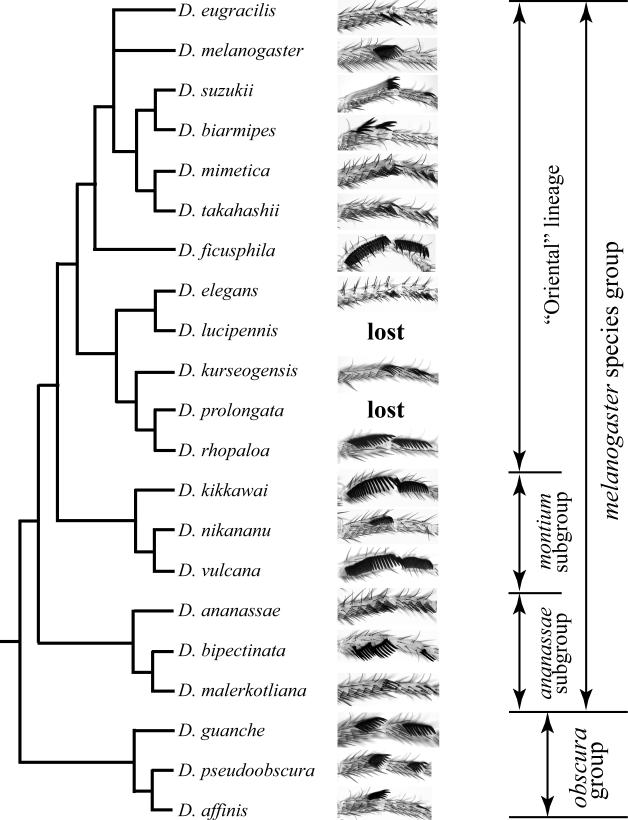
The sex comb is a recently evolved structure; in fact, the vast majority of Drosophila species lack sex combs. It is present only in the melanogaster, obscura, fima, and dentissima species groups of the subgenus Sophophora, and (possibly independently) in the genus Lordiphosa (Tsacas 1980; Tsacas 1981; Lakovaara and Saura 1982; Lemeunier et al. 1986; Hu and Toda 2000; Lachaise and Chassagnard 2002). Dramatic differences exist among closely related species in the orientation of the sex comb, the number of sex comb teeth, and the degree of their modification (Figure 2) (Kopp and True 2002; Barmina and Kopp 2007). In species that lack sex combs, mechanosensory bristle pattern on the T1 leg is identical in males and females, and the female pattern is conserved throughout Drosophilidae. This suggests that the sex comb evolved through sex-specific modification of a pre-existing developmental pathway. Drosophila males use their sex combs as part of stereotypical mating behavior but their exact function varies among species (Spieth 1952), suggesting that tactile interactions between males and females through the male’s sex comb are an important part of the mating ritual and offering a likely explanation for the rapid diversification of this structure.
Figure 2.
Phylogenetic analysis of sex comb evolution in the obscura and melanogaster species groups. The phylogeny, simplified from (Barmina and Kopp 2007), is based on a combined analysis of 14 loci and is strongly supported. Note the differences in sex comb size and orientation between closely related species, and the convergent origin of similar sex combs in distantly related species.
Genetic control of sex comb development: integration of multiple regulatory inputs
Segment specificity
Sex comb development in the T1 leg is promoted by the HOX gene Sex combs reduced (Scr). Sex comb is lost entirely in homozygous Scr null clones (Struhl 1982) and in trans-heterozygous combinations of strong hypomorphic mutations (Lewis et al. 1980; Pattatucci et al. 1991). A strong reduction in the number of sex comb teeth is seen in weaker hypomorphic combinations and in flies heterozygous for Scr nulls or deficiencies (Kaufman et al. 1980; Lewis et al. 1980; Pattatucci et al. 1991). Conversely, Scr duplications increase the number of sex comb teeth (Capdevila et al. 1986; Kennison and Russell 1987; Boube et al. 1997). Moreover, gain-of-function mutations that cause ectopic expression of Scr in T2 and T3 legs are sufficient to induce ectopic sex combs in these legs (Hannah-Alava 1964; Kaufman et al. 1980; Kennison and Tamkun 1988; Pattatucci and Kaufman 1991). Analysis of temperature-sensitive Scr alleles indicates that Scr is required for sex comb development during late third larval instar and early pupal development (Pattatucci et al. 1991). HOX proteins often bind DNA and regulate target genes in heterodimeric complexes with another transcription factor, Extradenticle (Exd) (Mann and Chan 1996). However, exd is not required for sex comb development, although it is essential for other Scr functions (Percival-Smith and Hayden 1998).
Sex specificity
Sexually dimorphic differentiation of most somatic tissues in Drosophila is controlled by a splicing cascade that culminates in the production of sex-specific isoforms of the transcription factor doublesex (dsx) (Baker and Ridge 1980; Baker and Wolfner 1988; Bell et al. 1988; McKeown 1992). The male-specific isoform (dsxM) promotes the development of male-specific structures, including the sex comb, and represses female-specific structures, while the female-specific isoform (dsxF) promotes female-specific and represses male-specific structures (Baker and Ridge 1980; Jursnich and Burtis 1993; Li and Baker 1998; Waterbury et al. 1999). DsxM and DsxF proteins share a common N-terminal DNA-binding domain, but have different C-terminal domains that have different effects on target gene expression (Burtis et al. 1991; Coschigano and Wensink 1993; Erdman and Burtis 1993; Narendra et al. 2002).
In dsx null mutants, both males and females develop a vestigial sex comb: the bristles of the most distal TBR assume morphology intermediate between sex comb teeth and regular bristles, and undergo a partial rotation (Hildreth 1965; Baker and Ridge 1980). A similar intermediate sex comb develops in dominant dsx mutants that express both male- and female-specific dsx isoforms (Baker and Ridge 1980; Nagoshi and Baker 1990). Overexpression of dsxM results in the development of ectopic sex comb teeth (Jursnich and Burtis 1993; Tanaka et al. submitted), whereas dsxF can reduce the number of sex comb teeth when expressed in males (Waterbury et al. 1999). dsx mutant clones induced at the end of larval development produce intermediate sex combs typical of dsx homozygotes, indicating that dsx function is cell-autonomous and required during the pupal stage (Baker and Ridge 1980).
Anterior-Posterior and Dorsal-Ventral axes
Each leg imaginal disc is subdivided into anterior and posterior compartments by the selector gene engrailed (en) and the Hedgehog (Hh) signaling pathway (Morata and Lawrence 1975; Tabata et al. 1995; Zecca et al. 1995; Lawrence and Struhl 1996). Two other signaling pathways, Wingless (Wg) and Decapentaplegic (Dpp), pattern the leg along the dorso-ventral axis, with Wg promoting ventral and Dpp dorsal pattern elements (Baker 1988; Couso et al. 1993; Brook and Cohen 1996; Jiang and Struhl 1996; Penton and Hoffmann 1996). The sex comb and TBRs develop in the ventro-lateral sector of the anterior compartment, in the lateral portion of the Wg-dependent domain. en acts cell-autonomously to restrict sex comb development to the anterior compartment (Tokunaga 1961). In en mutants, an ectopic sex comb and a set of TBRs develop in the posterior compartment in mirror-image to the normal structures; the ectopic sex comb also rotates in the opposite direction (Tokunaga 1961; Mukherjee 1965). Along the DV axis, sex comb development is promoted by wg and repressed by dpp. Sex comb teeth are lost, and tissue rotation fails, in wg hypomorphic mutants and in clones that are deficient for Wg signal transduction (Sharma and Chopra 1976; Theisen et al. 1994). Conversely, ectopic wg expression can induce sex comb development in lateral and dorsal positions in the leg (Struhl and Basler 1993; Wilder and Perrimon 1995; Maves and Schubiger 1998). Ectopic dorsal sex combs also develop in dpp hypomorphs (Spencer et al. 1982; Held et al. 1994) and in mutants that are deficient for Dpp signal transduction (Jiang and Struhl 1996; Penton and Hoffmann 1996; Theisen et al. 1996).
Proximo-distal axis
The patterning of Drosophila leg along the proximo-distal (PD) axis is coordinated by the Wg, Dpp, and EGF signaling pathways, which induce the expression of several transcription factors in concentric domains that correspond to future PD regions of the leg (Diaz-Benjumea et al. 1994; Lecuit and Cohen 1997; Campbell 2002; Galindo et al. 2002). Different combinations of these transcription factors define the identity of specific leg segments (Cohen et al. 1989; Godt et al. 1993; Mardon et al. 1994; Lecuit and Cohen 1997; Duncan et al. 1998; Galindo and Couso 2000; Kojima 2004). The distal first tarsal segment (t1), where the sex comb develops in D. melanogaster, is characterized by coexpression of Distal-less (Dll), dachshund (dac), rotund (rn), and a low level of bric a brac (bab) (Agnel et al. 1992; Godt et al. 1993; Campbell and Tomlinson 1998; Couderc et al. 2002; Galindo et al. 2002). This combination of transcription factors is unique within the developing leg. The tibia, the leg segment just proximal to the tarsus, lacks rn and bab expression, while the second tarsal segment has much lower levels of dac but higher levels of bab (Godt et al. 1993; Abu-Shaar and Mann 1998; Chu et al. 2002; Couderc et al. 2002).
Dll and dac promote sex comb development, whereas bab exerts an inhibitory influence. Hypomorphic Dll alleles reduce the number of sex comb teeth (Cohen et al. 1989) and suppress the extra sex comb phenotype of Polycomb mutants (Kennison and Tamkun 1988). Gain-of-function dac alleles that cause ectopic expression of dac in the more distal tarsal joints induce the development of additional sex combs in these joints (Docquier et al. 1997). Loss or reduction of bab function in the leg leads to the development of ectopic sex combs on t2, t3, and sometimes even on t4 (Godt et al. 1993; Couderc et al. 2002). Conversely, overexpression of bab reduces the number of sex comb teeth (Couderc et al. 2002).
Joint-dependent positioning
In addition to its position along the global PD axis of the leg, the developing sex comb seems to “know” its position within each individual leg segment. In D. melanogaster, the sex comb normally forms only at the distal end of t1. However, mutations or ectopic expression of a variety of genes, including bab, dac, bowl, and sex combs distal lead to the development of ectopic sex combs on t2, and sometimes also t3 and t4 (Kennison 1992; Godt et al. 1993; Boube et al. 1997; Couderc et al. 2002; de Celis Ibeas and Bray 2003). In each case, the ectopic sex combs develop at the distal end of each tarsal segment, skipping its proximal part. Thus, the sex comb becomes discontinuous along the global PD axis of the leg, yet occupies correct PD position within each segment. This rule is only violated in some mutants that disrupt joint formation, leading to the fusion of neighboring leg segments. In such mutants, which include combgap (Datta and Mukherjee 1971), four-jointed (fj) (Tokunaga and Gerhart 1976), and components of the Jak/Stat and Rho/Rac signaling pathways (Kopp, unpublished), sex comb teeth sometimes “spill over” into the proximal t2.
