Abstract
Postmenopausal women make up one of the fastest growing populations in the United States. Women typically have a higher incidence of cardiovascular disease following menopause. One of the major risk factors for cardiovascular disease is hypertension, and after menopause, blood pressure (BP) increases progressively in women. Also after menopause, the progression of renal disease increases in women compared with aged matched men. However, the mechanism(s) responsible for the postmenopausal increase in BP and renal injury are yet to be elucidated. Moreover the best therapeutic options to treat postmenopausal hypertension in women are not clear. Hypertension in postmenopausal women are usually associated with other cardiovascular risk factors, such as dyslipidemias, visceral obesity and endothelial dysfunction. Recently it became apparent that in a large number of hypertensive postmenopausal women, their BP is not well controlled with conventional antihypertensive medications. A clear understanding of the complex pathogenesis of postmenopausal hypertension is needed in order to offer the best therapeutic options for these women.
Keywords: Aldosterone, angiotensin II, blood pressure, endothelin, menopause, sex steroids sympathetic, visceral obesity
INTRODUCTION
Prior to middle age, men are at greater risk for cardiovascular and renal disease than are women. Men also have higher blood pressure (BP) than women [1, 2]. However, these sex differences change following menopause, when the risk for cardiovascular disease and prevalence of hypertension increases in women [3].
There are few clinical data regarding the effectiveness of antihypertensive medications in controlling BP in postmenopausal hypertensive women. The Women’s Health Initiative (WHI) report was based on nearly 100,000 women, ages 50 to 79 years, and was the largest and best-characterized cohort of postmenopausal women in the United States. This report showed that although older hypertensive women (aged 70 to 79 years) were as likely to be on treatment for hypertension (63.2%) as younger women (64.2%), a substantially smaller percentage of them had their BP under control (i.e. < 140/90 mm Hg; 29.3% versus 41.3% for the older versus younger women, respectively) [4]. Similar findings were observed when the results from the National Health and Nutrition Estimation Survey (NHANES) III data set (ending 1994) were compared with NHANES IV data set (ending 2004) [5]. Thus the best therapeutic options for treatment of postmenopausal hypertension are not clear. In this review we will discuss the current information regarding mechanisms responsible for hypertension in aging individuals, both ours and that of other investigators, that shows that hypertension in male animals/men is most likely due to a activation of a single system, and is unlike what is seen in postmenopausal women/animals.
MECHANISMS RESPONSIBLE FOR POSTMENOPAUSAL HYPERTENSION AND RENAL INJURY: ROLE OF THE RENIN ANGIOTENSIN SYSTEM
A major system for controlling blood pressure and body fluid volume (i.e. pressure-natriuresis) is the renin-angiotensin system [6]. Angiotensin II (Ang II) increases proximal sodium reabsorption by the kidney by stimulating epithelial transport [6, 7]. Ang II is also a potent stimulator of aldosterone secretion [8], and is a strong vasoconstrictor of intrarenal microcirculation.
Postmenopausal women exhibit activation of the renin-angiotensin system (RAS) compared with premenopausal women [9], and with age, there is positive linear correlation between plasma renin activity and aldosterone levels in women but not in men [8]. We have published that plasma renin activity (PRA) is increased in a rodent model of postmenopausal hypertension, the aging female spontaneously hypertensive rat (PMR) [10].
