Abstract
Recent studies have shown that livestock can carry Staphylococcus aureus and transmit it to human caretakers. We conducted a pilot study to determine the prevalence and molecular epidemiology of S. aureus among rural Iowans, including individuals with livestock contact. Nasal and throat swabs were collected and plated onto selective media to isolate methicillin-susceptible and methicillin-resistant S. aureus (MRSA), followed by antibiotic resistance testing and molecular analysis of the isolates. While no MRSA was detected, overall, 23.7% (31/131) of participants were found to harbor S. aureus in their nose, throat, or both. Fifteen isolates displayed resistance to one or more tested antibiotics, and the Panton-Valentine leukocidin (PVL) genes were present at a high level (29% [9/31] of S. aureus-positive participants). Younger age and tobacco use were associated with increased risk of S. aureus carriage. Our results suggest that carriage of PVL-positive S. aureus is common among rural Iowans, even in the absence of detectable MRSA colonization.
Introduction
Staphylococcus aureus is a common bacterium that can cause a wide range of infections, ranging from superficial skin infections to severe, and potentially fatal, invasive disease. The emergence of antibiotic resistance has made S. aureus infections increasingly difficult to treat, particularly when they are resistant to beta-lactam antibiotics (a class that includes methicillin, leading these strains to be referred to as methicillin-resistant S. aureus [MRSA]). Approximately 20%–30% of the population is asymptomatically colonized with methicillin-susceptible S. aureus (MSSA).12,13 The most common site of colonization is the anterior nares (nostrils),41 though the throat, skin, gastrointestinal, and genital tracts can also harbor this organism. While colonization itself does not harm the host, it is a risk factor for developing subsequent symptomatic infections.9,13 Both asymptomatic carriers and infected individuals may transmit the bacterium to susceptible persons.41 S. aureus may also be acquired via contact with animal carriers37,38 or contaminated fomites.17
Numerous studies of S. aureus colonization and infection in the healthcare setting have been conducted.20,29,35 A smaller number of studies have assessed the ecology and transmission of S. aureus in the community,22 and most of these were conducted in metropolitan areas.6,15,16 Even fewer studies have examined S. aureus carriage and infection in rural areas.31 This type of analysis is critical given the fact that many rural Americans have exposures, such as livestock contact, that are qualitatively different from those people living in urban areas. In an effort to gain a more complete understanding of the epidemiology of S. aureus, we conducted a pilot study examining the prevalence and molecular characteristics of S. aureus carriage among a primarily rural sample of Iowa residents.
Materials and Methods
Study population
A cross-sectional prevalence study was conducted through a convenience sample of Iowa residents, primarily from small towns and rural areas of the state. Community organizations, including church groups and various clubs, across Iowa were contacted by mail to ask whether they would be willing to participate. Appointments for sample collection were scheduled with interested parties. Approval from the University of Iowa Institutional Review Board was given prior to sampling, and informed consent was obtained from all participants. Five community organizations participated between July 2009 and April 2010 with a total enrollment of 120 adults (18 years of age or older) and 11 minors (<18 years).
Sample collection and culture
Sterile swabs were used to collect nasal and pharyngeal samples from healthy volunteers. Culturing of samples from both the nares and pharynx has been shown to increase the sensitivity of detection2,25–27,34; however, to minimize discomfort, minors were only asked to provide a nasal swab. All samples were maintained in liquid Stuart's medium at 4°C following collection and processed within 24 hours of collection.
Swabs were inoculated into 5 mL enrichment broth, as previously described.37 Cultures were incubated for 24 hours at 35°C and subsequently inoculated onto Mannitol salt agar plates and selective MRSA agar plates (BBL CHROMagar MRSA; Becton, Dickinson and Company). The selective MRSA plates were incubated for 24–48 hours at 35°C and examined for colonies morphologically consistent with S. aureus. Potential S. aureus isolates were subcultured onto Columbia colistin-nalidixic agar (CNA) plates (Columbia CNA; Remel). Isolates were confirmed as S. aureus by Gram stain, catalase test, coagulase test, and S. aureus latex agglutination assay (Pastorex Staph-plus; Bio-Rad). Methicillin resistance was assessed in part by testing for the presence of penicillin binding protein PBP2 with the MRSA latex agglutination test (Oxoid Ltd.). Isolates were stored at −80°C in a glycerol broth solution.
