Abstract
Adherence to antiretrovirals by pregnant women (and postpartum women if breastfeeding) is crucial to effectively decrease maternal viral load and decrease the risk of mother-to-child transmission of HIV. Our objectives were to describe self-reported adherence to antiretrovirals during the antepartum (after 22 weeks of pregnancy) and postpartum periods (6–12 weeks and 6 months), and identify predictors of adherence among HIV-infected women enrolled and followed in a prospective cohort study from June 2008 to June 2010 at multiple sites in Latin America. Adherence was evaluated using the number of missed and expected doses during the 3 days before the study visit. At the pre-delivery visit, 340 of 376 women (90%) reported perfect adherence. This rate significantly decreased by 6–12 weeks (171/214 [80%]) and 6 months postpartum (163/199 [82%], p<0.01). The odds for less than perfect adherence at the pre-delivery visit was significantly higher for pregnant women with current tobacco use (odds ratio [OR]=2.9, 95% confidence interval [CI]: 1.46–6.14; p=0.0029). At 6–12 weeks postpartum, the probability of non-perfect adherence increased by 6% for each 1 year increase in age (OR=1.06, 95% CI: 1.00–1.12, p=0.0497). At 6 months postpartum, the odds of nonperfect adherence was higher for those who were currently using alcohol (OR=3.04, 95% CI: 1.34–6.90; p=0.0079). Although a self-report measure of adherence based on only 3 days may lead to overestimation of actual adherence over time, women with perfect adherence had lower viral loads and higher CD4 counts. Adherence to antiretrovirals decreased significantly postpartum. Interventions should target women at high risk for lower adherence during pregnancy and postpartum, including tobacco and alcohol users.
Introduction
Sustained, effective antiretroviral (ARV) therapy has been shown to transform HIV infection from a disease with limited options and a poor prognosis into a chronic infection with prolonged survival.1 Adherence to ARVs is also crucial to optimize the clinical, immunologic, and virologic effectiveness of these drugs among pregnant women in order to prevent mother-to-child transmission (MTCT) of HIV.2,3 Viral load has a strong direct correlation with the risk of MTCT of HIV; therefore, the lower the maternal viral load, the lower the risk of MTCT of HIV. However, lowering maternal viral load is challenging since pregnancy is a relatively short time period in which to both initiate and adjust ARV regimens as necessary.
The effectiveness of ARV therapy is a function of the extent and duration of viral suppression, and it has been postulated that high levels of adherence (≥90%) are necessary to achieve optimal treatment outcomes.4 Factors associated with imperfect adherence to ARVs include factors associated with the patient (e.g., education about and commitment to ARV treatment, psychiatric illness, use of alcohol or other drugs), with treatment (e.g., adverse events, pill burden, and number of daily doses), and with the health care system (e.g., confidentiality). Any intervention to improve adherence must consider the patient holistically, i.e., taking into account behavioral, cognitive, emotional, and social characteristics.5 However, relatively few studies of ARV adherence have included pregnant and postpartum women, and none were conducted in Latin America, although an estimated 1.4 million people were living with HIV infection in Latin America at the end of 2009, and slightly more than half of them were women and girls.6
Identification of HIV-infected women at greatest risk for nonadherence to ARVs during pregnancy and postpartum could help target resources for adherence counseling and support.
With this purpose in mind, adherence to ARVs during pregnancy and postpartum, as well as reasons for lack of adherence, was examined in a population of HIV-infected women at multiple sites in Latin America enrolled in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative (NISDI) prospective cohort study: Longitudinal Study in Latin American Countries (LILAC).
Materials and Methods
LILAC is a prospective cohort study of HIV-infected women and HIV-exposed, uninfected infants conducted at sites in Argentina, Brazil, and Peru,7 with enrollment beginning in June 2008. The primary objectives of the LILAC study are: (1) to describe the characteristics of enrolled women and infants, including utilization of interventions for prevention of MTCT of HIV, use of ARVs, and rates of MTCT of HIV and (2) to characterize adverse events according to use of and exposure to ARVs and mode of delivery. Women are enrolled after 22 weeks of pregnancy and followed through delivery and postpartum. Maternal and infant study visits are conducted at hospital discharge after delivery, and then at 6–12 weeks and 6 months after delivery. Subjects are followed every 6 months thereafter, for up to 5 years after delivery/birth. Study visits include a medical history, a physical examination, and collection of laboratory samples. ARVs are prescribed according to local guidelines and self-reported adherence is recorded at each study visit (see below). The indication for use of ARVs during pregnancy (treatment of the mother's own HIV infection or prevention of MTCT of HIV) is recorded for each ARV regimen. Self-reported use (ever or current) of alcohol, tobacco, marijuana, cocaine/crack or heroin/opiates was recorded at study visits. The protocol was approved by the ethical review board at each clinical site, as well as by Institutional Review Boards at the sponsoring institution (NICHD) and at the data management center (Westat). All subjects provided written informed consent for participation in the study.