What can activate the renin angiotensin system after menopause? Estrogen reduces the number of angiotensin type 1 (AT-1) receptors in many tissues, including the kidney, and attenuates tissue responsiveness to Ang II [11–14]. Estrogen can increase expression of the renin substrate, angiotensinogen, mainly in the liver, but it decreases PRA [14]. Compared to age-matched male spontaneously hypertensive rats (SHR), PMR have significantly higher levels of plasma angiotensinogen and intrarenal Ang II, the main effector of the RAS, in the kidney. Therefore, a decline in estrogen levels, a hallmark of menopause, will lead to activation of the RAS system. We have published that in the young SHR, males have higher BP than females, and the sex difference in BP is abolished by angiotensin converting enzyme (ACE) inhibition [15]. Furthermore, we have recently shown that AT-1 receptor blockade with losartan in 16 mos old male SHR normalized BP [10]. However, in age-matched PMR, while mean arterial BP was significantly reduced (from 180 mm Hg), it was not normalized and remained approximately 130 mm Hg. Clinical studies using monotherapy with candesartan, an AT-1 receptor antagonist, only controlled BP in 36% of women after 1 month of treatment [16]. These data suggest that although activation of RAS plays an important role in mediating a part of the hypertension in PMR and postmenopausal women, the likelihood that postmenopausal hypertension can be adequately treated with a single drug is very low.
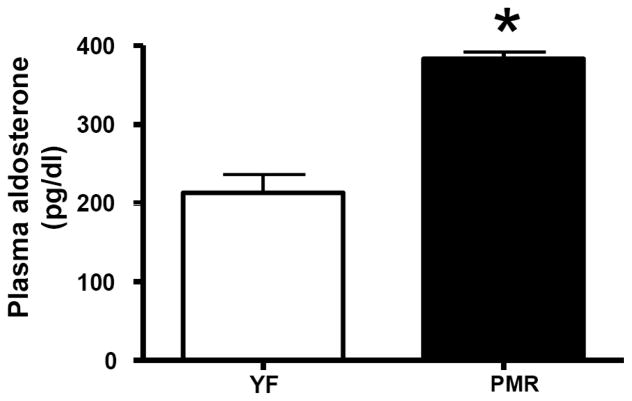
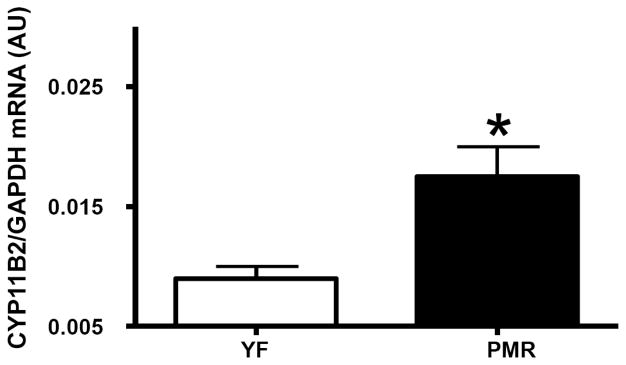
Recently there has been increased interest in the role of aldosterone in postmenopausal hypertension. Aldosterone is synthesized from cholesterol in the zona glomerulosa of the adrenal gland [17], and Ang II is the main stimulus for aldosterone release by the adrenal. The process requires the steroidogenic acute regulatory (StAR) protein to move cholesterol into the mitochondria. Following translocation to the mitochondria, cholesterol is converted to aldosterone by a series of enzymatic reactions catalyzed by dehydrogenases and mixed-function oxidases, such as the key enzyme, aldosterone synthase, which is encoded by the CYP11B2 gene, and determines circulating aldosterone levels [18]. In the PMR, we found that plasma aldosterone and adrenal gland aldosterone synthase expression are significantly elevated Figs. (1 and 2). These data suggest that anti-mineralocorticoid receptor antagonists may be useful in reducing BP in postmenopausal women. In support of this hypothesis, Drosperidone, when given with estradiol, significantly decreases BP in hypertensive postmenopausal women [19]. Drosperidone is a new progestin derived from 17α-spirolactone, that closely matches the pharmacological profile of natural progesterone, but is devoid of androgenic or glucocorticoid activities, and has a higher anti-mineralocorticoid potency than spironolactone [19]. Therefore, combination therapy with a minerolocorticoid receptor antagonist and estrogen maybe a promising therapeutic approach to treat postmenopausal hypertension.
Fig. (1).
Aldosterone plasma level is elevated in PMR.
Fig. (2).