Leg segmentation in Drosophila is controlled by the Notch signaling pathway (de Celis et al. 1998; Bishop et al. 1999; Rauskolb 2001), with an additional role played by a less characterized pathway that involves the secreted Fj protein and the abelson tyrosine kinase (Zeidler et al. 2000; Buckles et al. 2001). Notch signaling is activated in a series of rings located just proximal to each future joint (Bishop et al. 1999; de Celis Ibeas and Bray 2003; Hao et al. 2003). It is possible that Notch controls sex comb development by modifying the expression of PD patterning genes, many of which are modulated in a series of peaks and troughs that depend on Notch signaling (Godt et al. 1993; de Celis et al. 1998; Duncan et al. 1998; Bishop et al. 1999; Rauskolb and Irvine 1999). In particular, bab expression in the leg is partially regulated by bowl, a downstream target of Notch signaling (de Celis Ibeas and Bray 2003).
Integration of sex-specific, homeotic, and intrasegmental patterning cues
As this overview shows, the sex comb must integrate a wide variety of regulatory inputs to develop at the appropriate position. A central role in this integration is played by Scr and dsx (Barmina and Kopp 2007; Tanaka et al. submitted). Scr is expressed at a low level in most of the developing pupal leg, but is strongly upregulated in a ventral-anterior sector of the first tarsal segment and distal tibia, coincident with the future TBR and sex comb region. This expression domain is controlled by dac and bab along the PD axis, and by Wg signaling around the leg circumference (Barmina and Kopp 2007; Shroff et al. 2007; Randsholt and Santamaria 2008). Overexpression of Scr is sufficient to induce sex comb development in distal tarsal segments (Barmina and Kopp 2007). This indicates that quantitative modulation of Scr within the T1 leg defines the proximo-distal extent of the sex comb, and that the main function of dac, bab, and other PD patterning genes in sex comb development is to define the domain of high-level Scr expression. In contrast, even very high levels of Scr can only induce ectopic sex combs on the anterior-ventral leg surface, suggesting that Wg signaling, in addition to regulating Scr expression along the DV axis, is required in parallel with Scr to promote sex comb development (Barmina and Kopp 2007; Shroff et al. 2007).
In D. melanogaster, Scr expression in the T1 leg is sexually dimorphic. In males, Scr protein levels are highest in the distal part of t1 around the developing sex comb, whereas no such upregulation is observed in females (Barmina and Kopp 2007). This modulation is controlled by dsx, and, given the importance of precise quantitative control of Scr expression, plays an important role in determining sex comb morphology and position (Tanaka et al. submitted). dsx itself is expressed in the T1, but not in T2 or T3 legs, and is tightly localized to the presumptive sex comb region in the distal, anterior-ventral part of t1 (Robinett et al., 2010; Tanaka et al., submitted). This expression is activated by Scr during late third larval instar, and is further restricted along the PD axis so that dsx is expressed only in the distal-most portion of the high-level Scr domain. Ectopic expression of dsx in the more proximal parts of t1 is sufficient to induce ectopic sex comb teeth, indicating that sex comb position within the high Scr domain is defined by localized dsx expression (Tanaka et al. submitted).
Thus, Scr and dsx form a positive feedback loop: Scr activates dsx expression during late larval stage, and dsx modulates Scr expression quantitatively in the pupa. This autoregulatory module plays a key role in integrating the homeotic, sex-specific, proximo-distal, and circumferential regulatory inputs to induce sex comb development at a precisely defined position within the leg. The molecular mechanisms of this cross-regulation are not yet clear, and may be different in different cell types. Scr is required in the leg epidermis to delimit sex comb position and in the bristle precursor cells to determine the number of sex comb teeth. Similarly, dsx functions in the epidermis to position the sex comb and in the bristle cells to specify the male-specific morphology of sex comb teeth (Tanaka et al. submitted). Scr protein becomes undetectable in the differentiating sex comb teeth by the end of sex comb rotation, but is strongly upregulated in the adjacent epidermal cells. At the same stage, Dsx protein is present at a high level in the differentiating teeth, but disappears from most of the surrounding epithelium (Tanaka et al. submitted). This suggests that the regulatory interaction between dsx and Scr may be at least partly indirect and involve cell-cell signaling.
A key conclusion emerging from these studies is that the HOX and sex determination genes are not acting like “master regulatory genes” that modulate the output of the spatial patterning network from the outside. Rather, they are deeply integrated inside this network, where they coordinate multiple regulatory inputs and translate them into cell-type-specific differentiation.
Quantitative control of gene expression
An interesting aspect of sex comb development is that many of the regulatory genes including Scr, Dll, and bab (Kaufman et al. 1980; Kennison and Tamkun 1988; Cohen et al. 1989; Godt et al. 1993; Dworkin 2005) affect sex comb size and position in a dosage-sensitive manner. For example, males heterozygous for Scr null alleles have only half of the normal number of sex comb teeth (Kaufman et al. 1980), while males heterozygous for deficiencies that delete both bab paralogs have larger than normal sex combs on t1 as well as ectopic sex comb teeth on t2 (Godt et al. 1993; Couderc et al. 2002). These phenotypes are consistent with the observation that differences in Scr expression between distal and proximal t1, and between distal t1 and t2, are quantitative rather than absolute (Barmina and Kopp 2007; Tanaka et al. submitted). The positive feedback loop between Scr and dsx may be acting as an amplification mechanism that makes Scr and dsx expression levels, and thus sex comb development, particularly sensitive to subtle quantitative changes. This feature may have important implications for understanding the rapid diversification and convergent evolution of sex comb morphology (see below).
Evolution of sex comb morphology: phenotypic patterns and regulatory mechanisms
Rapid diversification and convergent evolution
In the melanogaster and obscura species groups, sex combs vary dramatically in size (the number of teeth), orientation (along or perpendicular to the proximo-distal leg axis), and tooth morphology (size, shape, color, and the presence of bracts) (Lemeunier et al. 1986; Kopp and True 2002). Phylogenetic analysis reveals many instances of rapid divergence and convergent evolution (Barmina and Kopp 2007) (Figure 2). Some of these changes have occurred on short evolutionary time scales. For example, D. bipectinata and D. malerkotliana are closely related, inter-fertile species that diverged well under one million years ago (Kopp and Barmina 2005). However, D. malerkotliana has a transverse sex comb consisting of only 5-6 teeth whose morphology is barely distinguishable from regular mechanosensory bristles, while D. bipectinata has a longitudinal sex comb containing up to 24 teeth that are greatly enlarged, curved, and melanized compared to other bristles (Figure 2). Conversely, the sex comb has been secondarily lost in D. prolongata, whose nearest relatives have some of the largest sex combs found in any species (Figure 2). Pervasive convergent changes affect the size, position, and orientation of the sex comb, as well as the morphology of individual teeth. In particular, large longitudinal sex combs occupying most of t1 and t2 evolved independently in the montium, ficusphila, and rhopaloa subgroups of the melanogaster species group and in some species of the obscura species group (Figure 2). This suggests that some sex-specific regulatory pathways have been reinvented, or at least reestablished, several times.
Evolving expression of Scr and dsx is associated with sex comb diversity
There is a strong correlation between the expression of Scr and dsx and the presence, size, and morphology of the sex comb. In species that primitively lack sex combs, Scr expression is only weakly modulated in pupal T1 legs, and does not differ between males and females (Barmina and Kopp 2007). A similar pattern is seen is species with transverse sex combs, and in species that have secondarily lost sex combs. On the other hand, in species with longitudinal sex combs, Scr is upregulated in the presumptive sex comb region. The distal boundary of high Scr expression corresponds to sex comb position: in species that have sex combs on both t1 and t2, Scr is also upregulated in both segments, while in species that have sex combs only on t1 Scr is upregulated only in t1. The presence of sex combs and high Scr expression in t2 is the ancestral condition for the melanogaster and obscura species groups, and was lost independently in five different clades (Barmina and Kopp, 2007).
dsx shows an equally close correlation with sex comb diversity. In flies that primitively lack sex combs, dsx is not expressed in the homologous region in either sex, while in the melanogaster and obscura species groups the domains of dsx expression in males coincide with sex comb position (Tanaka et al. submitted). For example, in species that have longitudinal sex combs along the entire t1 and t2 segments, dsx expression is expanded to the full length of these segments so that all sex comb teeth express Dsx. In all species, there is a close correspondence between dsx and high Scr expression (Tanaka et al. submitted). Moreover, in species with longitudinal sex combs Scr expression is sexually dimorphic so that both the spatial extent and the cell-by-cell levels of Scr are greater in males than in females (Barmina and Kopp, 2007). On the other hand, flies with transverse sex combs have sexually monomorphic Scr expression. Sex-specific expression of Scr has been gained and lost multiple times within the melanogaster species group, correlating with changes in dsx expression and with the gains and losses of longitudinal sex combs (Barmina and Kopp 2007; Tanaka et al. submitted).
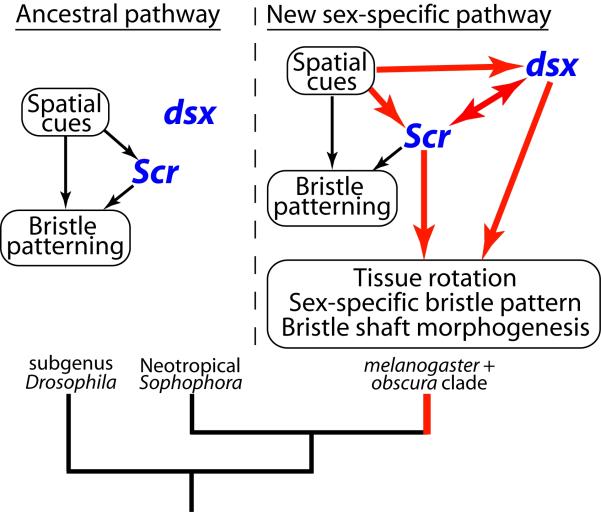
This correlation, combined with the experimental analysis of Scr and dsx functions in D. melanogaster, suggests that the cross-regulation between the HOX and sex determination genes is a general feature of sex comb development, and that changes in this interaction have played a key role in sex comb evolution (Figure 3). dsx expression was absent, and Scr expression was sexually monomorphic, in the ancestral condition. The origin of sex combs required the evolution of Scr-dependent dsx expression in the T1 leg, while the subsequent diversification of sex comb morphology involved repeated gains and losses of dsx-dependent modulation of Scr expression, as well as changes in the regulation of both genes by the PD patterning cues.