The study population for the present analysis comprised women enrolled in the LILAC study by June 30, 2010, during their first on-study pregnancy, who were prescribed ARVs for at least one of the three targeted visits (enrollment/antepartum, 6–12 weeks postpartum, and 6 months postpartum) and for whom a self-report adherence form for the visit was completed. If the subject had both an enrollment visit and another, subsequent antepartum visit, then data at the later of the two visits was analyzed. The enrollment/antepartum visit is hereafter referred to as the pre-delivery visit. For viral load values below the limit of detection, the viral load value was set to half of the lower limit of detection for calculation of mean and median values (e.g., a viral load value of less than 400 copies/mL was reassigned a value of 200 copies/mL).
Percent adherence was derived using information available for all ARVs included in the subject's regimen at a particular study visit and considered doses missed yesterday, 2 days ago, and 3 days ago. The total number of doses missed was divided by the total number of expected doses to be taken for these three time periods for all of the ARVs included in the subject's regimen at the time of the visit; the measure was then expressed in the form of the percent adherence as follows:
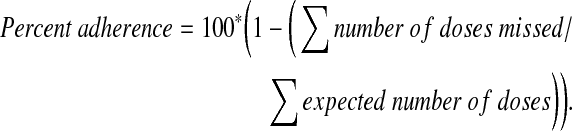 |
If the number of doses missed or the expected number of doses to be taken for a particular ARV for a particular time period (yesterday, 2 days ago, and 3 days ago) was missing, the adherence measure was set to missing. In instances where the reported number of doses missed for a visit was greater than the expected number of doses to be taken, the number of doses missed was set to the expected number of doses before calculating the adherence measures. An indicator of perfect adherence for each visit was derived from percent adherence to distinguish those that were 100% adherent over the 3-day time period from those that were not.
Information also was collected for a measure of long-term adherence that considered the last time an ARV dose was missed, as has been utilized previously.8 The response options were: never, during the previous 2 weeks, during the last month, over a month ago, or do not remember.
Descriptive statistics were used to describe demographic and clinical characteristics of the study subjects, as well as the frequency and patterns of responses to the adherence measures. Generalized estimation equations (GEE) were used to compare pre-delivery to postpartum perfect adherence rates. Associations between subject characteristics and adherence were examined using bivariable techniques, such as Fisher's exact test for categorical-scaled measures and Student's t test and nonparametric procedures (Kruskal-Wallis test) for continuous measures. The associations of perfect adherence with subject characteristics were examined at the pre-delivery, 6- to 12-week postpartum, and 6-month postpartum visits for purposes of identifying predictors of adherence for possible inclusion in multivariable modeling. Some subject characteristics were fixed at the time of enrollment, while information for others was updated at each study visit (e.g., substance use). For those characteristics that were measured at each visit, the value corresponding to the timing of the adherence measure was used in assessing the relationship to adherence (e.g., 6- to 12-week substance use for 6- to 12-week adherence analysis).
For a specific study visit, only those covariates associated with adherence at the α=0.10 level or less were considered as candidates for inclusion in the modeling, provided that their bivariable distributions would not lead to model failure due to small numbers or raise questions regarding the clinical importance of small differences between adherence groups. Logistic regression was used to model perfect adherence as a function of the characteristics meeting the above screening criteria, using forward stepwise selection.
Results
Size and characteristics of the study population
Among the 401 women enrolled in the LILAC study, none were enrolled for a second pregnancy on study. Five subjects were not prescribed ARVs at any of the three targeted visits, and another three did not complete the adherence form, even though they were prescribed ARVs at the time of the visit. Accounting for these exclusions, there were 393 subjects eligible for the study analyses at the pre-delivery visit (235 with the enrollment visit, but no subsequent antepartum visit; 158 with another antepartum visit after the enrollment visit). Characteristics of these 393 women are shown in Table 1. Of those using ARVs, 89.8% were using a three-drug regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI; 15.5%) or two NRTIs and one protease inhibitor (PI; 74.3%).
Table 1.