Aldosterone synthase (CYP11B2) mRNA expression is upregulated in PMR adrenal gland.
Estradiol is usually thought to be reno-protective. For example, in cultured renal glomerular mesangial cells, 17β-estradiol inhibits apoptosis and TGF-β activity and expression [20], which is responsible for mesangial expansion and glomerular injury. In addition, estradiol is anti-inflammatory [21–23]. Observational studies initially suggested a cardiovascular protective effect of hormone replacement therapy (HRT) in postmenopausal women. But controlled clinical trials, such as Heart and Estrogen/progestin Replacement Study (HERS) and the recently terminated WHI study, reported no protective cardiovascular effect of HRT, consisting of conjugated equine estrogen (CEE) plus medroxy-progesterone-acetate or estradiol alone for prevention of primary or secondary cardiovascular disease [24]. These findings highlight the importance of a re-evaluation of the cardiovascular action of estrogen in postmenopausal women. For example, under normal conditions, estradiol increases endothelial nitric oxide (NO) synthesis and activity and downregulates PRA and AT1 receptor expression. However, in the presence of low levels of NO and high levels of Ang II, estradiol exacerbates renal injury via upregulation of renal AT1 receptor expression [25]. In addition, oral contraceptives containing estrogen preparations can result in hypertension [26] and biopsy-proven renal damage in the absence of primary renal disease [27]. Thus with aging and menopause, the interactions between the RAS, one of the most powerful systems to control BP, and estradiol may change leading to promotion of hypertension and renal injury. Studies into the changes in these interactions after menopause remain to be fully understood.
MECHANISMS RESPONSIBLE FOR POSTMENOPAUSAL HYPERTENSION AND RENAL INJURY: ROLE OF BAROREFLEX SENSITIVITY (BRS) AND BP VARIABILITY
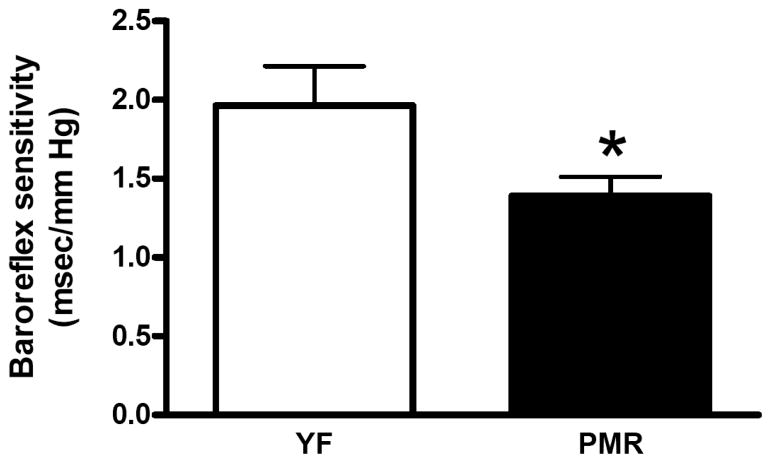
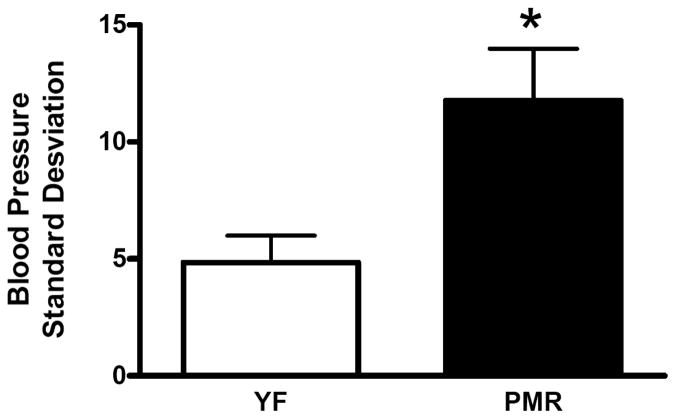
BP is not constant and spontaneous variations in BP exist. This variation is defined as BP variability. The baroreflex actively buffers the BP responses to pressor or depressor stimuli and thus plays an important role in preventing BP variability. BRS is reduced in individuals with hypertension, coronary heart disease or congestive heart failure [28]. In addition, BRS is reduced with advancing age, even in healthy subjects [29, 30]. However, in women, postmenopausal hypertension is often associated with cardiovascular autonomic alterations, such as a predominance of sympathetic control of the heart rate and decreases in BRS [31], to a greater extent than in age-matched men [32]. In rodents, ovariectomy (ovx) decreases BRS and estradiol supplementation restores it [33]. We have found that in PMR, BRS is impaired Fig. (3). Acute systemic administration of aldosterone reduces baroreceptor discharge from the carotid sinuses in dogs [34], and can cause impairment in the baroreflex control of heart rate in healthy human subjects [35]. Therefore, in postmenopausal women, one of the beneficial effects of drosperidone, the mineralocorticoid receptor antagonist discussed above, may be due to the inhibition of aldosterone action on BRS.