Figure 3.
A model of developmental changes involved in the origin of sex combs. Ancestral regulatory interactions are indicated in black, and newly evolved interactions in red. In the ancestral condition (left), leg patterning genes lay down the basic bristle pattern and establish a domain of high Scr expression on the ventral-anterior surface of the distal Ti and ta1. High levels of Scr organize the ventral-anterior bristles into TBRs. dsx is not expressed in the TBRs so they develop in a sexually monomorphic manner. In the melanogaster-obscura clade (right), dsx was recruited into the TBR development pathway under the control of both Scr and leg patterning genes. Scr activates dsx in T1 at the late larval stage, while dsx modulates Scr at the pupal stage to make its expression sexually dimorphic in some species. Both genes have acquired new downstream targets involved in bristle patterning and morphogenesis.
It is conceivable that the positive cross-regulation between Scr and dsx facilitated the rapid divergence and convergence of sex combs. Any mutation affecting the expression of either gene would be amplified by this feedback loop to produce a noticeable phenotypic change in the size and orientation of the sex comb and become “visible” to selection. Male-specific traits involved in courtship and mating are often gained and lost on short evolutionary timescales (Wiens 1999; Kimball et al. 2001; Wiens 2001). In many cases, the rapid evolution of male sexual characters reflects equally rapid changes in female preferences for these traits (Wiens 2001; Wong and Rosenthal 2006). It is tempting to speculate that selection for larger or smaller sex combs has often reversed direction in the melanogaster and obscura species groups, and that the positive cross-regulation between Scr and dsx has allowed these species to respond rapidly to changes in selective pressure.
Did sex combs evolve more than once?
Although sex combs are best described in a monophyletic lineage composed of the melanogaster and obscura species groups in the subgenus Sophophora, these structures are also found in the fima and dentissima species groups, whose phylogenetic positions within Sophophora are not entirely clear (Tsacas 1980; Tsacas 1981; Tsacas and Lachaise 1981b; Tsacas and Lachaise 1981a; Lachaise and Chassagnard 2002), as well as in some species of the little-studied genus Lordiphosa (Gao et al., submitted; Hu and Toda, 2000). In particular, males in the L. miki species group have large longitudinal sex combs that are remarkably similar to those found in the montium and rhopaloa subgroups in Sophophora (Figure 4 B-D, compare with Figure 2), while species in the L. denticeps group have weakly developed transverse sex combs. Phylogenetic analyses using molecular and morphological data suggest that Lordiphosa are the sister group of the Neotropical Sophophora lineage, which includes the willistoni and saltans species groups that lack sex combs (Figure 4 A) (Gao et al., submitted; Hu and Toda, 2000; Katoh et al., 2000). Interestingly, the miki group occupies a clearly derived position within Lordiphosa (Gao et al., submitted). This raises an intriguing possibility that sex combs evolved independently two or three times – once in the common ancestor of the melanogaster and obscura species groups, and once or twice in Lordiphosa (Figure 4 A). Alternatively, sex combs may have originated once in the common ancestor of Sophophora and Lordiphosa, and subsequently lost in several lineages: once in the Neotropical Sophophora, and two or more times within Lordiphosa. The rapid evolution of developmental mechanisms underlying sex comb morphology in the melanogaster and obscura groups makes both of these scenarios plausible. Analysis of sex comb development in the L. miki species group could help resolve this question, but unfortunately no Lordiphosa species are currently available in culture.
Figure 4.
Sex comb morphology and evolution in Lordiphosa. A. Phylogenetic relationships among Lordiphosa and Sophophora lineages (simplified from (Gao et al., submitted; Hu and Toda, 2000; Katoh et al., 2000)). On branches marked with long oblique lines, at least some species have well-developed longitudinal sex combs. On the branch marked with shorter transverse lines, only simple transverse sex combs are present in some species. All other lineages lack sex combs. (B-D) Sex combs in the Lordiphosa miki species group. B. L. clarofinis. C. L. stackelbergi. D. L. magnipectinata.
Cellular processes underlying sex comb diversity
Different sex combs develop from homologous precursors
The most obvious interspecific differences in sex comb morphology concern their orientation: longitudinal (along the PD axis) vs transverse (perpendicular to the PD axis). Due to this distinction, the sex combs of closely related species can differ dramatically despite developing from homologous TBRs. For example, the t1 sex combs of two close relatives, D. mimetica and D. biarmipes, develop from the two most distal TBRs in both species, and have the same number of teeth (9-10 in D. biarmipes vs 9-11 in D. mimetica) (Figure 5). However, in D. mimetica the bristles recruited into the sex comb remain organized into TBRs, so that the only differences between males and females concern the morphology of individual bristles (Figure 5 A, C, E). In D. biarmipes, bristle rows that make up the sex comb are turned 90 degrees relative to the remaining TBRs, while the area normally occupied by these rows is denuded of bristles (Figure 5 B, D, F). This difference is produced by cell rearrangement in the epithelial region surrounding the sex comb in D. biarmipes (Atallah et al. 2009b; Tanaka et al. 2009). Initially, bristle precursor cells at the distal end of t1 are arranged into two TBRs in both males and females of this species. In males, these TBRs undergo a coordinated migration in the anterior-dorsal direction, rotating 90° and coming to rest along the PD leg axis. In contrast, no such movement takes place in D. mimetica. Thus, the sex comb of D. biarmipes is homologous to female TBR bristles and to the transverse sex combs of other Drosophila species. A similar mechanism produces longitudinal sex combs in distantly related species belonging to the obscura species group and to the melanogaster, ananassae, and rhopaloa subgroups of the melanogaster species group (Figure 2) (Atallah et al. 2009b; Tanaka et al. 2009).
Figure 5.
Differences in sex comb development between D. mimetica and D. biarmipes (see Fig. 2 for phylogenetic relationships). A. SEM of D. mimetica male T1 leg. B. SEM of D. biarmipes T1 leg. Arrows in A and B point to the sex combs; note the absence of the t2 comb in D. biarmipes (arrowhead). C, D. Arrangement of TBRs in D. mimetica (C) and D. biarmipes (D) females, shown in orthogonal coordinates. Bristles that develop into sex comb teeth in males are highlighted in black. The orthogonal arrangement of TBR bristles is highly conserved, allowing the homology of sex comb teeth to be established across species. E, F. Schematic representation of adult sex comb morphology in D. mimetica (E) and D. biarmipes (F). Sex comb teeth are shown as solid grey circles. Arrows in F show the path of migrating sex comb precursors. Letters and numbers denote bristle identity according to the orthogonal coordinate system defined in C and D.
Similar sex combs develop from non-homologous precursors
In other species, the presence of longitudinal sex combs is due to a completely different mechanism. D. ficusphila and most species in the montium subgroup have sex comb teeth arranged in a single row along the PD leg axis (Figure 6 B, D-G). The entire ventral-anterior surface of the t1 and t2 segments, which is occupied by 8-10 TBRs in females, is devoid of bristles in males, leaving only the vestiges of the two most proximal TBRs. This difference is not produced by bristle migration, which does not occur in these species. Instead, bristle precursor cells are specified at different positions in males vs females (Atallah et al. 2009b; Tanaka et al. 2009). From this point of view, the sex combs of D. ficusphila and the montium subgroup species are not homologous to the female TBRs, or to the transverse and actively rotating sex combs of other species.
Figure 6.
Differences in sex comb morphology between D. eugracilis and D. ficusphila (see Fig. 2 for phylogenetic relationships). A. SEM of D. eugracilis male T1 leg. Arrows point to sex comb teeth, which are always two in number in this species. B. SEM of D. ficusphila male T1 leg: anterior, ventral, and dorsal views. Think arrows point to the main sex comb row; longer and thinner arrows point to the sparser secondary row; and arrowheads indicate chemosensory bristles. C, D. Arrangement of TBRs in D. eugracilis males (C) and D. ficusphila females (D), shown in orthogonal coordinates. Black bristles in C correspond to the sex comb teeth of D. eugracilis. E. Arrangement of TBRs (grey) and sex comb teeth (black) on the t1 segment in males of D. ficusphila. In this species, sex comb teeth are not homologous to TBR bristles (compare D and E). F, G. Bristle arrangements on the t2 segment in females (F) and males (G) of D. ficusphila. Note the that the number of sex comb teeth in males (black circles in G) is greater than the number of bristles in the homologous region in females (grey circles in F).
Thus, longitudinal sex combs can develop through either sex-specific patterning of bristle precursor cells, or through male-specific migration of sexually monomorphic precursors. These two modes of development must be based on different molecular changes. Coordinated epithelial movement in species with rotating sex combs must require sex-specific regulation of cytoskeletal and cells adhesion molecules. On the other hand, sex comb development in D. ficusphila and the montium subgroup most likely involves changes in the spatial patterning of bristle precursors by proneural genes (Orenic et al. 1993; Joshi 2006). Despite these fundamental differences, the two mechanisms can produce similar adult structures, for example in D. kikkawai and D. rhopaloa, or in D. nikananu and D. melanogaster (Figure 2). Moreover, each mechanism has evolved repeatedly in different clades (Tanaka et al. 2009), suggesting that selection can recruit not only different genes, but completely distinct developmental pathways to meet similar functional requirements in different species.
Multiple mechanisms contribute to variation in sex comb size
The number of sex comb teeth varies from two in D. eugracilis and D. dispar to over 40 in D. ficusphila and most species of the montium subgroup. In species with transverse sex combs, interspecific variation in sex comb size is due solely to the different number of TBR bristles that undergo male-specific modification. In fact, the arrangement of TBRs is so regular and invariant that it is possible to establish the homology of individual sex comb teeth across species (Figures 5, 6). The recruitment of teeth always proceeds from anterior-distal to posterior-proximal bristles. For example, in D. eugracilis only the most anterior bristles in the two most distal TBRs on t1 are enlarged to produce sex comb teeth (Figure 6 A, C). On the other hand, in D. mimetica all bristles in these two TBRs are recruited into the sex comb (Figure 5 A, C, E), while in D. ananassae the sex comb occupies parts of the four most distal TBRs (Figure 2).
In species with rotating sex combs, differences in sex comb size also reflect sex-specific patterning of bristle precursors. In these species, males have fewer TBRs but more bristles per TBR in the presumptive sex comb region. For example, D. melanogaster males have an average of seven TBRs in t1 compared to eight rows in females, but the most distal TBR contains 10-12 bristles in males compared to 3-5 bristles in females. This difference is more extreme in species with larger sex combs. In D. guanche males, the most distal t1 TBR has over 20 bristles, while the more proximal part of the segment carries 3-4 TBRs. In contrast, females have twice as many TBRs in t1 but the distal-most TBR contains only 2-4 bristles. Sexual dimorphism in the number and pattern of bristle precursors is even more pronounced in t2. Males of D. guanche have 20-22 sex comb teeth in this segment that originate as a single TBR (Tanaka et al. 2009), whereas females have only 6-7 bristles arranged in three TBRs. Similar levels of sexual dimorphism are found in species with non-rotating longitudinal sex combs. In D. ficusphila, the number of sex comb teeth in t2 greatly exceeds the number of TBR bristles in females (18-21 vs 9-10) (Figure 6 F, G). In other species with large rotated sex combs, such as D. kikkawai or D. rhopaloa, the number of sex comb teeth on t2 is also 1.5 – 2 fold greater than the number of female bristles.