Characteristics of Study Population (N=393) at the Pre-Delivery (Enrollment/Antepartum) Visit
| Characteristic | n (%) | Mean (SD) median |
|---|---|---|
| Country | ||
| Brazil | 312 (79.4) | |
| Argentina | 37 (9.4) | |
| Peru | 44 (11.2) | |
| Age at enrollment (years) | 29.0 (6.0) | |
| 29.0 | ||
| Race | ||
| Black of African heritage | 83 (21.1) | |
| White | 180 (45.8) | |
| Mestizo/Mulato | 130 (33.1) | |
| Parity | ||
| 0 | 70 (17.8) | |
| 1–2 | 213 (54.2) | |
| >2 | 110 (28.0) | |
| Educational level (years completed) | 8.3 (3.2) | |
| 8.0 | ||
| Number of persons living in subject's household | 4.0 (2.0) | |
| 4.0 | ||
| Gainfully employed outside of home | ||
| Yes | 142 (36.1) | |
| No | 251 (63.9) | |
| Mode of acquisition of HIV infection | ||
| Heterosexual transmission with or without other modes | 365 (92.9) | |
| Other | 5 (1.3) | |
| Unknown | 23 (5.9) | |
| CD4 count at visit (cells/mm3) | 499.6 (273.1) | |
| 470 | ||
| CD4 absolute count at visit (cells/mm3) | ||
| <200 | 51 (13.0) | |
| 200–499 | 158 (40.2) | |
| ≥500 | 184 (46.8) | |
| CD4% at visit: | 28.7 (10.5) | |
| 29.0 | ||
| Missing | 64 | |
| CD4% at visit | ||
| <14% | 24 (7.3) | |
| 14–28% | 131 (39.8) | |
| >28% | 174 (52.9) | |
| Missing | 64 | |
| Viral load at visit | 5478 (30,445) | |
| (copies/mL) | 200 | |
| Viral load at visit (log10 | 2.37 (0.86) | |
| copies/mL) | 2.30 | |
| Viral load at visit (copies/mL) | ||
| <400 (includes “below detection limit”) | 306 (78.1) | |
| ≥400 | 86 (21.9) | |
| Substance use—ever | ||
| Ever used substances | 206 (52.6) | |
| Never | 186 (47.4) | |
| Substance use—current | ||
| Alcohol | 71 (18.1) | |
| Tobacco | 88 (22.4) | |
| Marijuana | 16 (4.1) | |
| Cocaine/crack | 17 (4.3) | |
| Heroin/opiates | 7 (1.8) | |
| Hospitalized in last year or since last study visit | ||
| Yes | 27 (6.9) | |
| No | 366 (93.1) | |
| Duration of HIV diagnosis | 2.4 (3.1) | |
| at visit (years) | 1.0 | |
| ARV history at visit: n (%) | ||
| ARV-naïve | 9 (2.3) | |
| ARV-experienced | 384 (97.7) | |
| ARV regimen:n (%) | ||
| None | 12 (3.1) | |
| 1 NRTI | 4 (1.0) | |
| 2 NRTIs+1 NNRTI | 59 (15.0) | |
| 2 NRTIs+1 PI | 283 (72) | |
| Other | 35 (8.9) | |
| Duration of use of ARVs at visit (months) | 13.9 (22.9) | |
| 4.0 | ||
| Reason for ARV use at visit | ||
| Prophylaxis | 203 (53.0) | |
| Treatment | 180 (47.0) | |
| Current CDC clinical classification | ||
| A | 323 (82.2) | |
| B | 34 (8.7) | |
| C | 36 (9.2) | |
| Current CDC immunologic classification | ||
| Category 1 (CD4≥500 cells/mm3; CD4%≥29%) | 117 (29.8) | |
| Category 2 (CD4 200–499 cells/mm3; CD4% 14–28%) | 177 (45.0) | |
| Category 3 (CD4<200 cells/mm3; CD4%<14%) | 99 (25.2) | |
| Current WHO clinical classification | ||
| Stage 1 | 348 (88.5) | |
| Stage 2 | 15 (3.8) | |
| Stage 3 | 16 (4.1) | |
| Stage 4 | 14 (3.6) | |
| Current WHO immunological classification | ||
| None or not significant (CD4>500 cells/mm3) | 168 (42.9) | |
| Mild (CD4 350–499 cells/mm3) | 75 (19.1) | |
| Advanced (CD4 200–349 cells/mm3) | 89 (22.7) | |
| Severe (AIDS) (CD4<200 cells/mm3) | 60 (15.3) | |
ARV, antiretroviral; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; CDC, Centers for Disease Control and Prevention; SD, standard deviation; WHO, World Health Organization.
Antepartum and postpartum adherence to antiretrovirals
The number and proportion of women with a current prescription for ARVs and with a completed adherence form at each of the three visits was assessed. Of the 393 women with a pre-delivery visit, 383 had a current ARV prescription at that visit, and 376 had available adherence data. The mean adherence at the pre-delivery visit was 96.2% (standard deviation ([SD]: 15.8), and 340 women (90.4%) reported perfect adherence. Similarly, of the 383 women with a 6- to 12-week postpartum visit, 218 had a current ARV prescription at that visit, and 214 had available adherence data. The mean adherence at the 6- to 12-week postpartum visit was 89.1% (SD: 28.0), and 171 (79.9%) reported perfect adherence. Finally, of the 380 women with a 6 month postpartum visit, 206 had a current ARV prescription at that visit, and 199 had available adherence data. The mean adherence at the 6 month postpartum visit was 90.7% (SD: 25.0), and 163 women (81.9%) reported perfect adherence. Overall, percent adherence and the proportion of subjects with perfect adherence differed significantly across study visits when tested in the GEE model (p≤0.0065; data not shown). Additionally, postpartum adherence was significantly lower than antepartum adherence (p≤0.0082), but adherence did not vary significantly between the 6- to 12-week and 6-month postpartum visits (p>0.49; data not shown). Similar findings were observed when the models were fit to the data restricted to subjects with adherence measured at all three study visits.
Factors associated with perfect adherence to antiretrovirals
Table 2 presents the results of bivariable analyses of perfect adherence for each of the three time periods. At the pre-delivery visit, the following factors were associated with perfect adherence: mode of acquisition of HIV infection, CD4 percent, viral load, substance abuse (ever), current use of alcohol, current use of tobacco, and duration of use of ARVs. Due to small numbers of subjects with modes of acquisition of HIV infection other than heterosexual transmission, this variable was not considered a candidate variable for multivariable modeling. CD4 percent and viral load were considered to more likely be outcomes of adherence rather than risk factors for nonperfect adherence; therefore, these variables were not included in multivariable modeling. The remaining four variables (substance use [ever), current use of alcohol, current use of tobacco, and duration of use of ARVs] were candidate variables for the pre-delivery adherence model, but only current tobacco use remained in the model based on forward stepwise selection. The probability of nonperfect adherence was almost three times higher among those with current use of tobacco than among those not currently using tobacco (odds ratio [OR]=2.9; 95% confidence interval [CI]: 1.46–6.14; p=0.0029).