Fig. (3).
Baroreflex sensitivity is decreased in PMR.
MECHANISMS RESPONSIBLE FOR POSTMENOPAUSAL HYPERTENSION AND RENAL INJURY: ROLE OF ENDOTHELIN
Activation of the endothelin system is present in postmenopausal hypertension, and in one study a positive correlation was found between endothelin level and increasing levels of testosterone. Along the same lines, in female-to-male transsexuals, testosterone treatment increases plasma endothelin levels [36]. We have found that in PMR levels of testosterone are 4-fold increased compared to the young female SHR. In DOCA-salt rats, renal injury in ovx females is ameliorated by endothelin (ETA) receptor antagonism [37]. After warm ischemia, the early recovery of renal blood flow is delayed in male rats compared to females, due to a greater increase in renal vascular resistance in males, likely mediated in part by increased endothelin, since preproendothelin mRNA was elevated in kidneys of males but not females [38]. In uninephrectomized spontaneously hypertensive-stroke prone rats (SHRsp), ovx females exhibited significantly greater glomerular damage and greater endothelin expression than estradiol-treated animals [39]. Therefore, we hypothesized that endothelin receptor antagonists may provide a useful therapeutic option in postmenopausal hypertension.
In support of this notion, we previously showed that a portion of the hypertension in PMR is mediated by activation of the endothelin system, since the endothelin type A receptor antagonist, ABT627, decreases BP [40]. However, the use of ABT627 only reduced BP about 20 mm Hg and BP remained significantly higher in the aged PMR than in young female rats. Ang II has been shown to increase expression of preproendothelin in the kidney [41]. We found that the levels of endothelin are elevated in the kidney in PMR compared to the young females. Since androgens increase angiotensinogen and PRA, it is possible that androgens could increase Ang II leading to increased endothelin, and subsequent hypertension and renal injury. Alternatively, since estradiol can downregulate AT1 receptor expression, estrogen replacement therapy should prevent the increase in endothelin and protect against renal injury.
MECHANISMS RESPONSIBLE FOR POSTMENOPAUSAL HYPERTENSION AND RENAL INJURY: ROLE OF OBESITY
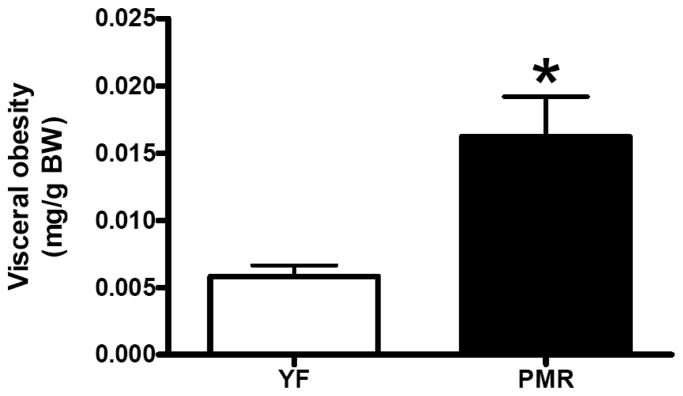
The menopause transition, as well as the early postmenopausal period, is associated with an increase in total and central obesity. Increased visceral fat is associated with insulin resistance, and this preferential storage of abdominal fat may contribute to cardiovascular disease and type II diabetes that is often present in postmenopausal women. Weight gain and obesity is thought to drive the increased prevalence of hypertension and metabolic syndrome in postmenopausal women. We have found that in PMR there is a significant increase in fat deposition in their abdominal cavity Fig. (5). Obesity can also cause sympathetic nerve activation [42], and thus further contribute to increase in BP in postmenopausal women.
