Abstract
Background
The diagnostic limitations of fine needle aspiration (FNA), like the indeterminate category, can be partially overcome by molecular analysis. As PAX8/PPARG and RET/PTC rearrangements have been detected in follicular thyroid carcinomas (FTCs) and papillary thyroid carcinomas (PTCs), their detection in FNA smears could improve the FNA diagnosis. To date, these rearrangements have never been analyzed in routine air-dried FNA smears, but only in frozen tissue, formalin-fixed paraffin-embedded (FFPE) tissue, and in fresh FNA material. Fixed routine air-dried FNA samples have hitherto been judged as generally not suitable for testing these rearrangements in a clinical setting. Therefore, the objective of the present study was to investigate the feasibility of extracting RNA from routine air-dried FNA smears for the detection of these rearrangements with real-time polymerase chain reaction (RT-PCR).
Methods
A new method for RNA extraction from routine air-dried FNA smears was established, which allowed analysis for the presence of four variants of PAX8/PPARG and RET/PTC 1 and RET/PTC 3, which were analyzed in 106 routine FNA smears and the corresponding surgically obtained FFPE tissues using real-time quantitative PCR (RT-qPCR). To assess RNA quality, an intron-spanning PAX8 cDNA was amplified.
Results
Acceptable RNA quality was obtained from 95% of the FNA samples and 92% of the FFPE samples. PAX8/PPARG was detected in 4 of 96 FFPEs and in 6 of 96 FNAs. PAX8/PPARG was present in 4 of 10 FTCs and in 3 of 42 follicular adenomas (FAs). Similarly, RET/PTC was found in 3 of 96 FFPEs and in 4 of 96 FNAs. Two of 21 PTC samples and 3 of 42 FA samples carried this rearrangement.
Conclusion
These data are the first to show the feasibility of extracting RNA from routine air-dried FNA smears for the detection of PAX8/PPARG and RET/PTC rearrangements with RT-qPCR. These promising methodological advances, if confirmed in larger series of FNA and FFPE samples, may lead to the introduction of molecular analysis of routine air-dried FNA smears in everyday practice.
Introduction
The majority of thyroid cancers are well differentiated and originate from the thyroid follicular cells. Fine needle aspiration (FNA) biopsy is widely recommended in the further selection of patients, where malignancy is suspected or cannot be ruled out (1,2). The most important inherent limitation of the sensitivity and specificity of FNA of thyroid nodules is the indeterminate/follicular proliferation cytology result. It is found in 20% of the FNA biopsies. Diagnostic lobectomy is required to resolve the nature of most nodules with this FNA diagnosis. However, molecular FNA analysis for a panel of somatic mutations, including rearrangements, significantly increases the number of definitive diagnoses in this cytology category (3).
The most prevalently described rearrangements are PAX8/PPARG and RET/PTC. RET/PTC rearrangements were reported in 13–43% of papillary thyroid carcinomas (PTCs) and in 10–45% of follicular adenomas (FAs) (4–6). PAX8/PPARG rearrangements have been identified in 25–63% of follicular thyroid carcinomas (FTCs), 11% of FAs, 13% of the follicular variant of PTCs, and 2% of oncocytic follicular thyroid carcinomas (oFTCs). These rearrangements are absent in classical PTCs and anaplastic thyroid cancers (7). Searching for RET/PTC rearrangements in thyroid aspirates has been reported as a useful tool in the preoperative diagnosis of PTC (8–10).