Table 2.
Characteristics Associated with Perfect Adherence to Antiretrovirals at the Pre-Delivery, 6- to 12-week Postpartum, and 6-Month Postpartum Visit
| |
Perfect adherence at pre-delivery |
Perfect adherence at 6–12 weeks postpartum |
Perfect adherence at 6 months postpartum |
||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Yes |
No |
|
Yes |
No |
|
Yes |
No |
|
| Characteristic | (N=340) | (N=36) | p Valuea | (N=171) | (N=43) | p Valuea | (N=163) | (N=36) | p Valuea |
| Age (years) | |||||||||
| Mean (SD) | 29.0 (6.0) | 29.8 (6.1) | 0.45 | 29.5 (6.1) | 31.5 (5.0) | 0.06 | 30.4 (5.9) | 29.6 (5.5) | 0.47 |
| Median | 29.0 | 28.5 | 29.0 | 31.0 | 30.0 | 30.0 | |||
| Parity:n (%) | |||||||||
| 0 | 61 (91.0) | 6 (9.0) | 0.42 | 31 (83.8) | 6 (16.2) | 0.08 | 35 (94.6) | 2 (5.4) | 0.04 |
| 1–2 | 190 (91.8) | 17 (8.2) | 99 (83.9) | 19 (16.1) | 87 (81.3) | 20 (18.7) | |||
| >2 | 89 (87.3) | 13 (12.7) | 41 (69.5) | 18 (30.5) | 41 (74.5) | 14 (25.5) | |||
| Education (years completed) | |||||||||
| Mean (SD) | 8.2 (3.3) | 8.5 (2.8) | 0.61 | 8.4 (3.2) | 8.4 (2.9) | 0.95 | 8.7 (3.1) | 7.6 (3.4) | 0.06 |
| Median | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | |||
| Number of persons living in subject's household | |||||||||
| Mean (SD) | 4.0 (2.1) | 4.0 (2.5) | 0.93 | 4.1 (2.4) | 4.0 (2.3) | 0.87 | 4.0 (2.4) | 4.6 (2.4) | 0.08 |
| Median | 4.0 | 3.0 | 4.0 | 4.0 | 3.0 | 4.0 | |||
| Mode of HIV infection | |||||||||
| Heterosexual transmission with or without other modes of transmission | 322 (91.2) | 31 (8.8) | 0.03 | 159 (81.1) | 37 (18.9) | 0.13 | 154 (83.2) | 31 (16.8) | 0.05 |
| Other | 3 (60.0) | 2 (40.0) | 3 (100) | 0 | 3 (100) | 0 | |||
| Unknown | 15 (83.3) | 3 (16.7) | 9 (60.0) | 6 (40.0) | 6 (54.5) | 5 (45.5) | |||
| CD4 count (cells/mm3) at visit | |||||||||
| Mean (SD) | 510.8 (272.6) | 456.6 (262.8) | 0.26 | 531.5 (275.6) | 482.6 (275.3) | 0.30 | 520.7 (257.3) | 495.2 (429.6) | 0.06 |
| Median | 486.0 | 426.5 | 485.5 | 408.0 | 494.0 | 370.5 | |||
| CD4 count (cells/mm3) at visit | |||||||||
| <200 | 39 (83.0) | 8 (17.0) | 0.20 | 14 (73.7) | 5 (26.3) | 0.36 | 15 (71.4) | 6 (28.6) | 0.10 |
| 200–499 | 137 (91.3) | 13 (8.7) | 74 (77.1) | 22 (22.9) | 69 (78.4) | 19 (21.6) | |||
| ≥500 | 164 (91.6) | 15 (8.4) | 82 (83.7) | 16 (16.3) | 79 (87.8) | 11 (12.2) | |||
| CD4% at visit: | |||||||||
| Mean (SD) | 29.3 (10.4) | 24.6 (10.4) | 0.03 | 26.2 (9,7) | 23.9 (8.5) | 0.20 | 26.3 (8.5) | 29.8 (28.2) | 0.50 |
| Median | 30.0 | 25.5 | 25.0 | 24.5 | 27.0 | 23.0 | |||
| CD4% at visit:n (%) | |||||||||
| <14% | 18 (81.8) | 4 (18.2) | 0.07 | 13 (72.2) | 5 (27.8) | 0.50 | 12 (66.7) | 6 (33.3) | 0.16 |
| 14–28% | 112 (91.1) | 11 (8.9) | 79 (80.6) | 19 (19.4) | 78 (85.7) | 13 (14.3) | |||
| >28% | 161 (94.7) | 9 (5.3) | 61 (83.6) | 12 (16.4) | 62 (84.9) | 11 (15.1) | |||
| CDC immunologic classification:n (%) | |||||||||
| Category 1 | 100 (89.3) | 12 (10.7) | 0.52 | 18 (90.0) | 2 (10.0) | 0.59 | 16 (94.1) | 1 (5.9) | 0.08 |
| Category 2 | 156 (92.3) | 13 (7.7) | 80 (79.2) | 21 (20.8) | 76 (86.4) | 12 (13.6) | |||
| Category 3 | 84 (88.4) | 11 (11.6) | 73 (78.5) | 20 (21.5) | 71 (75.5) | 23 (24.5) | |||
| Viral load (log10 copies/mL) at visit | |||||||||
| Mean (SD) | 2.3 (0.7) | 2.5 (1.1) | 0.22 | 2.3 (0.9) | 2.9 (1.4) | 0.06 | 2.4 (1.0) | 3.0 (1.4) | 0.01 |
| Median | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.6 | |||
| Viral load (copies/mL) at visit:n(%) | |||||||||
| <400 | 282 (92.5) | 23 (7.5) | 0.01 | 140 (84.8) | 25 (15.2) | 0.002 | 128 (87.1) | 19 (12.9) | 0.003 |
| ≥400 | 57 (81.4) | 13 (18.6) | 31 (63.3) | 18 (36.7) | 35 (67.3) | 17 (32.7) | |||
| Substance use—ever:n(%) | |||||||||
| Yes | 171 (87.2) | 25 (12.8) | 0.04 | 92 (75.4) | 30 (24.6) | 0.39 | 84 (76.4) | 26 (23.6) | 0.27 |