Fig. (5).
Visceral obesity is increased in PMR.
MECHANISMS RESPONSIBLE FOR POSTMENOPAUSAL HYPERTENSION AND RENAL INJURY: ROLE OF TESTOSTERONE
The kidney of postmenopausal women can synthesize testosterone and dihydrotestosterone since it contains the cytochrome P450 enzymes that are necessary for its biosynthesis [43]. Increases in testosterone can mediate renal injury in female rats. Increases in testosterone can also elevate BP, as found in women with polycystic ovary syndrome (PCOS) (). Testosterone supplements increased expression of androgen receptor (AR) and TGF-β 1 in glomeruli of ovx B6 mice [44], and AR antagonism protects against renal injury in female TGR(mREN2)27 [45]. As mentioned previously, PMR plasma testosterone is increased 4-fold compared to young female SHR [46]. Androgens can exert both genomic and non-genomic actions [47–49]. The genomic actions are mediated through the classic AR, which is a 110 kDa protein composed of multiple domains for androgen binding, DNA binding and transactivation. Ligand-bound classic AR mainly functions as a transcription factor modulating the expression of AR target genes. In addition to this genomic mode of action, androgens also exert non-genomic effects. The molecular identity of the AR mediating these non-genomic actions has not been elucidated yet. Androgen non-genomic actions include increase in intracellular calcium and activation of protein kinases, such as Src tyrosine kinase (c-Src), extracellular signal-regulated kinase 1/2 (ERK 1/2) and phosphatidylinositol 3-kinase (PI3K) [50–55]. Although the non-genomic actions of androgen are rapid, they can have long-lasting effects since the pathways that include the kinases mentioned above can have transcription regulatory effects. We previously showed that androgens can stimulate the production of aldosterone, a stimulatory action that was not blocked by flutamide, an AR antagonist. In our study we found that the non-classical AR-mediated actions of DHT were mediated by calmodulin/CaMK and protein kinase C, which opens new avenues for investigation into the mechanisms responsible for the non-classical receptor mediated effects of androgens. We can speculate then that the increase in aldosterone that we found in PMR may be an effect of androgens through non-genomic mechanisms. Further studies need to be done in order to fully understand the non-genomic mechanisms of testosterone in postmenopausal hypertension.
SUMMARY
Postmenopausal hypertension is a complex disease with multiple contributing mechanisms involved. The Joint National Committee on Treatment of Hypertension (JNC) VI and JNC VII Guidelines recommend the use of diuretics and beta-blockers in uncomplicated hypertensive patients. However, postmenopausal hypertension is often associated with more cardiovascular risk factors than found in patients with essential hypertension. At the present many postmenopausal hypertensive women are treated with calcium channel blockers, diuretics, or a combination of the two [4]. Treatment with calcium channel blockers is associated with increased morbidity and mortality in some cohorts [56, 57] and also may increase the transmission of the systemic pressure to the glomeruli, exacerbating renal injury [58], and thus further complicating hypertension. In addition, diuretics can worsen metabolic alterations often found in postmenopausal hypertension. Therefore, physicians should take into consideration that postmenopausal hypertension is a complex disease caused by an intricate combination of mechanisms working synergistically to increase BP, and that postmenopausal women need to be treated with blockers specific for the systems that mediate the hypertension rather than non-specific treatment with calcium channel blockers and diuretics that may cause more harm than good. Finally, more research is needed to determine the best treatment regimen for postmenopausal women.