While the DNA-based search for the point mutations is methodologically well established in routine air-dried FNA smears, the detection of RET/PTC and PAX8/PPARG is RNA based and has hitherto been judged unfeasible both for routine air-dried FNA and for formalin fixed-paraffin-embedded tissue (FFPE), even when using ultrasensitive conditions to avoid profound RNA degradation (6,11). Despite technical difficulties, the added diagnostic value of RET/PTC and PAX8/PPARG detection is considerable. This was demonstrated by Cantara et al. (8) and Nikiforov et al. (12) in fresh FNA material since, in these two studies, nearly all RET/PTC- or PAX8/PPARG-positive FNA samples were malignant by histologic analysis. To our knowledge, the rearrangements have never been analyzed in routine air-dried FNA smears [for more details see (3)]. Routine air-dried FNA samples have been judged to be generally not suitable for rearrangement analysis (6,11). However, molecular diagnosis of these rearrangements in routine air-dried smears would have major advantages. Achievement of this would obviate the need to prepare additional FNA material for RNA preservation or to store FNA material until completion of cytological diagnosis to see if the readings were indeterminate, thus prompting the need for molecular analysis. This ability would also obviate the need for a second FNA for molecular diagnostic studies. Thereby, the burden to the patient and society would be reduced, specifically because there would be no need for parallel morphologic and molecular diagnostics, and there would be less diagnostic surgeries (3). Therefore, the objective of this methodological study was to evaluate the feasibility of extracting RNA from routine air-dried FNA smears for the detection of PAX8/PPARG and RET/PTC rearrangements with real-time-quantitative polymerase chain reaction (RT-qPCR).
Methods
Patients, FNA and FFPE samples
Routine air-dried FNA smears from 106 patients who underwent surgery for thyroid nodules at the Odense University Hospital were retrospectively analyzed together with the corresponding FFPE material from the same patients. All FNA samples were graded by an experienced pathologist (A.K.) according to the 2006 American Thyroid Association thyroid cancer guidelines in one of the following category: non-neoplastic, indeterminate, malignant, or nondiagnostic (13). The cytological and histological evaluation of the paired 106 FNA smears and FFPE samples are shown in Table 1. The study was approved by the local ethics committee and was conducted in accordance with the Danish law for scientific ethics committees.
Table 1.
Cytological and Histological Evaluation of the Paired 106 Fine Needle Aspiration Smears and Formalin-Fixed Paraffin-Embedded Samples
| Cytology | Histology | Number of cases |
|---|---|---|
| Non-neoplastic (n=15) | ||
| Goiter | 7 | |
| FTC | 3 | |
| FA | 5 | |
| Indeterminate (n=61) | ||
| FTC | 12 | |
| FA | 38 | |
| oFA | 10 | |
| PTC | 1 | |
| Malignant (n=29) | ||
| FTC | 5 | |
| PTC | 24 | |
| Nondiagnostic (n=1) | FA | 1 |
| Total | 106 | |
FTC, follicular thyroid carcinoma; FA, follicular adenoma; oFA, oncocytic follicular adenoma; PTC, papillary thyroid carcinoma.
RNA extraction from FNA and FFPE slides
Routine air-dried FNA smears were incubated in xylene for 4–5 days to remove the cover slips. Thereafter, the slides were air-dried. Subsequently, 700 μL QIAzol was added to the slide according to the miRNeasy Mini Kit protocol (Qiagen), and the cells on the slide were detached within the QIAzol using a scalpel. The detached cells were transferred to a new tube, homogenized by pipetting up and down/vortexing. Then, 240 μL chloroform was added, mixed for 15 seconds, and subsequently incubated at room temperature for 3 minutes. Following this, the homogenate was centrifuged at 12,000 g at 4°C for 15 minutes. The upper aqueous phase containing the RNA was transferred to a new tube and extraction was continued according to the miRNeasy Mini Kit protocol.
The FFPE slides were incubated in xylene for 5 minutes. Then the slides were transferred to a 96% ethanol bath for a further 5 minutes. After drying the slides at room temperature, a 150 μL PKD buffer (miRNeasy FFPE Kit; Qiagen) was added onto the slides. The cells were scraped from the slide and transferred into a fresh tube. After adding 10 μL proteinase K, the tube was vortexed and then incubated for 15 minutes at 55°C. Subsequently, the tube was incubated at 80°C for another 10 minutes. Three hundred twenty microliter RBC solution (miRNeasy FFPE Kit; Qiagen) was added and mixed gently. After this, the solution was transferred into the gDNA elimination spin column (miRNeasy FFPE Kit; Qiagen) and centrifuged at 8000 g for 30 seconds. Then, 1120 μL 96% EtOH was added to the flow-through and mixed well. Further RNA extraction was done according to the mirNeasy FFPE kit protocol.