| No | 168 (93.9) | 11 (6.1) | 33 (82.5) | 7 (17.5) | 35 (85.4) | 6 (14.6) | |||
| Alcohol use—current:n(%) | |||||||||
| Yes | 54 (80.6) | 13 (19.4) | 0.005 | 19 (73.1) | 7 (26.9) | 0.43 | 23 (65.7) | 12 (34.3) | 0.01 |
| No | 286 (92.6) | 23 (7.4) | 152 (80.9) | 36 (19.1) | 140 (85.4) | 24 (14.6) | |||
| Tobacco use—current:n(%) | |||||||||
| Yes | 68 (81.0) | 16 (19.0) | 0.002 | 28 (75.7) | 9 (24.3) | 0.50 | 25 (67.6) | 12 (32.4) | 0.02 |
| No | 272 (93.2) | 20 (6.8) | 143 (80.8) | 34 (19.2) | 138 (85.2) | 24 (14.8) | |||
| Duration of use of ARVs at visit (in months) | |||||||||
| Mean (SD) | 14.7 (23.1) | 10.5 (22.6) | 0.03 | 25.8 (27.6) | 22.3 (27.4) | 0.47 | 32.0 (29.2) | 26.6 (24.2) | 0.30 |
| Median | 4.0 | 3.0 | 13.0 | 8.0 | 19.0 | 18.5 | |||
The p values for continuous characteristics were obtained from the Student's t test and nonparametric testing (Kruskal-Wallis test); p values for categorical characteristics are based on Fisher's exact test.
SD, standard deviation; ARVs, antiretrovirals.
The following factors were associated with perfect adherence at the 6- to 12-week postpartum visit: age, parity, and viral load. As delineated above, since viral load was considered more likely to represent an outcome of adherence rather than a risk factor for nonperfect adherence, this variable was not included in multivariable modeling. Thus, age and parity were candidate variables for the 6- to 12-week postpartum adherence model, but only age was independently associated with adherence. The probability of nonperfect adherence increased by 6% for each 1-year increase in age (OR=1.06; 95%CI: 1.00–1.12; p=0.0497).
Variables associated with perfect adherence at the 6 month postpartum visit included: parity, education, number of persons living in the household, mode of acquisition of HIV infection, CD4 count, viral load, current use of alcohol, current use of tobacco, and CDC immunologic classification. As before, CD4 count, viral load, and CDC immunologic classification (based on CD4 count) were considered outcomes of adherence rather than risk factors for nonperfect adherence and not included in multivariable modeling. Thus, the remaining five variables (parity, education, number of persons living in the household, current use of alcohol, current use of tobacco) were candidate variables for multivariable modeling, but only current use of alcohol was independently associated with adherence. The probability of non-perfect adherence was over threefold higher for those who were currently using alcohol at the time of the 6-month visit (OR=3.04; 95% CI: 1.34–6.90; p=0.0079).
Percent adherence and plasma viral load
As noted above, plasma viral load was considered an outcome of adherence rather than a risk factor for nonperfect adherence. Further analyses of the association between percent adherence and plasma viral load were conducted utilizing data from 375, 214, and 199 women at the pre-delivery, 6- to 12-week postpartum, and 6-month postpartum visits, respectively. At the pre-delivery visit, 70 women with a detectable viral load (≥400 copies per milliliter) had a mean adherence of 88.6 (SD: 29.3) and 305 with a nondetectable viral load had a mean adherence of 97.9 (SD: 9.9; p=0.0029). Similarly, at the 6- to 12-week postpartum visit, 49 women with a detectable viral load had a mean adherence of 70.1 (SD: 44.5) and 165 with a nondetectable viral load had a mean adherence of 94.7 (SD: 17.3; p=0.0001). Finally, at the 6 month postpartum visit, 52 women with a detectable viral load had a mean adherence of 80.1 (SD: 36.2) and 147 with a nondetectable viral load had a mean adherence of 94.5 (SD: 18.3; p=0.0008). Thus, at each time point, those women with detectable viral loads had lower mean percent adherence.