Fig. (4).
Blood pressure variability is increased in PMR.
Acknowledgments
The authors acknowledge the support of the National Institutes of Health RO1s HL66072 and HL69194 and PO1 HL51971, and the American Heart Association Scientific Development Award #0830239N.
References
- 1.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 2.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8(10 Pt 1):978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB. The Framingham Study: historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med. 2002;5(2):27–37. [PubMed] [Google Scholar]
- 4.Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and its treatment in postmenopausal women: baseline data from the Women’s Health Initiative. Hypertension. 2000;36(5):780–789. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 5.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51(4):1142–1148. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol. 1999;10(Suppl 12):S258–265. [PubMed] [Google Scholar]
- 7.Hall JE, Guyton AC, Brands MJ. Control of sodium excretion and arterial pressure by intrarenal mechanisms and the renin-angiotensin system. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. Raven Press; New York: 1995. pp. 1451–1475. [Google Scholar]
- 8.Giacche M, Vuagnat A, Hunt SC, Hopkins PN, Fisher ND, Azizi M, Corvol P, Williams GH, Jeunemaitre X. Aldosterone stimulation by angiotensin II : influence of gender, plasma renin, and familial resemblance. Hypertension. 2000;35(3):710–716. doi: 10.1161/01.hyp.35.3.710. [DOI] [PubMed] [Google Scholar]
- 9.Trenkwalder P, James GD, Laragh JH, Sealey JE. Plasma renin activity and plasma prorenin are not suppressed in hypertensives surviving to old age. Am J Hypertens. 1996;9(7):621–627. doi: 10.1016/0895-7061(96)00022-2. [DOI] [PubMed] [Google Scholar]
- 10.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R383–390. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
- 11.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97(22):2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 12.Owonikoko TK, Fabucci ME, Brown PR, Nisar N, Hilton J, Mathews WB, Ravert HT, Rauseo P, Sandberg K, Dannals RF, Szabo Z. In vivo investigation of estrogen regulation of adrenal and renal angiotensin (AT1) receptor expression by PET. J Nucl Med. 2004;45(1):94–100. [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R794–799. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 14.Klett C, Hellmann W, Hackenthal E, Ganten D. Modulation of tissue angiotensinogen gene expression by glucocorticoids, estrogens, and androgens in SHR and WKY rats. Clin Exp Hypertens. 1993;15(4):683–708. doi: 10.3109/10641969309041637. [DOI] [PubMed] [Google Scholar]
- 15.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35(1 Pt 2):480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Vega F, Abellan J, Vegazo O, De Vinuesa SG, Rodriguez JC, Maceira B, de Castro SS, Nicolas RR, Luno J. Angiotensin II type 1 receptor blockade to control blood pressure in postmenopausal women: influence of hormone replacement therapy. Kidney Int Suppl. 2002;(82):S36–41. doi: 10.1046/j.1523-1755.62.s82.8.x. [DOI] [PubMed] [Google Scholar]
- 17.Stewart PM. The Adrenal Cortex. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. Elsevier; Philadelphia: 2003. pp. 491–551. [Google Scholar]
- 18.Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186(1):1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- 19.White WB, Hanes V, Chauhan V, Pitt B. Effects of a new hormone therapy, drospirenone and 17-beta-estradiol, in postmenopausal women with hypertension. Hypertension. 2006;48(2):246–253. doi: 10.1161/01.HYP.0000232179.60442.84. [DOI] [PubMed] [Google Scholar]