Synthesis of cDNA complimentary to RNA extracted from FNA and FFPE samples
cDNA was synthesized using the miScript Reverse Transcription Kit (Qiagen) according to the manufacturers protocol. In brief, 7.5 μL template RNA was added to a master mix consisting of 2 μL 5×miScript RT buffer and 0.5 μL miScript Reverse Transcriptase Mix and incubated for 60 minutes at 37°C. Subsequently, the miScript Reverse Transcriptase Mix was inactivated for 5 minutes at 95°C.
Quantitative PCR
To check the RNA quality, an intron-spanning 122-bp fragment of PAX8 mRNA (exon 5–6) was analyzed by qPCR with subsequent fluorescence melting curve analysis on a Roche LightCycler 480 using FastStart SYBR Green Master chemistry (Roche). PAX8/PPARG, RET/PTC1, and RET/PTC3 rearrangements were detected by modified real-time PCR using previously described primers and probes flanking the fusion points (see Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy) and the LightCycler FastStart DNA MasterPlus HybProbe chemistry (Roche) (12,14). To rule out any contamination of the PCR-product, LightCycler Uracil-DNA Glycosylase (Roche) 0.2 U was added to each sample. PCRs were processed through an initial incubation for 10 minutes at 40°C followed by denaturation at 95°C for 5 minutes and by 50 cycles of a three-step PCR, including 10 seconds of denaturation at 95°C, a 10 seconds annealing phase at 62°C (PAX8/PPARG) or 64°C (RET/PTC), and an elongation phase at 72°C for 7 seconds. cDNA from patient specimens known to carry PAX8/PPARG or RET/PTC rearrangements were used as positive controls in each analysis. Samples tested positive were analyzed by capillary gel electrophoresis using the BigDye Terminator Kit on an ABI 3100 Genetic Analyzer (Applied Biosystems).
Capillary electrophoresis
Samples tested positive were analyzed by capillary electrophoresis using a high-resolution gel cartridge on a QIAxcel system (Qiagen). Aliquots (1–3 μL) of PCR products were mixed with the DNA dilution buffer to a total volume of 10 μL, which were loaded onto the QIAxcel system.
Results
The qPCR assays were developed and optimized with RNA from rearrangement positive tissue samples using SYBR Green chemistry. Subsequently, the detection of the rearrangements was done using more specific hydrolysis probes in FNA and FFPE samples.
RNA quality assessment
Total RNA was extracted from 106 routine air-dried FNA smears and from 106 corresponding FFPE slides. The quality of the extracted RNA was assessed by RT-qPCR amplification of a 122-bp PAX8 cDNA. The quality of the cDNA was deemed acceptable when the melting curve of the control fragment showed a specific peak for the PAX8 cDNA. Based on this criterion, 101 FNA samples (95%) and 98 FFPE samples (92%) were considered acceptable for the detection of the rearrangements and labeled cDNA-positive. Five FNA samples (5%) and eight FFPE samples (8%), which did not show a specific PAX8 amplification, were considered as cDNA-negative. Only the cDNA-positive paired samples were analyzed obtaining 96 matched cDNA- positive FFPE and FNA samples.
PAX8/PPARG detection
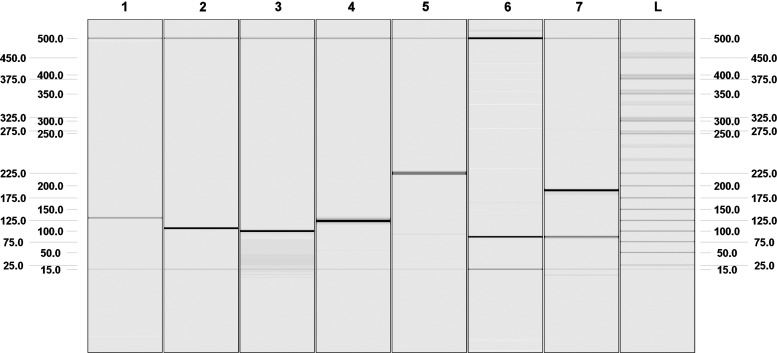
PAX8/PPARG were detected by RT-qPCR in 6 of 96 cDNA-positive FNA samples (6.2%) and in 4 of 96 cDNA-positive FFPE samples (4.2%) (Table 2). PAX8/PPARG was present in 4 of 10 (40%) cDNA-positive FTCs and in 3 of 42 cDNA-positive FAs (7.1%). No rearrangement was detected in oncocytic FA (oFA, n=7), oFTC (n=9), goiter (n=7), or PTC (n=21). The most frequent fusion variant, in the FNA samples, was PAX8 exons 1–8 juxtaposed to PPARG exon 1 (71%), followed by PAX8 exons 1–9 juxtaposed to PPARG exon 1 and PAX8 exons 1–10 juxtaposed to PPARG exon 1 (28%). All positive samples were verified by sequencing and also analyzed by capillary gel electrophoresis on a QIAxcel. Two samples carried two or more splicing variants (Fig. 1). Representative electropherograms for the different rearrangement variations are shown in Figure 1. In the FFPE samples, the most frequent splicing variant was PAX8 exons 1–10 juxtaposed to PPARG exon 1.