Molecular and susceptibility testing
Genomic DNA was extracted from each isolate using the Wizard Genomic DNA preparation kit as described for Gram-positive bacteria (Promega). The presence of the mecA and Panton-Valentine leukocidin (PVL) genes was determined by polymerase chain reaction (PCR) as previously described.4,19 For PVL, the primer pair luk-PV1 and luk-PV2 was utilized.
Amplification of the spa repeat region was performed using primers spa 1113f and spa 1514r, as previously described.36 PCR products were purified using the Qiagen PCR Purification Kit (Qiagen) and sequenced with the same primers used for amplification. Sequences were analyzed using the Ridom StaphType software package (Ridom GmbH) to obtain spa types, which were grouped into spa cluster complexes (spa-CCs) using the Based Upon Repeat Pattern (BURP) clustering algorithm.23,24 Pairwise cost distances less than or equal to six were utilized to define spa-CCs. spa types containing five or fewer repeat regions were excluded.
Using the broth dilution method described by the Clinical and Laboratory Standards Institute,7 isolates were tested for susceptibility to a panel of antibiotics, including penicillin, oxacillin, tetracycline, erythromycin, clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), gentamicin, levofloxacin, moxifloxacin, linezolid, daptomycin, vancomycin, quinupristin/dalfopristin, rifampin, inducible clindamycin, and high-level mupirocin. A change in the susceptibility testing panel was implemented at the University of Iowa hospital lab during the course of the study. Penicillin resistance testing was discontinued after the first 15 samples, and testing for inducible clindamycin resistance was initiated at that time.
Questionnaire data
Each adult participant and parents of participating minors were asked to fill out a questionnaire. Data obtained from the questionnaire included demographic information, data that could indicate presence of risk factors (e.g., recent hospitalization, ownership of pets, and participation in contact sports), and history of S. aureus infections. Questionnaire data were used to evaluate risk factors for S. aureus carriage.
Statistical analysis
Questionnaire and laboratory results were merged using SAS 9.2 software (SAS Institute, Inc.). Fisher's exact test and exact logistic regression were used to assess potential risk factors for S. aureus carriage in univariate analysis. Because of limitations of sample size, some exposures were analyzed separately as well as grouped together in broader categories. For example, all exposures to a hospital setting (either directly as a patient, through hospital visits, or through a family member) were analyzed both individually and as a grouped variable. Variables with p-values <0.15 during univariate analyses were entered into a full multivariate logistic regression model, with the exception of variables with 12 or fewer respondents per category. A final model, which included participant age and tobacco usage, was defined through manual backward selection and likelihood ratio tests at a significance level of 0.05. Risk factors among adults were evaluated separately from minors because of differences in S. aureus sampling procedures and questionnaires used for the two groups.
Results
One hundred and twenty adults and 11 minors were enrolled from five enrollment sites. Approximately 50% of participants were men (60/118 [50.8%] of responding adults and 6/11 [54.5%] of minors). The average age of the adults was 54.9 years (range 21–93), and the average age of the minors was 7.7 years (range 6 months–15 years). One person (0.8%) classified themselves as American Indian/Alaskan Native; the rest of the participants classified themselves as White. Among adults, 41.7% of participants classified their current residence as “rural,” 35.7% as “small town,” 13.0% as “suburban,” and 9.6% as “urban,” while 27.3% of children's residences were classified as “rural” and 72.7% as “small town.”
Twenty-nine {24.2% [95% confidence interval (CI), 17.4%–32.6%]} adults and two (18.2% [95% CI, 2.3%–51.8%]) minors were found to be colonized with S. aureus. All S. aureus isolates were negative for mecA by PCR, and were therefore classified as MSSA. Out of the 29 colonized adults, 16 (55.2%) had nasal carriage only, 8 (27.6%) had throat carriage only, and 5 (17.2%) were colonized in both their nose and throat, yielding 34 total isolates (Table 1). No statistically significant difference for the proportion of S. aureus carriage was observed across the five community groups (Fisher's exact test, p=0.055). In univariate analysis of risk factors for the adults, no association was observed between livestock exposure and S. aureus carriage. Additionally, no statistically significant association was observed between S. aureus carriage and gender, family income, education level, or children attending daycare (Table 2 and data not shown). In contrast, age was significantly associated with S. aureus carriage. In the final multivariate model, participant age and current or past usage of tobacco products were retained as significant risk factors for S. aureus colonization, with lower risk of S. aureus carriage for older age (adjusted odds ratio [OR], 0.96; 95% CI, 0.93–0.99) and increased risk for tobacco use (adjusted OR, 3.03; 95% CI, 1.10–8.44). No increased risk for S. aureus carriage was observed for current tobacco users when compared with past tobacco users (data not shown).