- 20.Negulescu O, Bognar I, Lei J, Devarajan P, Silbiger S, Neugarten J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int. 2002;62(6):1989–1998. doi: 10.1046/j.1523-1755.2002.00679.x. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez A, Hermenegildo C, Issekutz AC, Esplugues JV, Sanz MJ. Estrogens inhibit angiotensin II-induced leukocyte-endothelial cell interactions in vivo via rapid endothelial nitric oxide synthase and cyclooxygenase activation. Circ Res. 2002;91(12):1142–1150. doi: 10.1161/01.res.0000046018.23605.3e. [DOI] [PubMed] [Google Scholar]
- 22.Mori M, Tsukahara F, Yoshioka T, Irie K, Ohta H. Suppression by 17beta-estradiol of monocyte adhesion to vascular endothelial cells is mediated by estrogen receptors. Life Sci. 2004;75(5):599–609. doi: 10.1016/j.lfs.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez E, Lopez R, Paez A, Masso F, Montano LF. 17Beta-estradiol inhibits the adhesion of leukocytes in TNF-alpha stimulated human endothelial cells by blocking IL-8 and MCP-1 secretion, but not its transcription. Life Sci. 2002;71(18):2181–2193. doi: 10.1016/s0024-3205(02)01999-9. [DOI] [PubMed] [Google Scholar]
- 24.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166(3):357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 25.Oestreicher EM, Guo C, Seely EW, Kikuchi T, Martinez-Vasquez D, Jonasson L, Yao T, Burr D, Mayoral S, Roubsanthisuk W, Ricchiuti V, Adler GK. Estradiol increases proteinuria and angiotensin II type 1 receptor in kidneys of rats receiving L-NAME and angiotensin II. Kidney Int. 2006;70(10):1759–1768. doi: 10.1038/sj.ki.5001897. [DOI] [PubMed] [Google Scholar]
- 26.Ribstein J, Halimi JM, du Cailar G, Mimran A. Renal characteristics and effect of angiotensin suppression in oral contraceptive users. Hypertension. 1999;33(1):90–95. doi: 10.1161/01.hyp.33.1.90. [DOI] [PubMed] [Google Scholar]
- 27.Boyd WN, Burden RP, Aber GM. Intrarenal vascular changes in patients receiving oestrogen-containing compounds--a clinical, histological and angiographic study. Q J Med. 1975;44(175):415–431. [PubMed] [Google Scholar]
- 28.Parati G, Di Rienzo M, Mancia G. Dynamic modulation of baroreflex sensitivity in health and disease. Ann N Y Acad Sci. 2001;940:469–487. doi: 10.1111/j.1749-6632.2001.tb03699.x. [DOI] [PubMed] [Google Scholar]
- 29.Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol. 2000;529(Pt 1):263–271. doi: 10.1111/j.1469-7793.2000.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minami N, Mori N, Nagasaka M, Ito O, Ogawa M, Kurosawa H, Kanazawa M, Kohzuki M. Exercise training fails to modify arterial baroreflex sensitivity in ovariectomized female rats. Tohoku J Exp Med. 2007;211(4):339–345. doi: 10.1620/tjem.211.339. [DOI] [PubMed] [Google Scholar]
- 31.Lavi S, Nevo O, Thaler I, Rosenfeld R, Dayan L, Hirshoren N, Gepstein L, Jacob G. Effect of aging on the cardiovascular regulatory systems in healthy women. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R788–793. doi: 10.1152/ajpregu.00352.2006. [DOI] [PubMed] [Google Scholar]
- 32.Huikuri HV, Pikkujamsa SM, Airaksinen KE, Ikaheimo MJ, Rantala AO, Kauma H, Lilja M, Kesaniemi YA. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation. 1996;94(2):122–125. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 33.el-Mas MM, Abdel-Rahman AA. Estrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Can J Physiol Pharmacol. 1998;76(4):381–386. [PubMed] [Google Scholar]
- 34.Oelkers W, Foidart JM, Dombrovicz N, Welter A, Heithecker R. Effects of a new oral contraceptive containing an an-timineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clin Endocrinol Metab. 1995;80(6):1816–1821. doi: 10.1210/jcem.80.6.7775629. [DOI] [PubMed] [Google Scholar]
- 35.Riegger GA, Bouzo H, Petr P, Munz J, Spacek R, Pethig H, von Behren V, George M, Arens H. Improvement in exercise tolerance and symptoms of congestive heart failure during treatment with candesartan cilexetil. Symptom, tolerability, response to exercise trial of candesartan cilexetil in heart failure (STRETCH) investigators. Circulation. 1999;100(22):2224–2230. doi: 10.1161/01.cir.100.22.2224. [DOI] [PubMed] [Google Scholar]
