Abstract
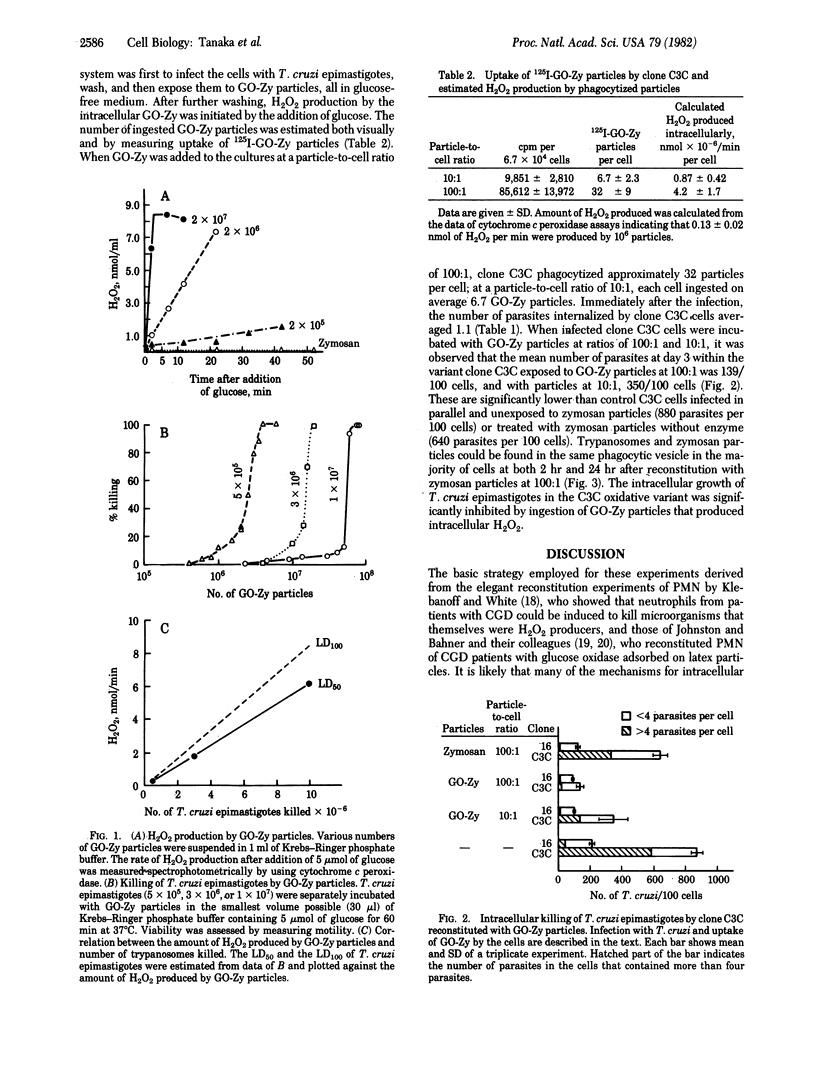
A variant clone, C3C, derived from the cloned macrophage cell line J774.16 lacks the capacity to produce O2- or H2O2 after appropriate stimulation. When the parental and variant cell lines were infected with epimastigotes of Trypanosoma cruzi, the parasites were killed or their growth was inhibited by the parental line, but they grew readily in the variant clone C3C. It was possible to reconstitute the variant cell line with an enzyme system targeted to the lysosomal compartment capable of generating a single oxygen metabolite, H2O2. This was accomplished by allowing the cells to phagocytize zymosan particles covalently coupled with glucose oxidase (GO-Zy particles). Approximately one-third of the H2O2 theoretically expected to be produced by the ingested GO-Zy particles could be detected outside the cells by the cytochrome c peroxidase assay; this fraction may represent the efficiency of extracellular assays for H2O2 production. When T. cruzi-infected clone C3C cells were reconstituted with GO-Zy particles, upon addition of glucose, intracellular killing of the parasites occurred. It was possible to estimate the level of H2O2 production required to kill a single parasite (8.7 x 10(-7) nmol/min) by GO-Zy particles in suspension and to formulate a first approximation of the killing potency of the reconstituted cells--i.e., number of parasites expected to be killed--that correlated well with the observed growth of the parasites intracellularly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Diamond B., Muschel R., Rosen N., Schneck J., Damiani G., Rosen O., Scharff M. Genetic approaches to the mechanism of macrophage functions. Fed Proc. 1978 Nov;37(13):2765–2771. [PubMed] [Google Scholar]
- Cheson B. D., Curnette J. T., Babior B. M. The oxidative killing mechanisms of the neutrophil. Prog Clin Immunol. 1977;3:1–65. [PubMed] [Google Scholar]
- Damiani G., Kiyotaki C., Soeller W., Sasada M., Peisach J., Bloom B. R. Macrophage variants in oxygen metabolism. J Exp Med. 1980 Oct 1;152(4):808–822. doi: 10.1084/jem.152.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff R. Killing in vitro of Trypanosoma cruzi by macrophages from mice immunized with T. cruzi or BCG, and absence of cross-immunity on challege in vivo. J Exp Med. 1975 Aug 1;142(2):299–311. doi: 10.1084/jem.142.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Baehner R. L. Improvement of leukocyte bactericidal activity in chronic granulomatous disease. Blood. 1970 Mar;35(3):350–355. [PubMed] [Google Scholar]
- Johnston R. B., Jr Oxygen metabolism and the microbicidal activity of macrophages. Fed Proc. 1978 Nov;37(13):2759–2764. [PubMed] [Google Scholar]
- Karnovsky M. L. Chronic granulomatous disease--pieces of a cellular and molecular puzzle. Fed Proc. 1973 Apr;32(4):1527–1533. [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- Kress Y., Bloom B. R., Wittner M., Rowen A., Tanowitz H. Resistance of Trypanosoma cruzi to killing by macrophages. Nature. 1975 Oct 2;257(5525):394–396. doi: 10.1038/257394a0. [DOI] [PubMed] [Google Scholar]
- Kress Y., Tanowitz H., Bloom B., Wittner M. Trypanosoma cruzi: infection of normal and activated mouse macrophages. Exp Parasitol. 1977 Apr;41(2):385–396. doi: 10.1016/0014-4894(77)90110-2. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G. Pathology of bactericidal power of neutrophils. Semin Hematol. 1975 Apr;12(2):143–160. [PubMed] [Google Scholar]
- Roos D., Weening R. S. Defects in the oxidative killing of microorganisms by phagocytic leukocytes. Ciba Found Symp. 1978 Jun 6;(65):225–262. [PubMed] [Google Scholar]