Table 1.
Molecular Typing and Antibiotic Susceptibility Profiles of Staphylococcus aureus Isolates
| Participant | Colonization site | AB resistance | PVL | spa type |
|---|---|---|---|---|
| 1 | Throat | P | − | t008 |
| 2 | Nose | P | − | t078 |
| 3 | Throat | None | − | t216 |
| 4 | Nose | P, E, TMP-SMX | − | t1892 |
| 5 | Nose | None | − | t012 |
| 5 | Throat | None | − | t4032 |
| 6 | Nose | P | + | t2104 |
| 7 | Throat | P | + | t2104 |
| 8 | Throat | P | + | t4106 |
| 9 | Throat | P | + | t021 |
| 10 | Nose | P | + | t015 |
| 11 | Nose | P | − | t021 |
| 12 | Nose | P | + | t021 |
| 12 | Throat | None | + | t021 |
| 13a | Nose | P | − | t216 |
| 14 | Nose | None | − | t233 |
| 15 | Throat | None | + | t3274 |
| 16 | Nose | None | + | t008 |
| 17 | Nose | TMP-SMX | − | t037 |
| 17 | Throat | None | − | t037 |
| 18 | Nose | None | − | t021 |
| 19 | Nose | None | − | t037 |
| 20 | Nose | None | + | t279 |
| 20 | Throat | None | + | t279 |
| 21 | Nose | None | − | t359 |
| 22 | Throat | None | − | t2612 |
| 23 | Nose | None | − | t209 |
| 23 | Throat | None | − | t209 |
| 24 | Throat | TMP-SMX | − | t024 |
| 25 | Nose | None | − | t084 |
| 26 | Nose | E, C, L | − | t002 |
| 27 | Nose | None | − | t548 |
| 28 | Nose | None | − | t4158 |
| 29 | Nose | None | − | t162 |
| 30 | Nose | E, IC | − | t002 |
| 31 | Nose | None | − | t021 |
Penicillin resistance testing was discontinued after this isolate.
PVL, Panton-Valentine leukocidin; P, penicillin resistance; E, erythromycin resistance; TMP-SMX, trimethoprim-sulfamethoxazole resistance; C, clindamycin resistance; L, levofloxacin resistance; IC, inducible clindamycin resistance.
Table 2.
Staphylococcus aureus Carriage Among Adults (n=120), by Demographic and Epidemiologic Characteristics
| Risk factor | % (Positive/total)a | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Age (years) | ||||
| 20–40 | 50.0 (12/24) | Ref. | — | — |
| 41–60 | 13.5 (7/52) | 0.16 | 0.05–0.47 | 0.001 |
| >60 | 19.0 (8/42) | 0.24 | 0.07–0.70 | 0.011 |
| Gender | ||||
| Male | 26.7 (16/60) | Ref. | ||
| Female | 19.0 (11/58) | 0.64 | 0.26–1.53 | 0.321 |
| Residence | ||||
| Urban | 18.2 (2/11) | Ref. | — | — |
| Suburban | 46.7 (7/15) | 3.94 | 0.70–31.9 | 0.144 |
| Small town | 19.5 (8/41) | 1.03 | 0.21–7.60 | 0.974 |
| Rural | 22.9 (11/48) | 1.34 | 0.29–9.63 | 0.733 |
| Family income | ||||
| <$40,000 | 35.0 (7/20) | Ref. | — | — |
| $40,000–$59,999 | 19.2 (5/26) | 0.44 | 0.12–1.69 | 0.684 |
| $60,000–$79,999 | 17.4 (4/23) | 0.39 | 0.10–1.61 | 0.529 |
| $80,000–$99,999 | 33.3 (5/15) | 0.93 | 0.23–3.82 | 0.254 |