Table 2.
PAX8/PPARG Rearrangement Positive Samples
| |
|
|
PAX8/PPARG detection in the FNA samples |
PAX8/PPARG detection in the FFPE samples |
||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | Histology | Cytology | PAX8 (exon 8)/PPARG | PAX8 (exon 9)/PPARG | PAX8 (exon 10)/PPARG | PAX8 (exon 8)/PPARG | PAX8 (exon 9)/PPARG | PAX8 (exon 10)/PPARG |
| 23 | FTC | Indeterminate | Positive | Negative | Negative | Negative | Negative | Negative |
| 132 | FTC | Indeterminate | Positive | Negative | Negative | Negative | Negative | Negative |
| 133 | FTC | Indeterminate | Positive | Negative | Negative | Negative | Negative | Negative |
| 161 | FTC | Indeterminate | Positive | Negative | Negative | Positive | Negative | Negative |
| 29 | FA | Indeterminate | Negative | Negative | Negative | Negative | Negative | Positive |
| 50 | FA | Indeterminate | Positive | Positive | Positive | Positive | Positive | Positive |
| 81 | FA | Indeterminate | Negative | Positive | Positive | Negative | Negative | Positive |
In three samples, it was possible to match PAX8/PPARG expression between corresponding FNA and FFPE samples (two FA and one FTC). In one patient, in whom the FFPE sample was positive for the rearrangement, the rearrangement could not be detected in the corresponding FNA sample, and in three patients showing positive FNA results, the rearrangement could not be identified in the corresponding FFPE sample.
FNA, fine needle aspiration; FFPE, formalin-fixed paraffin-embedded.
FIG. 1.
Digital electropherogram from the QIAxcel system representing different rearrangement variants. Lane 1: intron-spanning 122-bp fragment of PAX8 mRNA (exon 5–6) (control fragment); lane 2: RET/PTC3 amplicon (106 bp) using the primer set RET/PTC3-F/RET/PTC3-R; lane 3: PAX8/PPARG amplicon comprising PAX8 (exon 10)/PPARG (exon 1) (98 bp) using the primer set PAX8-E10-F/PPARG-E1-R; lane 4: PAX8/PPARG amplicon comprising PAX8 (exon 9)/PPARG (exon 1) (117 bp) using the primer set PAX8-E9-F/PPARG-E1-R; lane 5: PAX8/PPARG amplicon comprising PAX8 (exon 9–10)/PPARG (exon 1) (219 bp) using the primer set PAX8-E9-F/PPARG-E1-R; lane 6: PAX8/PPARG amplicon comprising PAX8 (exon 8)/PPARG (exon 1) (84 bp) using the primer set PAX8-E8-F/PPARG-E1-R; lane 7: PAX8/PPARG amplicons comprising PAX8 (exon 8)/PPARG (exon 1) (84 bp) and PAX8 (exon 8, 10)/PPARG (exon 1) (186 bp) using the primer set PAX8-E8-F/PPARG-E1-R; lane L: ladder.