This dimorphism suggests that Scr and dsx act in conjunction with proximo-distal patterning genes to regulate the specification of bristle precursor cells. In D. melanogaster, overexpression of Scr in t2 induces the development of sex combs on that segment in males, and of supernumerary bristles arranged into small TBRs in females. Comparative evidence also suggests that the development of large rotated sex combs on t2 in some species is caused, at least in part, by distal expansion of Scr expression. In some sense, this can be viewed as a male-specific homeotic transformation of the t2 towards t1 segment identify.
Sculpting the bristles
Sex comb rotation shows a strong correlation with the modification of individual teeth. In species that have transverse sex combs, the teeth do not differ greatly from their homologous female bristles. In such species, which include most members of the ananassae, takahashii, and elegans subgroups, sex comb teeth are somewhat enlarged and thickened, and are usually slightly blunter and darker than other t1 bristles. However, they remain completely or nearly straight and are only weakly melanized, so that the difference between sex comb teeth and “normal” mechanosensory bristles is one of degree rather than kind. On the other hand, in species with longitudinal sex combs the difference between sex comb teeth and regular bristles is qualitative and unmistakable. In such species, which include all members of the montium and melanogaster subgroups, most members of the rhopaloa subgroup, D. ficusphila, D. bipectinata, and several other species, the teeth are usually completely black. They are also enlarged and curved, sometimes dramatically, and in contrast to normal mechanosensory bristles are often rounded at the tips. In some species of the montium subgroup, sex comb teeth have a peculiar flattened or “figure 8” – shaped cross-section (Figure 7).
Figure 8.
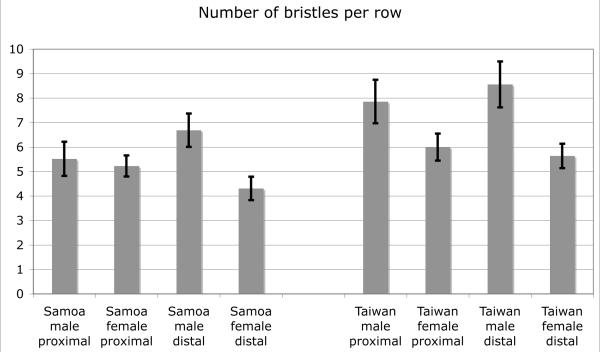
The numbers of sex comb teeth and bristles in homologous TBRs in two strains of D. bipectinata.
Figure 7.
The morphology of sex comb teeth in D. paralutea (takahashii species subgroup), D. suzukii (suzukii subgroup), and D. truncata (montium subgroup). Note the differences in the shape of the bristle cross-section, the number of actin bundles composing the base of each tooth (seen by the grooves along the bristle shaft), the sharpness of bristle tips, and the presence or absence of bracts at the base of sex comb teeth.
We know enough about bristle development to hazard some tentative guesses about the cellular processes that may shape the evolution of sex comb teeth. Bristle size, shape, and curvature are determined by directed deposition and crosslinking of actin filaments (Tilney et al. 1996; Tilney et al. 2004; Tilney and DeRosier 2005). The bristle shaft is a cytoplasmic extension of a single cell (trichogen), and is supported by massive actin bundles positioned around its circumference. The number and arrangement of these bundles can be determined from the regular ridges they form on the bristle surface. Typical mechanosensory bristles on Drosophila legs are composed of ~12-20 such bundles. However, sex comb teeth of many species contain a much greater number of bundles – well over 40 in the case of D. ficusphila and most montium subgroup species. Bristle elongation occurs at the tip, where actin filaments form numerous dynamic microvilli that eventually merge into larger actin bundles that become stabilized by crossbridging and binding to the cell membrane. Variation in the thickness of sex comb teeth is presumably due to differences in the number of actin microvilli and the pattern of their merger into larger, stable bundles.
Differences in actin filament dynamics at the tip of the bristle shaft may also be responsible for interspecific variation in the shape of sex comb teeth. In species with sharply tapered and pointed teeth, as for example in D. suzukii (Figure 7), the rate at which microvilli are initiated may decline steadily as the shaft elongates. In its close relative D. biarmipes, which has rounded sex comb teeth (Figure 5 B), microvilli initiation may occur at a constant rate until the very end of tooth elongation, and then cease abruptly.
In many species, a major distinction between sex comb teeth and homologous female bristles is their strong curvature. The actin bundles that surround the bristle shaft are not contiguous. Rather, they are composed of shorter modules stacked end to end. These modules are arranged in register with each other, so that all the bundles around the circumference of the bristle are interrupted at roughly the same locations along the proximo-distal axis of the bristle shaft. The curvature of the bristle is determined by the relative lengths of the modules on different shaft surfaces (Tilney et al. 1996; Tilney et al. 2004; Tilney and DeRosier 2005). If actin bundles form at the same rate on all bristle surfaces, the bristle will be straight. If, however, bundle elongation occurs at a higher rate on one of the surfaces, the bristle shaft will curve in the opposite direction. The curvature of sex comb teeth must then be determined by the relative rate of actin bundle formation on the superior and inferior shaft surfaces. This rate appears to be controlled by the capping and crosslinking of actin filaments, which stabilizes the bundles against disassembly. If so, the shape of sex comb teeth may be controlled by the expression of capping and crosslinking proteins associated with the actin cytoskeleton.
The nuclei of trichogen cells are polyploid. This is probably necessary for producing the large amounts of actin and actin-associated cytoskeletal proteins that comprise the bristle shaft. It is reasonable to suppose that the size of the bristle is determined, at least in part, by the degree of polyploidization of the trichogen nucleus. If this is the case, interspecific differences in the size of sex comb teeth may reflect the control of endoreplication by Scr, dsx, and other spatial patterning mechanisms. HOX genes have been shown to control both cell size and cell proliferation (Hombria and Lovegrove, 2003; Pavlopoulos and Akam, 2011; Roch and Akam, 2000), so it is not much of a stretch to imagine that one of the functions of Scr and dsx is to control the endoreplication of trichogen cells that make up the sex comb. In contrast to thoracic macrochaetes (Skaer et al., 2002), differences in the timing of precursor formation are unlikely to account for the increased size of sex comb teeth, since they are specified at the same time as most other mechanosensory leg bristles
Linking pattern formation to cell differentiation and morphogenesis
As this overview shows, evolutionary diversification of sex comb morphology is due to changes in a wide range of cellular processes, including cell proliferation, specification of bristle precursor cells, cytoskeleton remodeling and differential cell adhesion required for cell migration, endoreplication, actin cytoskeleton dynamics, and cell-cell signaling. All of these processes show instances of rapid divergence and convergent evolution in the melanogaster and obscura species groups (Figure 2). This implies that the HOX and sex determination genes must regulate, directly or indirectly, a large number of target genes, and that the sets of these target genes are subject to rapid evolutionary turnover. Evolutionary changes may occur both in pattern formation (i.e., the interactions between Scr, dsx, and intrasegmental spatial cues), and in the linkages between pattern formation and morphogenesis (the regulation of downstream target genes by Scr and dsx). Unraveling of these evolutionary changes must necessarily await the identification and comparative analysis of target genes jointly regulated by dsx and Scr (Barmina et al. 2005).
Microevolution of sex combs: genetic architecture and selective forces
As the preceding sections show, the evolution of sex combs involved a number of radical changes in morphology, mode of development, and even the presence of these structures. An obvious question is how these transitions occurred in the course of species divergence. Interspecific differences, no matter how drastic, must originate as intraspecific variation. Although no extant species varies in such fundamental traits as the presence/absence or orientation of the sex combs, some species or complexes of closely related species show more subtle variation in sex comb size and the degree of sexual dimorphism. Analyzing the genetic and developmental basis of these differences may shed some light on the early stages of sex comb divergence.
Developmental basis of variation in sex comb size
Are differences in sex comb size due to changes in a male-specific developmental pathway, or do they simply reflect the formation of a greater number of bristle precursor cells in both sexes? D. bipectinata, a species widespread in Southeast Asia, has extensive heritable variation in sex comb size, with the mean number of teeth varying >1.5-fold among populations (13 – 20 teeth). The t1 sex comb in this species consists of two rows, which develop by active rotation and correspond to the two most distal TBRs in females. The number of sex comb teeth in both proximal and distal rows is higher in the Taiwan strain than in the Samoa strain (P = 10−14 – 10−17) (Figure 8), but the number of bristles in the homologous TBRs in females is also significantly greater in Taiwan than in Samoa (P = 0.0005 – 10−7). However, the number of sex comb teeth in the proximal row in Samoa males is not significantly greater than the number of bristles in the corresponding TBR in Samoa females (P = 0.082), whereas in the Taiwan strain this difference is highly significant (P = 10−10). Thus, variation in sex comb size in D. bipectinata is due to a combined effect of male-specific and non-sex-specific developmental mechanisms. A similar dual mechanism seems to operate in D. melanogaster, where artificial selection for increased or decreased sex comb size leads to a correlated but weaker change in the number of bristles in the homologous female TBR (Ahuja and Singh 2008).
Genetics of interspecific differences
Several pairs of closely related, partially inter-fertile Drosophila species differ in the number of sex comb teeth, providing an opportunity to investigate the genetic basis of interspecific divergence. A number of studies have shown that these differences are controlled by multiple loci. In D. simulans and D. mauritiana, the mean sex comb size is 10.12 vs 13.93 teeth, respectively. In an F2 backcross, most of this difference mapped to the 3rd chromosome, where at least two major QTL intervals were detected, while the X chromosome had little or no effect (True et al. 1997). A more detailed dissection has shown that one of these regions, which had the strongest phenotypic effect, contains at least two separate QTLs (Graze et al. 2007). In D. yakuba and D. santomea, in contrast, the X chromosome had the strongest effect on the interspecific difference in sex comb size (Coyne et al. 2004), although that study lacked sufficient power to estimate the number of contributing QTLs. In crosses between D. simulans and D. sechellia, at least 4 QTLs influencing the number of sex comb teeth were detected (Macdonald and Goldstein 1999). In this case, most of the interspecific difference maps to the 2nd chromosome, while the 3rd has no detectable effect. Interestingly, the X chromosome has an effect in the opposite direction to the overall species difference. Similarly, two major 3rd-chromosomal QTLs in the D. simulans – D. mauritiana cross also affect the phenotype in opposite directions (True et al. 1997). These observations suggest that the differences in sex comb size do not necessarily result from directional selection.