| >$100,000 | 12.5 (3/24) | 0.27 | 0.06–1.21 | 0.194 |
| Child in daycare | ||||
| No | 20.6 (22/107) | Ref. | — | — |
| Yes | 50.0 (4/8) | 3.86 | 0.85–17.6 | 0.07 |
| Tobacco use | ||||
| None | 19.8 (18/91) | Ref. | — | — |
| Current or past | 35.7 (10/28) | 2.25 | 0.87–5.68 | 0.087 |
| Diabetes | ||||
| No | 26.9 (29/108) | Ref. | — | — |
| Yes | 0.0 (0/12) | 0.13 | (0–0.87) | 0.016 |
| Gym visit within prior month | ||||
| No | 27.4 (20/73) | Ref. | — | — |
| Yes | 19.5 (8/41) | 0.64 | 0.24–1.58 | 0.35 |
| Recent influenza-like illness | ||||
| No | 27.5 (25/91) | Ref. | — | — |
| Yes | 11.5 (3/26) | 0.34 | 0.08–1.10 | 0.105 |
| Recent antibiotic use | ||||
| No | 26.6 (25/94) | Ref. | ||
| Yes | 13.0 (3/23) | 0.41 | 0.09–1.34 | 0.183 |
| Hospital exposure | ||||
| No | 27.1 (19/70) | Ref. | — | — |
| Yes | 19.1 (9/47) | 0.64 | 0.25–1.53 | 0.322 |
| Long-term care facility exposure | ||||
| No | 25.5 (16/102) | Ref. | — | — |
| Yes | 13.3 (2/15) | 0.45 | 0.07–1.77 | 0.313 |
| Eczema | ||||
| No | 22.0 (24/109) | Ref. | — | — |
| Yes | 50.0 (4/8) | 3.54 | 0.76–16.0 | 0.089 |
| Occupational exposure to animals | ||||
| No | 24.4 (20/82) | Ref. | — | — |
| Yes | 21.6 (8/37) | 0.86 | 0.32–2.11 | 0.742 |
| Antibiotic soap in the home | ||||
| No | 30.8 (8/26) | Ref. | ||
| Yes | 22.0 (20/91) | 0.64 | 0.22–1.95 | 0.435 |
| Alcohol-based sanitizers in the home | ||||
| No | 30.4 (17/56) | Ref. | ||
| Yes | 18.0 (11/61) | 0.51 | 0.19–1.30 | 0.134 |
Full demographic data were not available for all participants.
OR, odds ratio; CI, confidence interval.
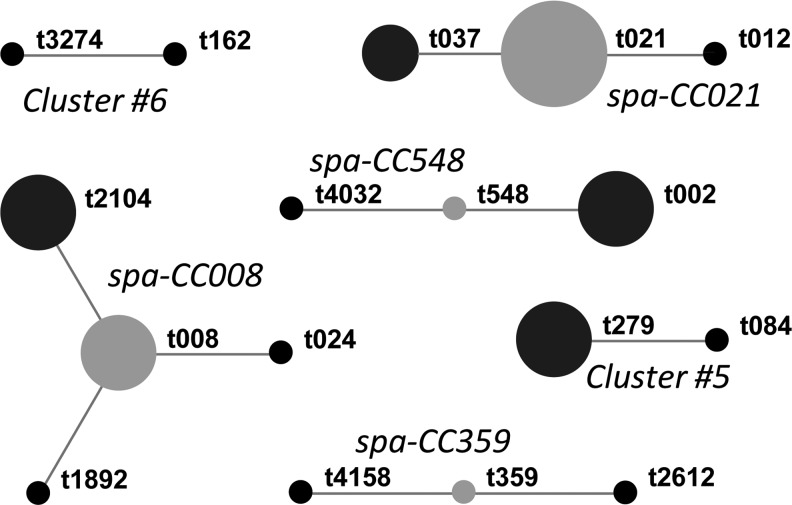
Twenty-three spa types were identified among the 36 adult and minor isolates (Table 1). Six isolates (16.7%) were t021, three isolates (8.3%) were t037, and two isolates each were t002, t008, t209, t216, t279, and t2104. Based upon the BURP clustering algorithm, the majority of isolates (28/36; 77.8%) were clustered into six spa-CCs (Fig. 1). Seven isolates (19.4%) from five spa types (t015, t078, t209, t216, and t4106) were too genetically distant to be placed into a spa-CC, whereas one isolate (2.8%) was excluded from the analysis due to a small number of tandem repeats. Eleven (32.4%) of the 34 adult isolates (or, 9/29 [31%] of S. aureus-carrying adult participants) were PVL positive, while both isolates from minors were PVL negative (Table 1). No association was observed between PVL positivity and spa-CC (Fisher's exact test, p=0.30). In univariate analyses, only participant age (OR, 0.95; 95% CI, 0.90–0.99) was found to be significantly associated with PVL-positive S. aureus carriage.