RET/PTC detection
RET/PTC rearrangements were detected in 4 of 96 cDNA-positive FNA samples (4%) and in 3 of 96 cDNA-positive FFPE samples (3.1%) (Table 3). RET/PTC rearrangements were present in 2 of 21 (8.7%) cDNA-positive PTCs and in 3 of 42 cDNA-positive FAs (7%). No rearrangement was detected in oFA (n=7), oFTC (n=9), goiter (n=7), or FTC (n=10). RET/PTC1 could be detected in three FNA samples and one FFPE sample, and RET/PTC3 could be detected in one FNA sample and two FFPE samples. Again, all positive samples were verified by sequencing and also analyzed by capillary gel electrophoresis on a QIAxcel.
Table 3.
RET/PTC Rearrangement Positive Samples
| |
|
|
RET/PTC detection in the FNA samples |
RET/PTC detection in the FFPE samples |
||
|---|---|---|---|---|---|---|
| Sample ID | Histology | Cytology | RET/PTC1 | RET/PTC3 | RET/PTC1 | RET/PTC3 |
| 8 | PTC | Malignant | Negative | Positive | Negative | Positive |
| 52 | PTC | Malignant | Positive | Negative | Positive | Negative |
| 30 | FA | Indeterminate | Positive | Negative | Negative | Negative |
| 40 | FA | Indeterminate | Positive | Negative | Negative | Negative |
| 48 | FA | Indeterminate | Negative | Negative | Negative | Positive |
Matching between the FNA smear and the FFPE sample results could be obtained in two patients. In one patient, in whom the FFPE sample was positive for the rearrangement, the rearrangement could not be detected in the corresponding FNA sample, and in two patients showing positive FNA results, the rearrangement could not be identified in the corresponding FFPE sample.
Sensitivity of detection
The sensitivity of the detection of the different rearrangements by qPCR with rearrangement-specific probes was tested by dilution curves. pGEM-T vectors carrying the respective rearrangement amplicons were spiked into genomic DNA from wild-type FFPE samples. The limit of detection for the different rearrangements was less than 100 copies (data not shown). Moreover, the sensitivity was also tested using rearrangement positive tested FFPE samples, which were spiked in different dilutions into wild-type FFPE samples covering the whole range of positive tested samples. Similar quantitation cycles were observed for positive tested FNA and FFPE samples.
Discussion
With the discovery of RET/PTC and PAX8/PPARG rearrangements in PTC and FTC, respectively, much knowledge has been added with regard to their frequency, detection method, and their applicability in medical diagnostics. Although, at present, FNA is the most sensitive method to diagnose nodules suspected of malignancy, molecular analysis by a panel of mutations, including RET/PTC and PAX8/PPARG rearrangements, has been demonstrated to be an important additional technique in raising the sensitivity of this method, especially regarding indeterminate samples (3,8,15,16).
The main novelty of our study is the demonstration, for the first time, of the feasibility of detecting the most common rearrangements (RET/PTC1, RET/PTC3, and three PAX8/PPARG rearrangements) in routine air-dried FNA smears as well as in corresponding FFPE samples. This is associated with a number of advantages for the patients and society alike.
RET/PTC and PAX8/PPARG rearrangements have been analyzed in several studies with different designs. The prevalence of these rearrangements varies with the geographic area, history of radiation exposure (17), and method. The latter being the most important factor for the variability. Moreover, for the RET/PTC rearrangements, Zhu et al. (11) demonstrated that the broad variation in frequency is not only due to different detection methods, but also due to the genetic heterogeneity of thyroid carcinomas. The accuracy has been comparable with all methods when the rearrangement has been present in a significant portion of the tumor (11). However, in the report by Algeciras-Schimnich et al., (14) a comparison of fluorescence in situ hybridization, immunohistochemistry, and RT-PCR for the detection of PAX8/PPARG demonstrated that the analysis using RT-PCR proved to be reliable, rapid, sensitive, and reproducible, although FISH is considered the gold standard for detection of rearrangements. As demonstrated here, the use of RT-qPCR for rearrangement analyses is possible, rapid, and sensitive. Moreover, it provides information about the specific breakpoints in the PAX8/PPARG fusion gene.