A major weakness of all these studies is that the differences between the parental strains for interspecific crosses are on the same order as among-strain variation within each species, and it is likely that most genetic variation segregates within species. Thus, the results of QTL mapping in interspecific crosses depend strongly on the choice of parental strains, and at least some of the QTLs identified in these crosses may in fact reflect intraspecific standing variation. Identification of these genes would tell us less about the evolution of developmental pathways than about the maintenance of (potentially deleterious or neutral) variation in these pathways.
Genetics of intraspecific variation
The genetic basis of sex comb size variation has also been studied within species. Using recombinant inbred lines in D. melanogaster, Nuzhdin and Reiwitch (Nuzhdin and Reiwitch 2000) found 2 significant QTLs, both located on the X chromosome and acting in opposite directions. However, these QTLs accounted for only 8% of the difference between parental strains, pointing to the existence of other, even weaker QTLs. Using a similar approach but different parental strains of D. melanogaster, Kopp et al. detected multiple QTLs on all chromosomes, all of them weak and many acting in opposite directions (Kopp et al. 2003). In crosses between two strains of D. simulans, several QTLs were found on the 2nd and 3rd chromosomes (Tatsuta and Takano-Shimizu 2006). Most of the difference was explained by two QTLs on the 3rd chromosome , both acting in same direction with large additive effects (44% and 48%); however, weaker QTLs acted in both directions. As in interspecific crosses, QTLs acting in the opposite direction to the overall strain difference appear to be common, suggesting that directional selection is unlikely to be the main cause of variation. The main conclusion emerging from these studies is that intraspecific variation in sex comb size is shaped by the segregation of a large number of small-effect QTLs. It is far from certain that these QTLs alleles provide the material for interspecific divergence, or even affect the same loci that are responsible for macroevolutionary changes in sex comb morphology.
Sex combs and mating behavior
An obvious hypothesis is that the rapid diversification of sex comb morphology is driven by sexual selection. Even in species that primitively lack sex combs, males use their front legs to grasp the base of the female’s wings or the abdomen during mounting and copulation. In species with particularly exaggerated sex combs, such as those in the montium subgroup, males aggressively grasp the female with their front legs, and the T1 tarsi with the sex combs are used to maintain the male’s hold on the female’s abdomen (Spieth, 1952). In contrast, in the obscura species group and in D. melanogaster and D. simulans, sex combs are not in contact with the female’s body once the male assumes the final copulatory position. In the obscura group, male grasps the dorsolateral surfaces of the female abdomen, but this contact is brief; afterwards, the male uses the tarsi to spread the female’s wings. In D. melanogaster and D. simulans, sex combs are used transiently to grasp the female genitalia. Overall, the functions of sex combs are quite different in different species, suggesting that sex comb morphology may coevolve with male and female mating behavior. During courtship and mating, male sex combs come into contact with mechanosensory bristles that cover the female abdomen and genitalia. It is possible that females use these mechanoreceptors to evaluate the size and shape of the male sex combs. Unfortunately, Drosophila courtship has not been videorecorded with sufficient resolution and speed to identify the female’s receptors that interact with sex combs, or to determine when this interaction first occurs during courtship.
The behavioral functions of sex combs have been tested in several species by surgical (Spieth 1952; Cook 1977; Coyne 1985) or genetic (Ng and Kopp 2008) removal. For example, in D. pseudoobscura and D. persimilis, surgical removal of the males’ front tarsi above the sex comb greatly reduces mating success, whereas removal below the sex comb has no effect. Direct observations showed that operated males court vigorously and attempt copulation repeatedly, but are unable to achieve it (Spieth 1952). More recently, Ng and Kopp (Ng and Kopp 2008) ablated sex combs genetically by expressing the female isoform of transformer in the tarsal segments. This treatment had no effect on the males’ vigor and courtship activity, but led to their rejection by females and a severe reduction in mating success.
Both surgical and genetic ablation experiments suggest that Drosophila females are capable of mate choice based partly on the presence of sex combs. However, it remains to be determined whether females can perceive subtle differences in sex comb size and morphology. Some clues are provided by field observations. In D. bipectinata, studies in a wild population have shown that males caught copulating with females had significantly more sex comb teeth than non-copulating males captured on the same fruit, suggesting that the sex comb is under sexual selection for increased size in this population (Polak et al. 2004). Similar experiments were performed in D. simulans and D. pseudoobscura. In D. simulans, copulating males had fewer sex comb teeth than non-copulating ones, while in D. pseudoobscura sex comb size did not differ significantly between copulating and non-copulating males (Markow et al. 1996). Comparison across species is difficult since each species was studied only once in a single population. It appears, however, that selective pressures may be different in different species, explaining the rapid diversification of sex combs. The role of sexual selection in sex comb evolution is still poorly understood and requires closer investigation.
Conclusions: lessons from sex comb evolution
As evolutionary innovations go, the sex comb is a modest one – no more than a set of modified bristles that have clear homologues in the ancestral condition. The very simplicity of this structure raises an exciting prospect of reconstructing the entire developmental pathway responsible for sex comb origin and diversification, from the upstream changes in pattern formation and cell fate to the downstream cellular processes involved in terminal differentiation. By following the assembly of this pathway through the Drosophila phylogeny, we may be able to trace how the gain of novel gene interactions led to the formation of a new structure. There is good reason to expect that the sex comb will provide a useful paradigm for understanding the genetic mechanisms of evolutionary innovations in general.
Innovation through cooption?
At the same time, this simplicity is relative. Sex comb development requires integration of diverse cellular processes, suggesting that the origin of this structure required evolutionary changes in the expression or function of many genes. An obvious question is how such changes can be achieved in a sufficiently coordinated fashion to yield a coherent developmental program. At least part of the solution may lie in the modular organization of development (von Dassow and Munro 1999; Schlosser and Wagner 2004; Monteiro and Podlaha 2009). Cell differentiation programs often exist as genetic modules where a small number of regulator genes control the expression of larger batteries of effector genes that are necessary and sufficient for cell-type-specific differentiation. In sex comb development, tissue rotation and the modification of bristle shafts are entirely different cellular processes that presumably involve different sets of genes, and it is easy to imagine that they evolved separately in other developmental contexts before being integrated into a common pathway. For instance, sex combs could have co-opted a pre-existing genetic module for making thick, blunt, highly melanized bristles such as those found on the genitalia and anal plates of many Drosophila species (Kopp and True 2002; Ashburner et al. 2005). Sex comb rotation occurs by a process of directional cell intercalation (Atallah et al. 2009a), which is a common and highly conserved cellular program deployed, for example, during germband extension in the Drosophila embryo (Zallen and Blankenship 2008). Thus, the novel developmental pathway responsible for the origin of sex combs could have evolved by bringing several older but previously unconnected developmental modules under the control of a common set of upstream regulators. A key advantage of such modules is that they come fully integrated so that innovation does not require a major increase in genetic complexity but is simply a matter of applying previously optimized cellular machinery for new purposes.
Diversification of morphological structures may also be facilitated by serial homology (Hall 1995; Ruvinsky and Gibson-Brown 2000; Monteiro 2008). In the sex comb, serial homology operates at three levels. First, each bristle represents a discrete unit yet all bristles share the same developmental program. Second, the TBRs are serially homologous and develop in similar ways. Finally, the first and second tarsal segments are also serially homologous units. Thus, sex comb morphology can be changed by applying the same developmental modifications to more or fewer of the available precursor modules. In other words, diversity can arise through individualization of previously identical serial homologs.
Innovation and homology
If evolving a sex comb requires nothing more than wiring together pre-existing genetic and cellular modules, is it truly “an innovation”? Evolutionary innovations are sometimes defined as traits that are “not homologous to any structure in the ancestral species” (Muller and Wagner 1991). However, this definition is easier to apply to morphological structures than to developmental processes that produce them. Homology relationships are often different depending on the level of biological organization one chooses to look at. We have seen, for example, that morphologically similar sex combs can develop from different precursor cells in different species. Such sex combs are homologous as adult structures, in the sense that the last common ancestor of these species had a sex comb, but not homologous as cell populations. Conversely, any morphological structure, no matter how novel, must necessarily evolve from a pre-existing cell population and thus will have a homolog at the cellular level. By focusing on cell populations or cell fates defined by the expression of conserved genetic modules (Wagner 2007), we can trace the probable evolutionary precursors even for such dramatic innovations as Lepidopteran wing scales (Galant et al. 1998; Zhou et al. 2009), insect wings (Averof and Cohen 1997; Damen et al. 2002), or the vertebrate neural crest (Sauka-Spengler and Bronner-Fraser, 2008; Yu, 2010). In this view, it is difficult to draw a sharp boundary between “innovation” and “modification” since many apparent innovations will occur through the cooption, modification, and integration of pre-existing developmental modules into novel pathways. Signatures of such assembly can be seen not only in genetic networks, but also in morphologies preserved in the fossil record (Vermeij 2006). The sex comb offers a good example of this process and may provide new insights into the mechanisms of the more dramatic innovations that occurred deeper in evolutionary time.
Master regulatory genes can evolve rapidly
In Drosophila and other insects, the HOX and sex determination genes are among the most upstream regulators of development, yet both have clearly evolved among closely related species and have played key roles in the origin and diversification of a structure that is absent even in most Drosophila species. In this context, both Scr and dsx can be viewed not so much as master regulators as “micromanagers” (Akam 1998). The involvement of these genes in sex comb evolution is made possible by the precise spatial control of their expression. Independent regulation of genes in different tissues or at different stages of development is one of the defining metazoan features that facilitates phenotypic evolution (Carroll 2008). Thus, any transcription factor can be regulated independently, and control different batteries of downstream genes, in different cell types. Since most if not all regulatory genes are re-used multiple times during development, the concept of a “master regulator” can only be applied to a particular cell type and stage of development, not to the organism as a whole. Thus, there is little reason to expect that any particular class of regulatory genes will be immune from rapid evolutionary change.
Functional shifts and morphological diversity
In lineages that primitively lack sex combs, the arrangement of bristles is virtually identical in all species, likely reflecting their common ancestral function as cleaning utensils. Yet many species in the melanogaster and obscura groups can be distinguished by their sex combs alone, suggesting that morphological diversification is linked to the acquisition of a new function. The same is likely true for all innovations, such as beetle horns (Emlen et al. 2007; Moczek et al. 2007), Lepidopteran wing scales (Nijhout 1991), or the abdominal appendages of Sepsid flies (Eberhard 2001; Bowsher and Nijhout 2007). An evolutionary innovation can be reasonably defined as “a newly evolved structure or condition that enables its phylogenetically derived bearer to perform a new function” (Vermeij 2006), so the study of evolutionary novelties should be undertaken together with the ecological and behavioral context in which these innovations originate and diversify.
