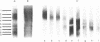Abstract
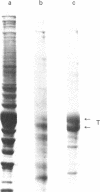
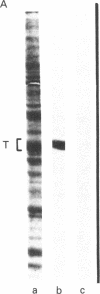
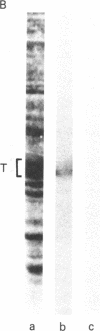
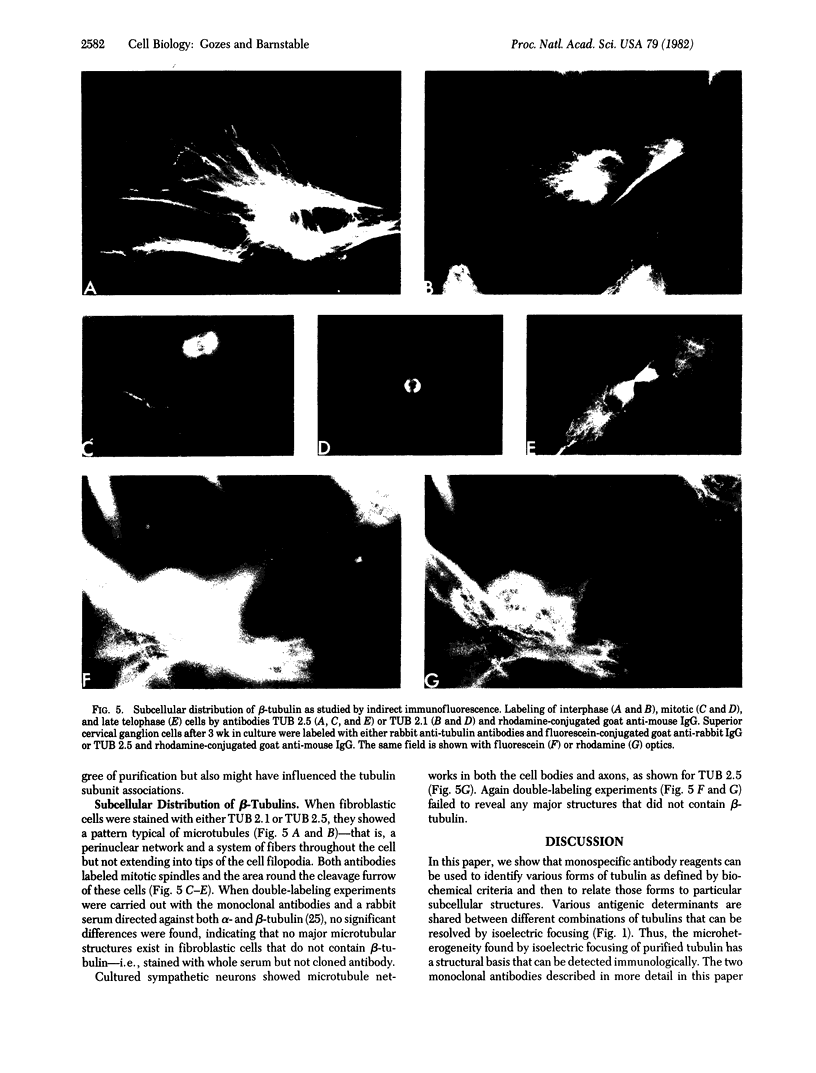
Anti-tubulin antibodies secreted by plasmacytoma NSI-spleen cell hybrids were detected by an indirect binding assay. Different antibodies bound to different combinations of the tubulins as resolved by isoelectric focusing. Two monoclonal antibodies (TUB 2.1 and TUB 2.5) labeled only (i) the tubulin band on a polyacrylamide electropherogram and (ii) beta-tubulins as resolved by isoelectric focusing. The fraction that was specifically bound and eluted from antibody affinity columns was enriched in beta-tubulins as compared with alpha-tubulins, suggesting the possibility of some soluble tubulin homodimers and alpha,beta-heterodimers. Double labeling experiments were used to show that all detectable microtubules contained beta-tubulin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980 Jul 17;286(5770):231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Weibel V. J. Changes in tubulin heterogeneity during postnatal development of rat brain. Biochem Biophys Res Commun. 1979 Feb 14;86(3):822–828. doi: 10.1016/0006-291x(79)91786-8. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Williams R. C. Reversible dissociation of the alpha beta dimer of tubulin from bovine brain. Biochemistry. 1978 Sep 19;17(19):3900–3907. doi: 10.1021/bi00612a002. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous A., Francon J., Lennon A. M., Nunez J. Microtubule assembly in vitro. Purification of assembly-promoting factors. Eur J Biochem. 1977 Aug 15;78(1):167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., de Baetselier A., Walker M. D., Behar L., Lehrach H., Frischauf A. M., Littauer U. Z. Brain tubulin and actin cDNA sequences: isolation of recombinant plasmids. Nucleic Acids Res. 1980 Aug 25;8(16):3553–3564. doi: 10.1093/nar/8.16.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z., Geiger B., Fuchs S. Immunochemical determination of tubulin. FEBS Lett. 1977 Jan 15;73(1):109–114. [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., de Baetselier A., Littauer U. Z. Translation in vitro of rat brain mRNA coding for a variety of tubulin forms. Eur J Biochem. 1980 Jan;103(1):13–20. doi: 10.1111/j.1432-1033.1980.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Patterson P. H. Long-term culture of dissociated sympathetic neurons. Methods Enzymol. 1979;58:574–584. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Ludueńa R. F., Shooter E. M., Wilson L. Structure of the tubulin dimer. J Biol Chem. 1977 Oct 25;252(20):7006–7014. [PubMed] [Google Scholar]
- Marotta C. A., Harris J. L., Gilbert J. M. Characterization of multiple forms of brain tubulin subunits. J Neurochem. 1978 Jun;30(6):1431–1440. doi: 10.1111/j.1471-4159.1978.tb10475.x. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Strocchi P., Gilbert J. M. Biosynthesis of heterogeneous forms of mammalian brain tubulin subunits by multiple messenger RNAs. J Neurochem. 1979 Jul;33(1):231–246. doi: 10.1111/j.1471-4159.1979.tb11725.x. [DOI] [PubMed] [Google Scholar]
- Nelles L. P., Bamburg J. R. Comparative peptide mapping and isoelectric focusing of isolated subunits from chick embryo brain tubulin. J Neurochem. 1979 Feb;32(2):477–489. doi: 10.1111/j.1471-4159.1979.tb00374.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Snary D., Barnstable C. J., Bodmer W. F., Goodfellow P. N., Crumpton M. J. Cellular distrubtion, purification, and molecular nature of human Ia antigens. Scand J Immunol. 1977;6(5):439–452. doi: 10.1111/j.1365-3083.1977.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F. Differentiation antigens of the lymphocyte cell surface. Contemp Top Mol Immunol. 1977;6:83–116. doi: 10.1007/978-1-4684-2841-4_3. [DOI] [PubMed] [Google Scholar]