To date, both rearrangement analyses have only been performed using RNA extracted from FFPE, frozen tissue samples, or fresh FNA material (8,12,14,17–20). Despite the advantages related to obtaining the molecular analyses directly from the air-dried FNA smear, this has never been done before, due to a severe degradation of the RNA by air-drying and the fixation (6,11). However, here we show the feasibility for an optimized RNA extraction, and the use of small PCR amplicons for also detecting rearrangements in air-dried FNA samples. The integrity of our samples could be succesfully assessed by the amplification of a 122-bp PAX8 fragment (from exons 5 and 6) in 95% of the FNA samples and 92% of the FFPE samples. Interestingly, the representative samples (Fig. 1, lanes 3, 5, and 7) show the PCR amplicons of sample number 50 (Table 2), which provided positive results in all three PAX8/PPARG PCRs. While PCRs per se do not allow a precise identification of the rearrangement variants occuring in the sample, the subsequent electrophoretic separation of the PCR products allows for this identification. The 84- and the 186-bp bands seen in lane 7 reflect the translocations between PAX8 (exon 8) and PPARG (exon 1) (84 bp) and PAX8 (exon 8, 10) and PPARG (exon 1) (186 bp). Moreover, the 219-bp band in lane 5 represents a translocation involving PAX8 (exon 9–10) and PPARG (exon 1). Therefore, sample number 50 contains three different PAX8/PPARG rearrangements: PAX8 (exon 8)/PPARG (exon 1), PAX8 (exon 8, 10)/PPARG (exon 1), and PAX8 (exon 8–10)/PPARG (exon 1). In addition to resolving the precise rearrangement variants by the electrophoretic separation of the positive samples, one can see the limitations of detection due to RNA degradation. Since in Figure 1, lane 5, a 219-bp band representing the amplicon of PAX8 (exon 9–10)/PPARG (exon 1) is visible, the PCR involving primers PAX8-E8-F/PPARG-E1-R (lane 7) should also show this variant. This should give a band of 375 bp size. However, this amplicon is not visible. Therefore, we can assume that the RNA integrity is larger than 220 bp, but smaller than 375 bp.
With both rearrangement analyses, we found five FNA samples that were positive, but the corresponding FFPE samples were negative. Interestingly, Unger et al. found a heterogeneous distribution of RET/PTC-positive cells within tumors, which means the rearrangement positive cells appeared as clusters mixed with rearrangement-negative tumor cells (21). Similar results have also been demonstrated for BRAF (22). Therefore, in the rearrangement positive FNA samples, the biopsy might stem from a rearrangement positive patch of a polyclonal nodule, while the rearrangement-negative FFPE material might stem from rearrangement-negative patches of the nodule. A further explanation for the positive FNA samples, which gave negative results in the corresponding FFPE samples, is most likely a better RNA quality in FNA samples compared to the FFPE material. This hypothesis is supported by a higher number of FNA samples that were positive in a PCR amplifying a PAX8 fragment (from exons 5 and 6 for RNA quality assessment), as compared to the FFPE samples (95% vs. 92%). Moreover, this hypothesis can explain the discrepant results obtained for sample 81 in PAX8/PPARG screening (Table 2). The electrophoretic separation of the PCR products allowed identifying the precise rearrangement variant in sample 81, which was PAX8 (exon 8–10)/PPARG (data not shown). While this variant could be detected using the forward primers located in exons 9 and 10 in the FNA sample (resulting in PCR products of 219 and 98 bp size, respectively), only the smaller fragment could be amplified in the FFPE sample, suggesting higher RNA fragmentation in the FFPE samples.
In two FFPE-positive samples, it was not possible to identify the rearrangement in the corresponding FNA sample. In this case, the larger available amount of RNA in FFPE samples, due to a bigger quantity of nodular tissue, could probably explain this difference.
The prevalence of PAX8/PPARG rearrangements varies from 25% to 63% in FTC and between 4% and 33% in FA (15,21,22). Based on our data, 40% of the FTC and 7% of the FA carried this rearrangement. RET/PTC was described to be present in 13–43% of PTC and in 10–45% of the FA (4–6). In our study, the RET/PTC rearrangement detection frequency was 8.7% and 7% in PTC and FA, respectively. Of importance, our samples are obtained from an area with normal iodine intake and from individuals with no history of radiation exposure.