The Drosophila sex comb achieves a happy balance – complex enough to be interesting, yet simple enough to be genetically tractable; raising important macroevolutionary questions yet also providing microevolutionary models; displaying spectacular interspecific diversity yet offering the advantages of the Drosophila genetic and molecular toolkit. Understanding the development, origin, and diversification of this structure can make an important contribution to our knowledge of the genetic mechanisms of evolutionary innovations.
ACKNOWLEDGEMENTS
I am grateful to Dr. M. Toda for providing Lordiphosa specimens and discussing his unpublished work, and to Joel Atallah for comments on the manuscript. Drosophila species were obtained from the US National Drosophila Species stock center or collected by members of our lab. Work in our lab is supported by NIH grant 5-R01GM082843-02 and NSF grant IOS-0815141.
REFERENCES
- Abouheif E, Wray GA. Evolution of the gene network underlying wing polyphenism in ants. Science. 2002;297(5579):249–252. doi: 10.1126/science.1071468. [DOI] [PubMed] [Google Scholar]
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125(19):3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Agnel M, Roder L, Griffin-Shea R, Vola C. The spatial expression of Drosophila rotund gene reveals that the imaginal discs are organised in domains along the proximal-distal axis. Roux Arch dev Biol. 1992;201(5):284–295. doi: 10.1007/BF00592109. [DOI] [PubMed] [Google Scholar]
- Ahuja A, Singh RS. Variation and evolution of male sex combs in Drosophila: nature of selection response and theories of genetic variation for sexual traits. Genetics. 2008;179(1):503–509. doi: 10.1534/genetics.107.086363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam M. Hox genes: from master genes to micromanagers. Curr Biol. 1998;8(19):R676–678. doi: 10.1016/s0960-9822(98)70433-6. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Golic KG, Hawley RS. Drosophila : a laboratory handbook. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2005. [Google Scholar]
- Atallah J, Liu NH, Dennis P, Hon A, Godt D, Larsen EW. Cell dynamics and developmental bias in the ontogeny of a complex sexually dimorphic trait in Drosophila melanogaster. Evol Dev. 2009a;11(2):191–204. doi: 10.1111/j.1525-142X.2009.00319.x. [DOI] [PubMed] [Google Scholar]
- Atallah J, Liu NH, Dennis P, Hon A, Larsen EW. Developmental constraints and convergent evolution in Drosophila sex comb formation. Evol Dev. 2009b;11(2):205–218. doi: 10.1111/j.1525-142X.2009.00320.x. [DOI] [PubMed] [Google Scholar]
- Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385(6617):627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- Baker BS, Ridge KA. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics. 1980;94(2):383–423. doi: 10.1093/genetics/94.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BS, Wolfner MF. A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster. Genes Dev. 1988;2(4):477–489. doi: 10.1101/gad.2.4.477. [DOI] [PubMed] [Google Scholar]
- Baker NE. Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development. 1988;102(3):489–497. doi: 10.1242/dev.102.3.489. [DOI] [PubMed] [Google Scholar]
- Barmina O, Gonzalo M, McIntyre L, Kopp A. Sex- and segment-specific modulation of gene expression profiles in Drosophila. Dev Biol. 2005;288:528–544. doi: 10.1016/j.ydbio.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Barmina O, Kopp A. Sex-specific expression of a HOX gene associated with rapid morphological evolution. Dev Biol. 2007;311(2):277–286. doi: 10.1016/j.ydbio.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Bell LR, Maine EM, Schedl P, Cline TW. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Bishop SA, Klein T, Arias AM, Couso JP. Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development. 1999;126(13):2993–3003. doi: 10.1242/dev.126.13.2993. [DOI] [PubMed] [Google Scholar]
- Boube M, Benassayag C, Seroude L, Cribbs DL. Ras1-mediated modulation of Drosophila homeotic function in cell and segment identity. Genetics. 1997;146(2):619–628. doi: 10.1093/genetics/146.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher JH, Nijhout HF. Evolution of novel abdominal appendages in a sepsid fly from histoblasts, not imaginal discs. Evol Dev. 2007;9(4):347–354. doi: 10.1111/j.1525-142X.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Brook WJ, Cohen SM. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila Leg. Science. 1996;273(5280):1373–1377. doi: 10.1126/science.273.5280.1373. [DOI] [PubMed] [Google Scholar]
- Buckles GR, Rauskolb C, Villano JL, Katz FN. Four-jointed interacts with dachs, abelson and enabled and feeds back onto the Notch pathway to affect growth and segmentation in the Drosophila leg. Development. 2001;128(18):3533–3542. doi: 10.1242/dev.128.18.3533. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Coschigano KT, Baker BS, Wensink PC. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. Embo J. 1991;10(9):2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature. 2002;418(6899):781–785. doi: 10.1038/nature00971. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125(22):4483–4493. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Botas J, Garcia-Bellido A. Genetic interactions between the Polycomb locus and the Antennapedia and Bithorax complexes of Drosophila. Roux Arch dev Biol. 1986;195(7):417–432. doi: 10.1007/BF00375746. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Evolution at two levels: on genes and form. PLoS Biol. 2005;3(7):e245. doi: 10.1371/journal.pbio.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- - Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134(1):25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Chu J, Dong PD, Panganiban G. Limb type-specific regulation of bric a brac contributes to morphological diversity. Development. 2002;129(3):695–704. doi: 10.1242/dev.129.3.695. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Bronner G, Kuttner F, Jurgens G, Jackle H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989;338(6214):432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Cook RM. Behavioral role of the sexcombs in Drosophila melanogaster and Drosophila simulans. Behav Genet. 1977;7(5):349–357. doi: 10.1007/BF01077448. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993;7(1):42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Couderc JL, Godt D, Zollman S, Chen J, Li M, Tiong S, Cramton SE, Sahut-Barnola I, Laski FA. The bric a brac locus consists of two paralogous genes encoding BTB/POZ domain proteins and acts as a homeotic and morphogenetic regulator of imaginal development in Drosophila. Development. 2002;129(10):2419–2433. doi: 10.1242/dev.129.10.2419. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259(5094):484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Coyne JA. Genetic studies of three sibling species of Drosophila with relationship to theories of speciation. Genet Res. 1985;46(2):169–192. doi: 10.1017/s0016672300022643. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Elwyn S, Kim SY, Llopart A. Genetic studies of two sister species in the Drosophila melanogaster subgroup, D. yakuba and D. santomea. Genet Res. 2004;84(1):11–26. doi: 10.1017/s0016672304007013. [DOI] [PubMed] [Google Scholar]
- Cresko WA, Amores A, Wilson C, Murphy J, Currey M, Phillips P, Bell MA, Kimmel CB, Postlethwait JH. Parallel genetic basis for repeated evolution of armor loss in Alaskan threespine stickleback populations. Proc Natl Acad Sci U S A. 2004;101(16):6050–6055. doi: 10.1073/pnas.0308479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen WG, Saridaki T, Averof M. Diverse adaptations of an ancestral gill: a common evolutionary origin for wings, breathing organs, and spinnerets. Curr Biol. 2002;12(19):1711–1716. doi: 10.1016/s0960-9822(02)01126-0. [DOI] [PubMed] [Google Scholar]
- Datta RK, Mukherjee AS. Developmental genetics of the mutant combgap in Drosophila melanogaster. I. Effect on the morphology and chaetotaxy of the prothoracic leg. Genetics. 1971;68(2):269–286. doi: 10.1093/genetics/68.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis Ibeas JM, Bray SJ. Bowl is required downstream of Notch for elaboration of distal limb patterning. Development. 2003;130(24):5943–5952. doi: 10.1242/dev.00833. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Tyler DM, de Celis J, Bray SJ. Notch signalling mediates segmentation of the Drosophila leg. Development. 1998;125(23):4617–4626. doi: 10.1242/dev.125.23.4617. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372(6502):175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- Docquier F, Randsholt NB, Santamaria P. Gain of function mutations of the dachshund (dac) gene induce proximo-distal leg transformations in Drosophila melanogaster. Int J Dev Biol. 1997;41(5):13S. [Google Scholar]
- Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12(9):1290–1303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin I. Evidence for canalization of Distal-less function in the leg of Drosophila melanogaster. Evol Dev. 2005;7(2):89–100. doi: 10.1111/j.1525-142X.2005.05010.x. [DOI] [PubMed] [Google Scholar]
- Eberhard WG. Multiple origins of a major novelty: moveable abdominal lobes in male sepsid flies (Diptera: Sepsidae), and the question of developmental constraints. Evol Dev. 2001;3(3):206–222. doi: 10.1046/j.1525-142x.2001.003003206.x. [DOI] [PubMed] [Google Scholar]
- Emlen DJ, Lavine L. Corley, Ewen-Campen B. On the origin and evolutionary diversification of beetle horns. Proc Natl Acad Sci U S A. 2007;104(Suppl 1):8661–8668. doi: 10.1073/pnas.0701209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Burtis KC. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. Embo J. 1993;12(2):527–535. doi: 10.1002/j.1460-2075.1993.tb05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant R, Skeath JB, Paddock S, Lewis DL, Carroll SB. Expression pattern of a butterfly achaete-scute homolog reveals the homology of butterfly wing scales and insect sensory bristles. Curr Biol. 1998;8(14):807–813. doi: 10.1016/s0960-9822(98)70322-7. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Bishop SA, Greig S, Couso JP. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science. 2002;297(5579):256–259. doi: 10.1126/science.1072311. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Couso JP. Intercalation of cell fates during tarsal development in Drosophila. Bioessays. 2000;22(9):777–780. doi: 10.1002/1521-1878(200009)22:9<777::AID-BIES1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Godt D, Couderc JL, Cramton SE, Laski FA. Pattern formation in the limbs of Drosophila: bric a brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119(3):799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- Graze RM, Barmina O, Tufts D, Naderi E, Harmon KL, Persianinova M, Nuzhdin SV. New candidate genes for sex comb divergence between Drosophila mauritiana and Drosophila simulans. Genetics. 2007 doi: 10.1534/genetics.106.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. Homology and embryonic development. In: M.K. H, R.J. M, M.T. C, editors. Evolutionary Biology. Plenum Press; New York: 1995. pp. 1–37. [Google Scholar]