Reasons for nonperfect adherence
Subjects reporting that they had any problems or situations making it difficult to take every dose of medication every day were asked to respond to a list of different problems or situations related to ARV adherence (Table 3). The most common problem related to taking ARVs was forgetting to take the ARV (35.8% pre-delivery, 52.0% at 6–12 weeks postpartum, and 64.5% at 6 months postpartum). The other commonly cited reasons were: being away from home, a change in the daily routine, and running out of ARVs/not coming for ARVs.
Table 3.
Problems or Situations Making it Difficult to Take Antiretrovirals in Each Study Visit
| |
Instances where problem identified |
||
|---|---|---|---|
| Problem or situation making it difficult to take ARVs | Pre-delivery visit (n=293) | 6- to 12-week postpartum visit (n=152) | 6-month postpartum visit (n=138) |
| Just forgot | 105 | 79 | 89 |
| Subject was away from home | 70 | 21 | 16 |
| I ran out of medicine; didn't come for medicine | 32 | 28 | 30 |
| There was a change in daily routine | 47 | 22 | 34 |
| Was worried about side effects | 22 | 11 | 11 |
| Subject was busy with other things | 13 | 12 | 8 |
| Medicine tastes bad | 10 | 5 | 5 |
| Too busy with baby/child | 0 | 23 | 15 |
| Did not want others to notice caregiver giving the medicine | 8 | 0 | 2 |
| Baby/child was ill | 1 | 0 | 1 |
| Family said someone told them not to give/take the medicine | 0 | 0 | 0 |
| Study subject was ill | 13 | 0 | 0 |
| Subject felt depressed | 9 | 7 | 10 |
| There was too much medicine to give | 0 | 2 | 0 |
| Other problems or situations | 39 | 34 | 16 |
Last time antiretrovirals were missed during and after pregnancy
At the pre-delivery visit, when asked about the last time medications were missed, 67.8% of women responded that they never forgot their medication. By the time of the 6- to 12-week and 6-month postpartum visits, 55.6% and 57.2% of women responded that they never forgot their medications, respectively. Long-term adherence varied over time (p=0.0004), with significant differences identified between the pre-delivery visit and the 6- to 12-week and 6-month postpartum visits; long-term adherence did not differ between postpartum visits.
Discussion
In this analysis of ARV adherence among pregnant and postpartum women in Latin America, there was a statistically significant decline in perfect adherence to ARVs during the postpartum period (80% at 6–12 weeks and 82% at 6 months) compared to reported adherence during pregnancy (90.4%). Current tobacco use was a predictor of non-perfect adherence to ARVs during pregnancy, and older age and current alcohol use were associated with nonperfect adherence at 6–12 weeks and 6 months postpartum, respectively. Our results confirm the association described previously between ARV adherence as measured by the simple and low-cost approach of self-report and positive clinical, immunologic, and virologic outcomes.9,10–13 That said, a self-report measure of adherence based on only 3 days may lead to overestimation of actual adherence over time.14 A very common reason for missing ARV doses cited by women was “just forgot.” However, this response may be a euphemism for some other situations associated with taking/giving medications (e.g., stigma, depression) that the participants do not want to discuss.
The adherence rates we observed among the study population during pregnancy and postpartum are similar to what has been reported in some studies,15 but higher than reported by others.16–18 However, all of these mentioned studies, as well as ours, reported higher adherence rates during pregnancy compared to postpartum. The differences between studies may reflect differences in population characteristics, access to care, and the complexity of ARV regimens used. Most subjects in our study used PI-containing regimens, with dosing schedules that required relatively few pills each day.
Substance use appears to be an important factor in ARV adherence during and after pregnancy. The rates of using alcohol (18.1%) and tobacco (22.4%) during pregnancy observed in our study population were higher than anticipated but were not different from what was observed postpartum, contrary to the expectation that women tend to have less substance use during pregnancy. The association between tobacco and alcohol use and nonperfect adherence has been previous reported.15,17,18 One recent study comparing HIV-infected female smokers to nonsmokers demonstrated increased mortality and decreased adherence to ARV treatment among current smokers.19 Several additional studies showed lower rates of virologic suppression in HIV-infected smokers compared to nonsmokers, and speculated that inferior adherence could have been a contributing factor.20,21 The reasons for poorer adherence among smokers are not precisely defined. Cigarette smoking is frequently linked to other behaviors that have been considered predictors of nonadherence, such as depression22–24 and illicit substance use.25,26 Possible reasons for poorer adherence to ARV treatment among HIV-infected smokers include indifference to health outcomes,27 a propensity for risk taking,28 and fatalism.29
An unexpected finding in our study was the association of older age with nonadherence at 6- to 12-week postpartum visit. Poorer ARV adherence among adolescents, even during pregnancy has been described.30 However, adolescents were under-represented in our study. Some studies have shown that HIV-infected women with children under 18 years old living with them experience increased demands compared to HIV-infected women without children, which subsequently results in poorer adherence to antiretrovirals.31,32 The association between older age and nonadherence in our study needs further exploration.