FIG. 1.
Based Upon Repeat Pattern clustering of Staphylococcus aureus isolates. Designated group founders of spa-CCs with more than three members are indicated in gray, while other group members are indicated in black. Dot sizes are proportional to the number of strains belonging to the corresponding spa type. spa types that were excluded from the analysis (t233) or not grouped into a spa-CC (t015, t078, t209, t216, and t4106) are not shown.
Of the 15 isolates tested for penicillin susceptibility, 4 were found to be sensitive (26.7%), 10 (66.7%) were resistant to penicillin alone, and 1 (6.7%) was resistant to penicillin, erythromycin, and TMP-SMX. Six of the isolates resistant to penicillin were also PVL positive (6/11; 54.5%). Of all 34 isolates tested, 2 were resistant to TMP-SMX only. One isolate was resistant to clindamycin, erythromycin, and levofloxacin (Table 1). One isolate from a minor (spa type t021) was susceptible to all antibiotics tested, while the other isolate (spa type t002) was resistant to erythromycin and displayed inducible clindamycin resistance (Table 1). No oxacillin resistance was detected for any of the isolates.
Discussion
In our study, approximately one-quarter of rural Iowans tested were positive for S. aureus carriage in the nose and/or throat, a prevalence that is lower than the reported national average.12,13,18,21 Population-based data from the National Health and Nutrition Examination Survey (NHANES), a study by the Centers for Disease Control and Prevention designed to assess various health indicators in a nationally representative sample of the U.S. population, indicated that S. aureus nasal colonization prevalence among healthy, noninstitutionalized Americans was ∼30% between 2001 and 2004. In contrast, we found 17.6% (95% CI, 11.5%–25.2%) positivity overall for nasal carriage in our study population (i.e., excluding exclusive throat carriers), including 17.5% positivity among adults and 18.2% positivity among minors. It is not clear whether this discrepancy was due to environmental heterogeneity, differences in the demographic composition of the study populations, or part of a larger trend in decreasing prevalence of S. aureus colonization, as the prevalence of MSSA colonization was shown to decline from 32.4% to 28.6% between 2001–2002 and 2003–2004.12 In our study, 6.7% of participants were exclusive throat carriers. Throat carriage was not evaluated in the NHANES, so the overall prevalence of S. aureus carriage in the general population may have been underestimated. Similar to what we observed, others have reported that inclusion of throat sampling with nasal sampling increases the sensitivity of detection by 25% or greater.14,25
We found that among adults, risk factors for S. aureus carriage included young age and current or past tobacco use. Consistent with these results, data from NHANES indicate that youth and young adults are at higher risk of carrying S. aureus, perhaps as a result of an increased number of social contacts.39 Contrary to other studies,3,13,21 we did not observe a significantly higher prevalence of carriage among men. Our data indicate that tobacco use, when controlling for age, is associated with increased risk for S. aureus carriage. Similarly, passive smoking was correlated with higher carriage prevalence in a cohort of children in The Netherlands.3 We were unable to address the effects of race, country of origin, education level, or income level, all of which have been previously associated with differences in S. aureus carriage, because of the high homogeneity of our study population with regard to these variables. Previous work from our group has demonstrated an elevated prevalence of MRSA carriage among swine workers in Iowa.37 In the current study, we did not observe a similar elevated carriage of MSSA among participants with occupational exposure to livestock (swine, cattle, or chickens), and no MRSA was detected. Owing to limitations of sample size, we cannot determine whether the prevalence of MRSA carriage in rural Iowans is different from the national population, as national surveys suggest that ∼1% of the population is positive for MRSA carriage; a similar prevalence in our population would have resulted in only one or two MRSA-positive participants.