- 36.van Kesteren PJ, Kooistra T, Lansink M, van Kamp GJ, Asscheman H, Gooren LJ, Emeis JJ, Vischer UM, Stehouwer CD. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost. 1998;79(5):1029–1033. [PubMed] [Google Scholar]
- 37.Montezano AC, Callera GE, Mota AL, Fortes ZB, Nigro D, Carvalho MH, Zorn TM, Tostes RC. Endothelin-1 contributes to the sexual differences in renal damage in DOCA-salt rats. Peptides. 2005;26(8):1454–1462. doi: 10.1016/j.peptides.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Muller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G, Tulassay T, Szabo AJ. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 2002;62(4):1364–1371. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 39.Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, Hamar P, Amann K, Ritz E. Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRsp Rats. J Am Soc Nephrol. 2004;15(2):348–358. doi: 10.1097/01.asn.0000105993.63023.d8. [DOI] [PubMed] [Google Scholar]
- 40.Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R229–233. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- 41.Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mRNA during chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1388–1392. doi: 10.1152/ajpregu.2001.280.5.R1388. [DOI] [PubMed] [Google Scholar]
- 42.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48(5):787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 43.Quinkler M, Bumke-Vogt C, Meyer B, Bahr V, Oelkers W, Diederich S. The human kidney is a progesterone-metabolizing and androgen-producing organ. J Clin Endocrinol Metab. 2003;88(6):2803–2809. doi: 10.1210/jc.2002-021970. [DOI] [PubMed] [Google Scholar]
- 44.Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, Karl M. Gender-specific effects of endogenous testosterone: female alpha-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007;72(4):464–472. doi: 10.1038/sj.ki.5002328. [DOI] [PubMed] [Google Scholar]
- 45.Baltatu O, Cayla C, Iliescu R, Andreev D, Bader M. Abolition of end-organ damage by antiandrogen treatment in female hypertensive transgenic rats. Hypertension. 2003;41(3 Pt 2):830–833. doi: 10.1161/01.HYP.0000048702.55183.89. [DOI] [PubMed] [Google Scholar]
- 46.Fortepiani LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckel-hoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension. 2003;41(3 Pt 2):640–645. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- 47.Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 48.Heinlein CA, Chang C. The roles of androgen receptors androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16(10):2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 49.Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25(3):139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- 50.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19(20):5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–730. [PubMed] [Google Scholar]
- 52.Guo Z, Benten WP, Krucken J, Wunderlich F. Nongenomic testosterone calcium signaling. Genotropic actions in androgen receptor-free macrophages. J Biol Chem. 2002;277(33):29600–29607. doi: 10.1074/jbc.M202997200. [DOI] [PubMed] [Google Scholar]
- 53.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J Biol Chem. 2003;278(44):42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94(6):1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 55.Sun YH, Gao X, Tang YJ, Xu CL, Wang LH. Androgens induce increases in intracellular calcium via a G protein-coupled receptor in LNCaP prostate cancer cells. J Androl. 2006;27(5):671–678. doi: 10.2164/jandrol.106.000554. [DOI] [PubMed] [Google Scholar]
- 56.Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, Rosendaal FR, Lemaitre RN, Smith NL, Wahl PW, Wagner EH, Furberg CD. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274(8):620–625. [PubMed] [Google Scholar]
- 57.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92(5):1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 58.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96(2):793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]