- Hannah-Alava A. Interaction of Non-Allelic Loci in Expression of the Extra-Sexcomb Phenotype in Drosophila Melanogaster. Z Vererbungsl. 1964;95:1–9. doi: 10.1007/BF00898179. [DOI] [PubMed] [Google Scholar]
- Hannah-Alavah A. Morphology and chaetotaxy of the legs of Drosophila melanogaster. J Morph. 1958;103:281–310. [Google Scholar]
- Hao I, Green RB, Dunaevsky O, Lengyel JA, Rauskolb C. The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev Biol. 2003;263(2):282–295. doi: 10.1016/j.ydbio.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Held LI., Jr. Bristles induce bracts via the EGFR pathway on Drosophila legs. Mech Dev. 2002;117(1-2):225–234. doi: 10.1016/s0925-4773(02)00212-5. [DOI] [PubMed] [Google Scholar]
- Held LI, Jr., Grimson MJ, Du Z. Proving an old prediction: The sex comb rotates at 16 to 24 hours after pupariation. D I S. 2004;87 [Google Scholar]
- Held LI, Jr., Heup MA, Sappington JM, Peters SD. Interactions of decapentaplegic, wingless, and Distal-less in the Drosophila leg. Roux’s Arch Dev Biol. 1994;203:310–319. doi: 10.1007/BF00457802. [DOI] [PubMed] [Google Scholar]
- Hildreth PE. Doublesex, Recessive Gene That Transforms Both Males and Females of Drosophila into Intersexes. Genetics. 1965;51:659–678. doi: 10.1093/genetics/51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombria JC, Lovegrove B. Beyond homeosis--HOX function in morphogenesis and organogenesis. Differentiation. 2003;71(8):461–476. doi: 10.1046/j.1432-0436.2003.7108004.x. [DOI] [PubMed] [Google Scholar]
- Hu Y-G, Toda MJ. Polyphyly of Lordiphosa and its relationships in Drosophilinae (Diptera: Drosophilidae) Systematic Entomology. 2000;25:1–17. [Google Scholar]
- Jiang J, Struhl G. Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell. 1996;86(3):401–409. doi: 10.1016/s0092-8674(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Joshi M, Buchanan K, Shroff S, Orenic TV. Delta and Hairy establish a periodic prepattern that positions sensory bristles in Drosophila legs. Dev Biol. 2006;293(1):64–76. doi: 10.1016/j.ydbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Jursnich VA, Burtis KC. A positive role in differentiation for the male doublesex protein of Drosophila. Dev Biol. 1993;155(1):235–249. doi: 10.1006/dbio.1993.1021. [DOI] [PubMed] [Google Scholar]
- Katoh T, Tamura K, Aotsuka T. Phylogenetic position of the subgenus Lordiphosa of the genus Drosophila (Diptera: Drosophilidae) inferred from alcohol dehydrogenase (Adh) gene sequences. J Mol Evol. 2000;51(2):122–130. doi: 10.1007/s002390010072. [DOI] [PubMed] [Google Scholar]
- Kaufman TC, Lewis RA, Wakimoto BT. Genetic analysis of chromosome 3 in Drosophila melanogaster: the homeotic gene complex in polytene chromosome interval 84A-B. Genetics. 1980;94:115–133. doi: 10.1093/genetics/94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison JA. Entry for scd. In: Lindsley DL, Zimm GG, editors. The genome of Drosophila melanogaster. Academic Press; San Diego: 1992. [Google Scholar]
- Kennison JA, Russell MA. Dosage-dependent modifiers of homeotic mutations in Drosophila melanogaster. Genetics. 1987;116:75–86. doi: 10.1093/genetics/116.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison JA, Tamkun JW. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc Natl Acad Sci U S A. 1988;85(21):8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys DN, Lewis DL, Selegue JE, Pearson BJ, Goodrich LV, Johnson RL, Gates J, Scott MP, Carroll SB. Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science. 1999;283(5401):532–534. doi: 10.1126/science.283.5401.532. [DOI] [PubMed] [Google Scholar]
- Kimball RT, Braun EL, Ligon DJ, Lucchini V, Randi E. A molecular phylogeny of the peacock-pheasants (Galliformes Polyplectron spp.) indicates loss and reduction of ornamental traits and display behaviours. Biological Journal of the Linnean Society. 2001;73(2):187–198. [Google Scholar]
- Kojima T. The mechanism of Drosophila leg development along the proximodistal axis. Dev Growth Differ. 2004;46(2):115–129. doi: 10.1111/j.1440-169X.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Kopp A, Barmina O. Evolutionary history of the Drosophila bipectinata species complex. Genet Res. 2005;85:23–46. doi: 10.1017/s0016672305007317. [DOI] [PubMed] [Google Scholar]
- Kopp A, Duncan I, Godt D, Carroll SB. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature. 2000;408(6812):553–559. doi: 10.1038/35046017. [DOI] [PubMed] [Google Scholar]
- Kopp A, Graze RM, Xu S, Carroll S, Nuzhdin SV. Quantitative trait loci responsible for variation in sexually dimorphic traits in Drosophila melanogaster. Genetics. 2003;163:771–787. doi: 10.1093/genetics/163.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A, True JR. Evolution of male sexual characters in the oriental Drosophila melanogaster species group. Evol Devel. 2002;4(4):278–291. doi: 10.1046/j.1525-142x.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- Lachaise D, Chassagnard MT. Divergence of sex comb phenotypes in the Drosophila fima species group and radiation on Afrotropical Ficus, including five new species from East Africa and Madagascar (Diptera: Drosophilidae) Annales de la Societe entomologique de France. 2002;38(1-2):79–99. [Google Scholar]
- Lakovaara S, Saura A. Evolution and speciation in the Drosophila obscura group. Ashburner, Carson, Thompson, 1981-1986. 1982:1–59. b. [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85(7):951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Morata G. Bristle patterns and compartment boundaries in the tarsi of Drosophila. J Embryol Exp Morphol. 1979;51:195–208. [PubMed] [Google Scholar]
- Layalle S, Ragone G, Giangrande A, Ghysen A, Dambly-Chaudiere C. Control of bract formation in Drosophila: poxn, kek1, and the EGF-R pathway. Genesis. 2004;39(4):246–255. doi: 10.1002/gene.20052. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388(6638):139–145. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- Lemeunier F, David J, Tsacas L, Ashburner M. The melanogaster species group. Ashburner, Carson, Thompson, 1981-1986. 1986:147–256. e. [Google Scholar]
- Lewis RA, Wakimoto BT, Denell RE, Kaufman TC. Genetic analysis of the Antennapedia gene complex (ANT-C) and adjacent chromosomal regions of Drosophila melanogaster. II. Polytene chromosome segments 84A-84B1,2. Genetics. 1980;95:383–397. doi: 10.1093/genetics/95.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Baker BS. hermaphrodite and doublesex function both dependently and independently to control various aspects of sexual differentiation in Drosophila. Development. 1998;125(14):2641–2651. doi: 10.1242/dev.125.14.2641. [DOI] [PubMed] [Google Scholar]
- Macdonald SJ, Goldstein DB. A quantitative genetic analysis of male sexual traits distinguishing the sibling species Drosophila simulans and D. sechellia. Genetics. 1999;153(4):1683–1699. doi: 10.1093/genetics/153.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann RS, Chan SK. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12(7):258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120(12):3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Markow TA, Bustoz D, Pitnick S. Sexual selection and a secondary sexual character in two Drosophila species. Anim Behav. 1996;52(4):759–766. [Google Scholar]
- Maves L, Schubiger G. A molecular basis for transdetermination in Drosophila imaginal discs: interactions between wingless and decapentaplegic signaling. Development. 1998;125(1):115–124. doi: 10.1242/dev.125.1.115. [DOI] [PubMed] [Google Scholar]
- McAlpine JF. Morphology and terminology - adults. In: McAlpine P, Shewell, Teskey, Vockeroth, Wood, editors. Manual of Nearctic Diptera. 1981. pp. 9–63. 1981. [Google Scholar]
- McKeown M. Sex differentiation: the role of alternative splicing. Curr Opin Genet Dev. 1992;2(2):299–303. doi: 10.1016/s0959-437x(05)80288-6. [DOI] [PubMed] [Google Scholar]
- Moczek AP, Andrews J, Kijimoto T, Yerushalmi Y, Rose DJ. Emerging model systems in evo-devo: horned beetles and the origins of diversity. Evol Dev. 2007;9(4):323–328. doi: 10.1111/j.1525-142X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- Moczek AP, Cruickshank TE, Shelby A. When ontogeny reveals what phylogeny hides: gain and loss of horns during development and evolution of horned beetles. Evolution Int J Org Evolution. 2006;60(11):2329–2341. [PubMed] [Google Scholar]
- Monteiro A. Alternative models for the evolution of eyespots and of serial homology on lepidopteran wings. Bioessays. 2008;30(4):358–366. doi: 10.1002/bies.20733. [DOI] [PubMed] [Google Scholar]
- Monteiro A, Podlaha O. Wings, horns, and butterfly eyespots: how do complex traits evolve? PLoS Biol. 2009;7(2):e37. doi: 10.1371/journal.pbio.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G, Lawrence PA. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975;255(5510):614–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee AS. The effects of sexcombless on the forelegs of Drosophila melanogaster. Genetics. 1965;51:285–304. doi: 10.1093/genetics/51.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller GB, Wagner GP. Novetly in evolution: Restructuring the concept. Annu Rev Ecol Syst. 1991;22:229–256. [Google Scholar]
- Nagoshi RN, Baker BS. Regulation of sex-specific RNA splicing at the Drosophila doublesex gene: cis-acting mutations in exon sequences alter sex-specific RNA splicing patterns. Genes Dev. 1990;4(1):89–97. doi: 10.1101/gad.4.1.89. [DOI] [PubMed] [Google Scholar]
- Narendra U, Zhu L, Li B, Wilken J, Weiss MA. Sex-specific gene regulation. The Doublesex DM motif is a bipartite DNA-binding domain. J Biol Chem. 2002;277(45):43463–43473. doi: 10.1074/jbc.M204616200. [DOI] [PubMed] [Google Scholar]
- Ng C-S, Kopp A. Sex combs are important for male mating success in Drosophila melanogaster. Behav Genet. 2008;38:195–201. doi: 10.1007/s10519-008-9190-7. [DOI] [PubMed] [Google Scholar]
- Nijhout HF. The development and evolution of butterfly wing patterns. Smithsonian Institution Press; Washington: 1991. [Google Scholar]
- Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Chapter 1. Gene regulatory networks in neural crest development and evolution. Curr Top Dev Biol. 2009;86:1–14. doi: 10.1016/S0070-2153(09)01001-1. [DOI] [PubMed] [Google Scholar]
- Nuzhdin SV, Reiwitch SG. Are the same genes responsible for intra- and interspecific variability for sex comb tooth number in Drosophila? Heredity. 2000;84(Pt 1):97–102. doi: 10.1038/sj.hdy.6886400. [DOI] [PubMed] [Google Scholar]
- Orenic TV, Held LI, Jr., Paddock SW, Carroll SB. The spatial organization of epidermal structures: hairy establishes the geometrical pattern of Drosophila leg bristles by delimiting the domains of achaete expression. Development. 1993;118(1):9–20. doi: 10.1242/dev.118.1.9. [DOI] [PubMed] [Google Scholar]