While overall prevalence of S. aureus carriage in our study population was lower than the national average, we did observe a disproportionately high percentage of positivity for the gene encoding the putative virulence factor PVL. While its role in S. aureus pathogenesis is still a matter of debate, PVL is a cytotoxin most commonly associated with community-associated-MRSA and has been epidemiologically linked to severe skin and soft-tissue infections and necrotizing pneumonia.1,8,11,40 We found that 11/36 (30.6%) S. aureus isolates were positive for PVL. In comparison, <1% of the MSSA samples (and ∼15% of asymptomatic MRSA carriers) from the 2001–2004 NHANES were shown to carry PVL.39 As MSSA cases are less frequently studied in a systematic manner, it is not known how widespread PVL-positive MSSA strains are in the nonhospitalized population, and most available data on PVL prevalence come from samples collected from individuals symptomatically infected with MRSA. In a study examining MSSA from abscesses of patients at Children's Hospital in Missouri, Orscheln et al. found that 14% of the MSSA isolates tested were PVL positive, and most of these were classified as multilocus sequence type 8 and were shown to be closely related to USA300.30 In that study, much greater diversity was seen among PVL-negative MSSA. In contrast, PVL positivity among MSSA isolates colonizing the nares of healthy children in the same community was reported to be much lower, at 2%.10,30 In our study, PVL positivity did not appear to be associated with a particular genetic lineage, as presence of the PVL gene was observed among diverse spa-CCs, including spa-CC008 (often associated with ST8/USA300) and spa-CC021 (often associated with ST30/USA1100). Other studies have indicated that PVL-positive MSSA strains are more genetically diverse than PVL-positive MRSA strains,32 suggesting that PVL-positive MRSA strains may arise when mec elements are acquired by pre-existing PVL-positive MSSA strains.5,28,32
The majority of the samples tested were resistant to penicillin, consistent with high rates of resistance reported for the United States from NHANES.39 We also found that >8% of isolates were resistant to TMP-SMX, a higher level than has typically been reported. One recent multicenter study reported a resistance rate of 1.6% to this antibiotic,33 and <1% of MSSA samples from the NHANES were TMP-SMX resistant.33,39 We did observe a lower prevalence of TMP-SMX resistance among participants reporting recent antibiotic usage (13.0% vs. 26.6%), although this difference was not statistically significant.
In conclusion, we found that while the prevalence of S. aureus carriage is relatively low in rural Iowa, the isolates identified are characterized by a high prevalence of PVL positivity and high genetic heterogeneity. Twenty-three distinct spa types were identified, some of which belong to sequence types often associated with community-associated MRSA. Typically, S. aureus surveys are conducted in a clinical setting and thus present a biased view of the genetic diversity in circulation, limiting our understanding of the mechanisms governing emergence and maintenance of both MSSA and MRSA. Larger population-based studies of nonhospitalized rural populations with diverse occupational and environmental exposures are necessary to identify risk factors for colonization and infection and to identify transmission pathways of pathogenic strains.
Acknowledgment
This research was supported in part by Grant Number 5 U50 OH007548-08 from CDC-NIOSH.
Disclosure Statement
No competing financial interests exist. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC, NIOSH, or the Great Plains Center for Agricultural Health.
References
- 1.Baba T. Takeuchi F. Kuroda M. Yuzawa H. Aoki K. Oguchi A. Nagai Y. Iwama N. Asano K. Naimi T., et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 2.Bignardi G.E. Lowes S. MRSA screening: throat swabs are better than nose swabs. J. Hosp. Infect. 2009;71:373–374. doi: 10.1016/j.jhin.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert D. van Belkum A. Sluijter M. Luijendijk A. de Groot R. Rumke H.C. Verbrugh H.A. Hermans P.W. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 4.Bosgelmez-Tinaz G. Ulusoy S. Aridogan B. Coskun-Ari F. Evaluation of different methods to detect oxacillin resistance in Staphylococcus aureus and their clinical laboratory utility. Eur. J. Clin. Microbiol. Infect. Dis. 2006;25:410–412. doi: 10.1007/s10096-006-0153-8. [DOI] [PubMed] [Google Scholar]