The strengths of our study include its prospective cohort study design, with questionnaires included to evaluate adherence. Our self-report measure is simple and low cost to implement, and correlated with viral load in this relatively high adherence population. Additionally, we evaluated adherence at two time points during the postpartum period, allowing us to examine changes in behavior during this complex time in women's lives. A limitation of the study is that it did not include measures of psychosocial factors, such as depression, stressful life events and time constraints, nor did it include objective measures of adherence. Additionally, the small numbers of women reporting hard drug use may have limited the power to assess this factor's association with adherence.
The results of this study confirm previous reports that women are more likely to adhere to ARVs during pregnancy than postpartum. This study highlights the vulnerability of HIV-infected women during the postpartum period and the need to design and evaluate adherence interventions containing a substance use cessation component. Although improved adherence during pregnancy has been critical to the successful reduction of MTCT of HIV, these successes will be tempered by increased maternal morbidity and mortality and a potential increase in orphans if we fail to implement interventions to maintain adherence to ARVs over the long term. Substance use cessation during gestation has well-known additional benefits from the neonatal perspective and should be a major goal in prenatal care.33,34 Interventions to provide adequate social support and integration with mental health programs should be explored as means to help women to adhere to ARVs.
Acknowledgments
Presented in part at the 19th Conference on Retroviruses and Opportunistic Infections. Seattle, WA: March 5–8, 2012 (abstract 1016).
The NISDI LILAC Study Team
Principal investigators, co-principal investigators, study coordinators, coordinating center representatives, and NICHD staff include: Argentina: Buenos Aires: Marcelo H. Losso, Irene Foradori, Alejandro Hakim, Erica Stankievich, Silvina Ivalo (Hospital General de Agudos José María Ramos Mejía); Brazil: Belo Horizonte: Jorge Pinto, Victor Melo, Fabiana Kakehasi (Universidade Federal de Minas Gerais); Caxias do Sul: Rosa Dea Sperhacke, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/ Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Beatriz Grinsztejn, Valdilea Veloso, Luis Eduardo Fernandes, Gisely Falco (Hospital Geral Nova de Iguacu-HIV Family Care Clinic); Porto Alegre: Rosa Dea Sperhacke, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Rosa Dea Sperhacke, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Regis Kreitchmann, Luis Carlos Ribeiro, Fabrizio Motta, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Geraldo Duarte, Adriana A. Tiraboschi Bárbaro, Conrado Milani Coutinho, Fabiana Rezende Amaral, Anderson Sanches de Melo (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Elizabeth S. Machado, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Leon Claude Sidi, Maria Leticia Santos Cruz, Maria Isabel Gouvêa, Ana Paula Antunes, Plinio Tostes Berardo (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Prescilla Chow (Escola Paulista de Medicina- Universidade Federal de São Paulo); Peru: Lima: Jorge Alarcón Villaverde (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiología, UNMSM), Carlos Velásquez Vásquez (Instituto Nacional Materno Perinatal), César Gutiérrez Villafuerte (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiología, UNMSM).
Data Management and Statistical Center: Yolanda Bertucci, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, James Korelitz, Margot Krauss, Kathryn Miller, Sharon Sothern de Sanchez, Sonia K. Stoszek (Westat, Rockville, MD).
NICHD: George K. Siberry, Rohan Hazra, Lynne M. Mofenson, Jennifer S. Read, Heather Watts (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland, USA).
Supported by NICHD Contract # N01-HD-3-3345 (2002–2007) and by NICHD Contract # HHSN267200800001C (NICHD Control # N01-HD-8-0001) (2007–2012).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cohen MS. Hellmann N. Levy JA. DeCock K. Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J Clin Invest. 2008;118:1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. May 24, 2010. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. [Feb 13;2012 ]. pp. 1–117.http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf
- 3.Brazilian Guidelines for Prevention of Mother to Child HIV Transmission. Brazilian Ministry of Health. 2010. www.aids.gov.br/publicacao/recomendacoes-consenso-gestante. [Feb 13;2012 ]. www.aids.gov.br/publicacao/recomendacoes-consenso-gestante
- 4.Bangsberg DR. Acosta EP. Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 5.Simoni JM. Amico KR. Smith L. Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7:44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. Report on the Global AIDS Epidemic. 2010. www.unaids.org/globalreport/Global_report.htm. [Mar 27;2012 ]. www.unaids.org/globalreport/Global_report.htm