- Pattatucci AM, Kaufman TC. The homeotic gene Sex combs reduced of Drosophila melanogaster is differentially regulated in the embryonic and imaginal stages of development. Genetics. 1991;129(2):443–461. doi: 10.1093/genetics/129.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattatucci AM, Otteson DC, Kaufman TC. A functional and structural analysis of the Sex combs reduced locus of Drosophila melanogaster. Genetics. 1991;129(2):423–441. doi: 10.1093/genetics/129.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton A, Hoffmann FM. Decapentaplegic restricts the domain of wingless during Drosophila limb patterning. Nature. 1996;382:162–165. doi: 10.1038/382162a0. [DOI] [PubMed] [Google Scholar]
- Percival-Smith A, Hayden DJ. Analysis in Drosophila melanogaster of the interaction between sex combs reduced and extradenticle activity in the determination of tarsus and arista identity. Genetics. 1998;150(1):189–198. doi: 10.1093/genetics/150.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak M, Starmer WT, Wolf LL. Sexual selection for size and symmetry in a diversifying secondary sexual character in Drosophila bipectinata Duda (Diptera: Drosophilidae) Evolution Int J Org Evolution. 2004;58(3):597–607. [PubMed] [Google Scholar]
- Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, Zon LI, Borowsky R, Tabin CJ. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat Genet. 2006;38(1):107–111. doi: 10.1038/ng1700. [DOI] [PubMed] [Google Scholar]
- Prud’homme B, Gompel N, Rokas A, Kassner VA, Williams TM, Yeh SD, True JR, Carroll SB. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature. 2006;440(7087):1050–1053. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]
- Randsholt NB, Santamaria P. How Drosophila change their combs: the Hox gene Sex combs reduced and sex comb variation among Sophophora species. Evol Dev. 2008;10(1):121–133. doi: 10.1111/j.1525-142X.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- Rauskolb C. The establishment of segmentation in the Drosophila leg. Development. 2001;128(22):4511–4521. doi: 10.1242/dev.128.22.4511. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Irvine KD. Notch-mediated segmentation and growth control of the Drosophila leg. Dev Biol. 1999;210(2):339–350. doi: 10.1006/dbio.1999.9273. [DOI] [PubMed] [Google Scholar]
- Roch F, Akam M. Ultrabithorax and the control of cell morphology in Drosophila halteres. Development. 2000;127(1):97–107. doi: 10.1242/dev.127.1.97. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Gibson-Brown JJ. Genetic and developmental bases of serial homology in vertebrate limb evolution. Development. 2000;127(24):5233–5244. doi: 10.1242/dev.127.24.5233. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Evolution of the neural crest viewed from a gene regulatory perspective. Genesis. 2008;46(11):673–682. doi: 10.1002/dvg.20436. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Wagner GP. Modularity in development and evolution. University of Chicago Press; Chicago: 2004. [Google Scholar]
- Shapiro MD, Marks ME, Peichel CL, Blackman BK, Nereng KS, Jonsson B, Schluter D, Kingsley DM. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature. 2004;428(6984):717–723. doi: 10.1038/nature02415. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48(2):461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Shroff S, Joshi M, Orenic TV. Differential Delta expression underlies the diversity of sensory organ patterns among the legs of the Drosophila adult. Mech Dev. 2007;124(1):43–58. doi: 10.1016/j.mod.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Spencer FA, Hoffmann FM, Gelbart WM. Decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell. 1982;28(3):451–461. doi: 10.1016/0092-8674(82)90199-4. [DOI] [PubMed] [Google Scholar]
- Spieth HT. Mating behavior within the genus Drosophila (Diptera) Bulletin of the American Museum of Natural History. 1952;99(7):395–474. [Google Scholar]
- Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci U S A. 1982;79(23):7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72(4):527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Sucena E, Stern DL. Divergence of larval morphology between Drosophila sechellia and its sibling species caused by cis-regulatory evolution of ovo/shaven-baby. Proc Natl Acad Sci U S A. 2000;97(9):4530–4534. doi: 10.1073/pnas.97.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalla BJ, Jeffery WR. Requirement of the Manx gene for expression of chordate features in a tailless ascidian larva. Science. 1996;274(5290):1205–1208. doi: 10.1126/science.274.5290.1205. [DOI] [PubMed] [Google Scholar]
- Szebenyi AL. Cleaning behaviour in Drosophila melanogaster. Anim Behav. 1969;17:641–651. [Google Scholar]
- Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development. 1995;121(10):3359–3369. doi: 10.1242/dev.121.10.3359. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Barmina O, Kopp A. Distinct developmental mechanisms underlie the evolutionary diversification of Drosophila sex combs. Proc Natl Acad Sci U S A. 2009;106(12):4764–4769. doi: 10.1073/pnas.0807875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Barmina O, Sanders LE, Arbeitman MN, Kopp A. Spatial regulation of doublesex by Scr controls the development and evolution of Drosophila sex combs. submitted.
- Tatsuta H, Takano-Shimizu T. Genetic architecture of variation in sex-comb tooth number in Drosophila simulans. Genet Res. 2006;87(2):93–107. doi: 10.1017/S0016672306008111. [DOI] [PubMed] [Google Scholar]
- Theisen H, Haerry TE, O’Connor MB, Marsh JL. Developmental territories created by mutual antagonism between Wingless and Decapentaplegic. Development. 1996;122(12):3939–3948. doi: 10.1242/dev.122.12.3939. [DOI] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120(2):347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Connelly P, Smith S, Guild GM. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J Cell Biol. 1996;135(5):1291–1308. doi: 10.1083/jcb.135.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Ruggiero L, Vranich KA, Guild GM, Derosier D. The role actin filaments play in providing the characteristic curved form of Drosophila bristles. Mol Biol Cell. 2004;15(12):5481–5491. doi: 10.1091/mbc.E04-06-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, DeRosier DJ. How to make a curved Drosophila bristle using straight actin bundles. Proc Natl Acad Sci U S A. 2005;102(52):18785–18792. doi: 10.1073/pnas.0509437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga C. The differentiation of a secondary sex comb under the influence of the gene engrailed in Drosophila melanogaster. Genetics. 1961;46(2):157–176. doi: 10.1093/genetics/46.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- - Cell lineage and differentiation on the male foreleg of Drosophila melanogaster. Dev Biol. 1962;4:489–516. doi: 10.1016/0012-1606(62)90054-4. [DOI] [PubMed] [Google Scholar]
- Tokunaga C, Gerhart JC. The effect of growth and joint formation on bristle pattern in D. melanogaster. J Exp Zool. 1976;198(1):79–95. doi: 10.1002/jez.1401980110. [DOI] [PubMed] [Google Scholar]
- True JR, Liu J, Stam LF, Zeng ZB, Laurie CC. Quantitative genetic analysis of divergence in male secondary sexual traits between Drosophila simulans and Drosophila mauritiana. Evolution. 1997;51:816–832. doi: 10.1111/j.1558-5646.1997.tb03664.x. [DOI] [PubMed] [Google Scholar]
- Tsacas L. Les especes montagnardes afrotropicales de Drosophilidae (Diptera) Annales de la Societe entomologique de France. 1980;16(4):517–540. [Google Scholar]
- - Une radiation montagnarde: Le groupe dentissima. Rapport d’Activite Scientifique Laboratoire de Biologie et Genetique Evolutives, Centre National de la Recherche Scientifique, Gif-sur-Yvette. 1981:54–56. [Google Scholar]
- Tsacas L, Lachaise D. Les especes au second article tarsal modifie du groupe afrotropical Drosophila fima (Diptera, Drosophilidae) Annales de la Societe entomologique de France. 1981a;17(3):395–415. [Google Scholar]
- - Une radiation coevolutive sur les sycones de Ficus: Le groupe fima. Rapport d’Activite Scientifique Laboratoire de Biologie et Genetique Evolutives, Centre National de la Recherche Scientifique, Gif-sur-Yvette. 1981b;1979-1981:56–57. [Google Scholar]
- Vermeij GJ. Historical contingency and the purported uniqueness of evolutionary innovations. Proc Natl Acad Sci U S A. 2006;103(6):1804–1809. doi: 10.1073/pnas.0508724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dassow G, Munro E. Modularity in animal development and evolution: elements of a conceptual framework for EvoDevo. J Exp Zool. 1999;285(4):307–325. [PubMed] [Google Scholar]
- Wagner GP. The developmental genetics of homology. Nat Rev Genet. 2007;8(6):473–479. doi: 10.1038/nrg2099. [DOI] [PubMed] [Google Scholar]
- Wang X, Chamberlin HM. Evolutionary innovation of the excretory system in Caenorhabditis elegans. Nat Genet. 2004;36(3):231–232. doi: 10.1038/ng1301. [DOI] [PubMed] [Google Scholar]
- Waterbury JA, Jackson LL, Schedl P. Analysis of the doublesex female protein in Drosophila melanogaster: role on sexual differentiation and behavior and dependence on intersex. Genetics. 1999;152(4):1653–1667. doi: 10.1093/genetics/152.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ. Phylogenetic evidence for multiple losses of a sexually selected character in phrynosomatid lizards. Proc R Soc Lond B Biol Sci. 1999;266:1529–1535. [Google Scholar]
- - Widespread loss of sexually selected traits: how the peacock lost its spots/ Trends Ecol Evol. 2001;16(9):517–523. [Google Scholar]
- Wilder EL, Perrimon N. Dual functions of wingless in the Drosophila leg imaginal disc. Development. 1995;121(2):477–488. doi: 10.1242/dev.121.2.477. [DOI] [PubMed] [Google Scholar]
- Wilkins AS. The evolution of developmental pathways. Sinauer Associates; Sunderland, Mass: 2002. [Google Scholar]
- Wong BB, Rosenthal GG. Female disdain for swords in a swordtail fish. Am Nat. 2006;167(1):136–140. doi: 10.1086/498278. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Stock DW, Jeffery WR. Hedgehog signalling controls eye degeneration in blind cavefish. Nature. 2004;431(7010):844–847. doi: 10.1038/nature02864. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Blankenship JT. Multicellular dynamics during epithelial elongation. Semin Cell Dev Biol. 2008;19(3):263–270. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121(8):2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228(2):181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Yu L, Shen X, Li Y, Xu W, Yi Y, Zhang Z. Homology of dipteran bristles and lepidopteran scales: requirement for the Bombyx mori achaete-scute homologue ASH2. Genetics. 2009;183(2):619–627. 611SI–613SI. doi: 10.1534/genetics.109.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]