- 5.Boyle-Vavra S. Daum R.S. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Investig. 2007;87:3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 6.Bratu S. Landman D. Gupta J. Trehan M. Panwar M. Quale J. A population-based study examining the emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 in New York City. Ann. Clin. Microbiol. Antimicrob. 2006;5:29. doi: 10.1186/1476-0711-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.[CLSI] Clinical, Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobially. eighth. CLSI; Wayne, PA: 2009. [Google Scholar]
- 8.David M.Z. Daum R.S. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz S.A. Epplin E.K. Garbutt J. Storch G.A. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J. Infect. 2009;59:394–401. doi: 10.1016/j.jinf.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz S.A. Garbutt J. Elward A. Shannon W. Storch G.A. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics. 2008;121:1090–1098. doi: 10.1542/peds.2007-2104. [DOI] [PubMed] [Google Scholar]
- 11.Gillet Y. Issartel B. Vanhems P. Fournet J.C. Lina G. Bes M. Vandenesch F. Piemont Y. Brousse N. Floret D., et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 12.Gorwitz R.J. Kruszon-Moran D. McAllister S.K. McQuillan G. McDougal L.K. Fosheim G.E. Jensen B.J. Killgore G. Tenover F.C. Kuehnert M.J. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 13.Graham P.L., 3rd Lin S.X. Larson E.L. A U.S. population-based survey of Staphylococcus aureus colonization. Ann. Int. Med. 2006;144:318–325. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- 14.Hamdan-Partida A. Sainz-Espunes T. Bustos-Martinez J. Characterization and persistence of Staphylococcus aureus strains isolated from the anterior nares and throats of healthy carriers in a Mexican community. J. Clin. Microbiol. 2010;48:1701–1705. doi: 10.1128/JCM.01929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hota B. Ellenbogen C. Hayden M.K. Aroutcheva A. Rice T.W. Weinstein R.A. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch. Intern. Med. 2007;167:1026–1033. doi: 10.1001/archinte.167.10.1026. [DOI] [PubMed] [Google Scholar]
- 16.Kourbatova E.V. Halvosa J.S. King M.D. Ray S.M. White N. Blumberg H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am. J. Infect. Control. 2005;33:385–391. doi: 10.1016/j.ajic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Kramer A. Schwebke I. Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuehnert M.J. Kruszon-Moran D. Hill H.A. McQuillan G. McAllister S.K. Fosheim G. McDougal L.K. Chaitram J. Jensen B. Fridkin S.K., et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J. Infect. Dis. 2006;193:172–179. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 19.Lina G. Piemont Y. Godail-Gamot F. Bes M. Peter M.O. Gauduchon V. Vandenesch F. Etienne J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 20.Lu P.L. Tsai J.C. Chiu Y.W. Chang F.Y. Chen Y.W. Hsiao C.F. Siu L.K. Methicillin-resistant Staphylococcus aureus carriage, infection and transmission in dialysis patients, healthcare workers and their family members. Nephrol. Dial. Transplant. 2008;23:1659–1665. doi: 10.1093/ndt/gfm806. [DOI] [PubMed] [Google Scholar]
- 21.Mainous A.G., 3rd Hueston W.J. Everett C.J. Diaz V.A. Nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus in the United States, 2001–2002. Ann. Fam. Med. 2006;4:132–137. doi: 10.1370/afm.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik S. Vranken P. Silio M. Ratard R. Van Dyke R. Prevalence of community-associated methicillin-resistant Staphylococcus aureus colonization outside the healthcare environment. Epidemiol. Infect. 2009;137:1237–1241. doi: 10.1017/S0950268809002222. [DOI] [PubMed] [Google Scholar]
- 23.Mellmann A. Weniger T. Berssenbrugge C. Keckevoet U. Friedrich A.W. Harmsen D. Grundmann H. Characterization of clonal relatedness among the natural population of Staphylococcus aureus strains by using spa sequence typing and the BURP (based upon repeat patterns) algorithm. J. Clin. Microbiol. 2008;46:2805–2808. doi: 10.1128/JCM.00071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellmann A. Weniger T. Berssenbrugge C. Rothganger J. Sammeth M. Stoye J. Harmsen D. Based Upon Repeat Pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 2007;7:98. doi: 10.1186/1471-2180-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertz D. Frei R. Jaussi B. Tietz A. Stebler C. Fluckiger U. Widmer A.F. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin. Infect. Dis. 2007;45:475–477. doi: 10.1086/520016. [DOI] [PubMed] [Google Scholar]