It has been demonstrated that RET/PTC and PAX8/PPARG rearrangements may be involved in malignant transformation (23). If these rearrangements are detected in encapsulated follicular tumors with nuclear atypia, the tumors are supposed to be premalignant lesions (24,25). In addition, PAX8/PPARG-positive FAs may very likely be precursors for PAX8/PPARG-positive FTCs, as also suggested by studies in transgenic models (26). Larger series of samples have to be analyzed to establish molecular FNA screening as a routine diagnostic procedure. However, based on these findings we would suggest that patients whose FNA sample is positive for a rearrangement should definitely undergo surgery.
In conclusion, this methodological study is the first to demonstrate the feasibility of extracting RNA from routine air-dried FNA smears for detecting PAX8/PPARG and RET/PTC rearrangements using RT-qPCR. Moreover, the combination of RT-qPCR and electrophoretic separation of the PCR products on a QIAxcel allows the precise identification of the rearrangement variants in the samples analyzed. However, to include the molecular analysis from air-dried smears in the management of thyroid nodules, it is imperative to analyze a statistically meaningful number of samples. After a successful validation (comprising also other mutations), the molecular analyses of routine air-dried FNA smears should be introduced in everyday practice. It will provide substantial improvements in the diagnosis of the indeterminate FNA category, thereby reducing the rate of superfluous diagnostic surgeries.
Supplementary Material
Acknowledgments
We thank B. Jarzab (Gliwice, Poland) for sending rearrangement positive tissue samples used as positive controls. This research was supported by Centro Nacional de Pesquisa e Tecnologia do Brasil (CNPq), Brazil by a Ph.D. scholarship to Carolina Ferraz (290023/2009-2), by a DFG grant to Markus Eszlinger (ES162/4-1), and by an unrestricted grant by the Novo Nordisk Foundation to Laszlo Hegedüs. Ralf Paschke is supported by DFG and Krebshilfe.
Disclosure Statement
All authors have nothing to declare. No competing financial interests exist.
References
- 1.Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 2.Paschke R. Hegedus L. Alexander E. Valcavi R. Papini E. Gharib H. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol. 2011;7:354–361. doi: 10.1038/nrendo.2011.1. [DOI] [PubMed] [Google Scholar]
- 3.Ferraz C. Eszlinger M. Paschke R. Current state and future perspective of molecular diagnosis of fine-needle aspiration biopsy of thyroid nodules. J Clin Endocrinol Metab. 2011;96:2016–2026. doi: 10.1210/jc.2010-2567. [DOI] [PubMed] [Google Scholar]
- 4.Giusti F. Falchetti A. Franceschelli F. Marini F. Tanini A. Brandi ML 2010 Thyroid cancer: current molecular perspectives. J Oncol. 2010:351679. doi: 10.1155/2010/351679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo T. Ezzat S. Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 6.Nikiforova MN. Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009;19:1351–1361. doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 7.Placzkowski KA. Reddi HV. Grebe SK. Eberhardt NL. McIver B 2008 The role of the PAX8/PPARgamma fusion oncogene in thyroid cancer. PPAR Res. 2008:672829. doi: 10.1155/2008/672829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantara S. Capezzone M. Marchisotta S. Capuano S. Busonero G. Toti P. Di Santo A. Caruso G. Carli AF. Brilli L. Montanaro A. Pacini F. Montanaro A. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365–1369. doi: 10.1210/jc.2009-2103. [DOI] [PubMed] [Google Scholar]