- 7.Read JS. Duarte G. Freimanis Hance L, et al. The NICHD International Site Development Initiative perinatal cohorts (2002-09) Int J Epidemiol. doi: 10.1093/ije/dyr024. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haberer JE. Cook A. Walker AS, et al. Excellent adherence to antiretrovirals in HIV+ Zambian children is compromised by disrupted routine, HIV nondisclosure, and paradoxical income effects. PLoS One. 2011;6:e18505. doi: 10.1371/journal.pone.0018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simoni JM. Kurth AE. Pearson CR. Pantalone DW. Merrill JO. Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieuwkerk PT. Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J Acquir Immune Defic Syndr. 2005;38:445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- 11.Sethi AK. Celentano DD. Gange SJ. Moore RD. Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37:1112–1118. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 12.Spire B. Duran S. Souville M. Leport C. Raffi F. Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: From a predictive to a dynamic approach. Soc Sci Med. 2002;54:1481–1496. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- 13.Chesney MA. Ickovics JR. Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care Jun. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 14.Wagner GJ. Rabkin JG. Measuring medication adherence: Are missed doses reported more accurately then perfect adherence? AIDS Care. 2000;12:405–408. doi: 10.1080/09540120050123800. [DOI] [PubMed] [Google Scholar]
- 15.Zorrilla CD. Santiago LE. Knubson D, et al. Greater adherence to highly active antiretroviral therapy (HAART) between pregnant versus non-pregnant women living with HIV. Cell Mol Biol (Noisy-le-grand) 2003;49:1187–1192. [PubMed] [Google Scholar]
- 16.Bardeguez AD. Lindsey JC. Shannon M, et al. Adherence to antiretrovirals among US women during and after pregnancy. J Acquir Immune Defic Syndr. 2008;48:408–417. doi: 10.1097/QAI.0b013e31817bbe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellins CA. Chu C. Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20:958–968. doi: 10.1080/09540120701767208. [DOI] [PubMed] [Google Scholar]
- 18.Cohn SE. Umbleja T. Mrus J. Bardeguez AD. Andersen JW. Chesney MA. Prior illicit drug use and missed prenatal vitamins predict nonadherence to antiretroviral therapy in pregnancy: Adherence analysis A5084. AIDS Patient Care STDs. 2008;22:29–40. doi: 10.1089/apc.2007.0053. [DOI] [PubMed] [Google Scholar]
- 19.Feldman JG. Minkoff H. Schneider MF, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: A report from the women's interagency HIV study. Am J Public Health. 2006;96:1060–1065. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFino M. Clark J. Mogyoros D. Shuter J. Predictors of virologic success in patients completing a structured antiretroviral adherence program. J Assoc Nurses AIDS Care. 2004;15:60–67. doi: 10.1177/1055329004269135. [DOI] [PubMed] [Google Scholar]
- 21.Purkayastha T. Wasi F. Shuter J. Factors associated with sustained virologic suppression in patients receiving antiretroviral therapy in an urban HIV care clinic. AIDS Patient Care STDs. 2005;19:785–793. doi: 10.1089/apc.2005.19.785. [DOI] [PubMed] [Google Scholar]
- 22.Arnsten JH. Demas PA. Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakimuli-Mpungu E. Bass JK. Alexandre P, et al. Depression, Alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: A systematic review. AIDS Behav. doi: 10.1007/s10461-011-0087-8. (in press). [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez JS. Batchelder AW. Psaros C. Safren SA. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58:181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg KM. Demas PA. Howard AA. Schoenbaum EE. Gourevitch MN. Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004;19:1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard AA. Arnsten JH. Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 27.Odum AL. Madden GJ. Bickel WK. Discounting of delayed health gains and losses by current, never- and ex-smokers of cigarettes. Nicotine Tob Res. 2002;4:295–303. doi: 10.1080/14622200210141257. [DOI] [PubMed] [Google Scholar]
- 28.Camenga DR. Klein JD. Roy J. The changing risk profile of the American adolescent smoker: implications for prevention programs and tobacco interventions. J Adolesc Health. 2006;39:121–110. doi: 10.1016/j.jadohealth.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Schnoll RA. Malstrom M. James C, et al. Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns. 2002;46:137–145. doi: 10.1016/s0738-3991(01)00157-4. [DOI] [PubMed] [Google Scholar]
- 30.Laine C. Newschaffer CJ. Zhang D. Cosler L. Hauck WW. Turner BJ. Adherence to antiretroviral therapy by pregnant women infected with human immunodeficiency virus: A pharmacy claims-based analysis. Obstet Gynecol. 2000;95:167–173. doi: 10.1016/s0029-7844(99)00523-2. [DOI] [PubMed] [Google Scholar]
- 31.Merenstein D. Schneider MF. Cox C, et al. Association of child care burden and household composition with adherence to highly active antiretroviral therapy in the Women's Interagency HIV Study. AIDS Patient Care STDs. 2009;23:289–296. doi: 10.1089/apc.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vyavaharkar M. Moneyham L. Tavakoli A, et al. Social support, coping, and medication adherence among HIV-positive women with depression living in rural areas of the southeastern United States. AIDS Patient Care STDs. 2007;21:667–680. doi: 10.1089/apc.2006.0131. [DOI] [PubMed] [Google Scholar]
- 33.Mengel MB. Searight HR. Cook K. Preventing alcohol-exposed pregnancies. J Am Board Fam Med. 2006;19:494–505. doi: 10.3122/jabfm.19.5.494. [DOI] [PubMed] [Google Scholar]
- 34.Lumley J. Chamberlain C. Dowswell T. Oliver S. Oakley L. Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001055.pub3/pdf/abstract. [Feb 27;2012 ]. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001055.pub3/pdf/abstract [DOI] [PMC free article] [PubMed]