- 26.Mertz D. Frei R. Periat N. Zimmerli M. Battegay M. Fluckiger U. Widmer A.F. Exclusive Staphylococcus aureus throat carriage: at-risk populations. Arch. Int. Med. 2009;169:172–178. doi: 10.1001/archinternmed.2008.536. [DOI] [PubMed] [Google Scholar]
- 27.Meurman O. Routamaa M. Peltonen R. Screening for methicillin-resistant Staphylococcus aureus: which anatomical sites to culture? J. Hosp. Infect. 2005;61:351–353. doi: 10.1016/j.jhin.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Monecke S. Slickers P. Ellington M.J. Kearns A.M. Ehricht R. High diversity of Panton-Valentine leukocidin-positive, methicillin-susceptible isolates of Staphylococcus aureus and implications for the evolution of community-associated methicillin-resistant S. aureus. Clin. Microbiol. Infect. 2007;13:1157–1164. doi: 10.1111/j.1469-0691.2007.01833.x. [DOI] [PubMed] [Google Scholar]
- 29.Naimi T.S. LeDell K.H. Como-Sabetti K. Borchardt S.M. Boxrud D.J. Etienne J. Johnson S.K. Vandenesch F. Fridkin S. O'Boyle C., et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 30.Orscheln R.C. Hunstad D.A. Fritz S.A. Loughman J.A. Mitchell K. Storch E.K. Gaudreault M. Sellenriek P.L. Armstrong J.R. Mardis E.R., et al. Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin. Infect. Dis. 2009;49:536–542. doi: 10.1086/600881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polgreen P.M. Beekmann S.E. Chen Y.Y. Doern G.V. Pfaller M.A. Brueggemann A.B. Herwaldt L.A. Diekema D.J. Epidemiology of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus in a rural state. Infect. Control Hosp. Epidemiol. 2006;27:252–256. doi: 10.1086/501537. [DOI] [PubMed] [Google Scholar]
- 32.Rasigade J.P. Laurent F. Lina G. Meugnier H. Bes M. Vandenesch F. Etienne J. Tristan A. Global distribution and evolution of Panton-Valentine leukocidin-positive methicillin-susceptible Staphylococcus aureus, 1981–2007. J. Infect. Dis. 2010;201:1589–1597. doi: 10.1086/652008. [DOI] [PubMed] [Google Scholar]
- 33.Richter S.S. Heilmann K.P. Dohrn C.L. Riahi F. Costello A.J. Kroeger J.S. Biek D. Critchley I.A. Diekema D.J. Doern G.V. Activity of ceftaroline and epidemiologic characterization of Staphylococcus aureus from 43 medical centers in the United States, 2009. Antimicrob. Agents Chemother. 2011;55:4154–4160. doi: 10.1128/AAC.00315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringberg H. Petersson A. Cathrine. Walder M. Hugo Johansson P.J. The throat: an important site for MRSA colonization. Scand. J. Infect. Dis. 2006;38:888–893. doi: 10.1080/00365540600740546. [DOI] [PubMed] [Google Scholar]
- 35.Shitrit P. Gottesman B.S. Katzir M. Kilman A. Ben-Nissan Y. Chowers M. Active surveillance for methicillin-resistant Staphylococcus aureus (MRSA) decreases the incidence of MRSA bacteremia. Infect. Control Hosp. Epidemiol. 2006;27:1004–1008. doi: 10.1086/507914. [DOI] [PubMed] [Google Scholar]
- 36.Shopsin B. Gomez M. Montgomery S.O. Smith D.H. Waddington M. Dodge D.E. Bost D.A. Riehman M. Naidich S. Kreiswirth B.N. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 1999;37:3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith T.C. Male M.J. Harper A.L. Kroeger J.S. Tinkler G.P. Moritz E.D. Capuano A.W. Herwaldt L.A. Diekema D.J. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PloS One. 2009;4:e4258. doi: 10.1371/journal.pone.0004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith T.C. Pearson N. The emergence of Staphylococcus aureus ST398. Vector Borne Zoonot. Dis. 2011;11:327–339. doi: 10.1089/vbz.2010.0072. [DOI] [PubMed] [Google Scholar]
- 39.Tenover F.C. McAllister S. Fosheim G. McDougal L.K. Carey R.B. Limbago B. Lonsway D. Patel J.B. Kuehnert M.J. Gorwitz R. Characterization of Staphylococcus aureus isolates from nasal cultures collected from individuals in the United States in 2001 to 2004. J. Clin. Microbiol. 2008;46:2837–2841. doi: 10.1128/JCM.00480-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandenesch F. Naimi T. Enright M.C. Lina G. Nimmo G.R. Heffernan H. Liassine N. Bes M. Greenland T. Reverdy M.E., et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wertheim H.F. Melles D.C. Vos M.C. van Leeuwen W. van Belkum A. Verbrugh H.A. Nouwen J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]