- 9.Cheung CC. Carydis B. Ezzat S. Bedard YC. Asa SL. Analysis of ret/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J Clin Endocrinol Metab. 2001;86:2187–2190. doi: 10.1210/jcem.86.5.7504. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore G. Giannini R. Faviana P. Caleo A. Migliaccio I. Fagin JA. Nikiforov YE. Troncone G. Palombini L. Basolo F. Santoro M. Analysis of BRAF point mutation and RET/PTC rearrangement refines the fine-needle aspiration diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2004;89:5175–5180. doi: 10.1210/jc.2003-032221. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z. Ciampi R. Nikiforova MN. Gandhi M. Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006;91:3603–3610. doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 12.Nikiforov YE. Steward DL. Robinson-Smith TM. Haugen BR. Klopper JP. Zhu Z. Fagin JA. Falciglia M. Weber K. Nikiforova MN. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 13.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Sherman SI. Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 14.Algeciras-Schimnich A. Milosevic D. McIver B. Flynn H. Reddi HV. Eberhardt NL. Grebe SK. Evaluation of the PAX8/PPARG translocation in follicular thyroid cancer with a 4-color reverse-transcription PCR assay and automated high-resolution fragment analysis. Clin Chem. 2010;56:391–398. doi: 10.1373/clinchem.2009.134015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eszlinger M. Paschke R. Molecular fine-needle aspiration biopsy diagnosis of thyroid nodules by tumor specific mutations and gene expression patterns. Mol Cell Endocrinol. 2010;322:29–37. doi: 10.1016/j.mce.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Moses W. Weng J. Sansano I. Peng M. Khanafshar E. Ljung BM. Duh QY. Clark OH. Kebebew E. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. 2010;11:2589–2594. doi: 10.1007/s00268-010-0720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikiforova MN. Biddinger PW. Caudill CM. Kroll TG. Nikiforov YE. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Castro P. Rebocho AP. Soares RJ. Magalhaes J. Roque L. Trovisco V. Vieira de Castro I. Cardoso-de-Oliveira M. Fonseca E. Soares P. Sobrinho-Simoes M. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:213–220. doi: 10.1210/jc.2005-1336. [DOI] [PubMed] [Google Scholar]
- 19.Marques AR. Espadinha C. Catarino AL. Moniz S. Pereira T. Sobrinho LG. Leite V. Expression of PAX8-PPAR gamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2002;87:3947–3952. doi: 10.1210/jcem.87.8.8756. [DOI] [PubMed] [Google Scholar]
- 20.Musholt TJ. Fottner C. Weber MM. Eichhorn W. Pohlenz J. Musholt PB. Springer E. Schad A. Detection of papillary thyroid carcinoma by analysis of BRAF and RET/PTC1 mutations in fine-needle aspiration biopsies of thyroid nodules. World J Surg. 2010;11:2595–2603. doi: 10.1007/s00268-010-0729-4. [DOI] [PubMed] [Google Scholar]
- 21.Unger K. Zitzelsberger H. Salvatore G. Santoro M. Bogdanova T. Braselmann H. Kastner P. Zurnadzhy L. Tronko N. Hutzler P. Thomas G. Heterogeneity in the distribution of RET/PTC rearrangements within individual post-Chernobyl papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4272–4279. doi: 10.1210/jc.2003-031870. [DOI] [PubMed] [Google Scholar]
- 22.Guerra A. Sapio MR. Marotta V. Campanile E. Rossi S. Forno I. Fugazzola L. Budillon A. Moccia T. Fenzi G. Vitale M. The primary occurrence of BRAF(V600E) is a rare clonal event in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:517–524. doi: 10.1210/jc.2011-0618. [DOI] [PubMed] [Google Scholar]
- 23.Arora N. Scognamiglio T. Zhu B. Fahey TJ., III Do benign thyroid nodules have malignant potential? An evidence-based review. World J Surg. 2008;32:1237–1246. doi: 10.1007/s00268-008-9484-1. [DOI] [PubMed] [Google Scholar]
- 24.Dwight T. Thoppe SR. Foukakis T. Lui WO. Wallin G. Hoog A. Frisk T. Larsson C. Zedenius J. Involvement of the PAX8/peroxisome proliferator-activated receptor gamma rearrangement in follicular thyroid tumors. J Clin Endocrinol Metab. 2003;88:4440–4445. doi: 10.1210/jc.2002-021690. [DOI] [PubMed] [Google Scholar]
- 25.Fusco A. Chiappetta G. Hui P. Garcia-Rostan G. Golden L. Kinder BK. Dillon DA. Giuliano A. Cirafici AM. Santoro M. Rosai J. Tallini G. Assessment of RET/PTC oncogene activation and clonality in thyroid nodules with incomplete morphological evidence of papillary carcinoma: a search for the early precursors of papillary cancer. Am J Pathol. 2002;160:2157–2167. doi: 10.1016/S0002-9440(10)61164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CS. Zhu X. Lessons from mouse models of thyroid cancer. Thyroid. 2009;19:1317–1331. doi: 10.1089/thy.2009.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.