Abstract
Characterization of the matrix metalloproteinase-2 (MMP-2) substrates and understanding of its function remain difficult because up to date preparations containing minor amounts of other eukaryotic proteins that are co-purified with MMP-2 are still used. In this work, the expression of a soluble and functional full-length recombinant human MMP-2 (rhMMP-2) in the cytoplasm of Escherichia coli is reported, and the purification of this metalloproteinase is described. Culture of this bacterium at 18 °C culminated in maintenance of the soluble and functional rhMMP-2 in the soluble fraction of the E. coli lysate and its purification by affinity with gelatin-sepharose yielded approximately 0.12 mg/L of medium. Western Blotting and zymographic analysis revealed that the most abundant form was the 72-kDa MMP-2, but some gelatinolytic bands corresponding to proteins with lower molecular weight were also detected. The obtained rhMMP-2 was demonstrated to be functional in a gelatinolytic fluorimetric assay, suggesting that the purified rhMMP-2 was correctly folded. The method described here involves fewer steps, is less expensive, and is less prone to contamination with other proteinases and MMP inhibitors as compared to expression of rhMMP-2 in eukaryotic tissue culture. This protocol will facilitate the use of the full-length rhMMP-2 expressed in bacteria and will certainly help researchers to acquire new knowledge about the substrates and biological activities of this important proteinase.
Keywords: Matrix metalloproteinase-2, Gelatinase A, Bacteria, Protein expression, Escherichia coli
1. Introduction
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases commonly known for their ability to cleave components of the connective tissue in physiological and pathological processes (Visse and Nagase, 2003). MMP-2, also known as gelatinase A/type IV collagenase, is secreted as a zymogen of 72 kDa and becomes activated after removal of the propeptide (Ra and Parks, 2007). This proteinase has the capacity to degrade several extracellular matrix components such as type IV collagens as well as all nonfibrillar collagens, laminin, fibronectin, casein, elastin, and even fibrillar collagens (Back et al., 2010; Kwan et al., 2004; Page-McCaw et al., 2007). In recent years, it has been shown that MMP-2 can also cleave many non-matrix endogenous molecules, playing an important role in different biological processes such as cell-adhesion (Page-McCaw et al., 2007), platelet aggregation (Sawicki et al., 1997), and vascular tone control by cleavage of big endothelin-1 (Fernandez-Patron et al., 1999) and the calcitonin gene-related peptide (Ito et al., 1998). Furthermore, MMP-2 has been detected inside cardiac myocytes, where it has been described to cleave troponin I (Gao et al., 2003), poly-(ADP-ribose) polymerase (Kwan et al., 2004), and titin (Ali et al., 2010). So far, a large spectrum of substrates has been attributed to MMP-2, and an ever growing number of functional roles has been ascribed to it (Nagase et al., 2006).
In order to study the substrate specificity of MMP-2, infer its role in physiological or pathological processes, and characterize it from a crystallographic viewpoint, it is absolutely necessary to have a highly purified and functional full-length (72 kDa) MMP-2, which is not easy to obtain. Classically, MMP-2 has been purified from human plasma (Steffensen et al., 2011) or from the conditioned medium of eukaryotic culture supernatants (Morrison et al., 2001) by affinity chromatography. However, since in most of these systems MMP-2 forms a tight, noncovalent complex with the tissue inhibitor of metalloproteinase-2 (TIMP-2), it is many times co-purified with the latter molecule (Chen et al., 1991; Itoh et al., 1995). Small amounts of other human proteinases, which co-purify (Morodomi et al., 1992) with MMP-2 are also an obstacle to substrate investigation assays when human MMP-2 purified by affinity chromatography is employed. Some other molecules such as fibronectin also co-purify with MMP-2 on gelatin columns (Steffensen et al., 2011). All these contaminant proteins could lead to erroneous results in kinetic assays or mass spectrometry analyses of enzyme fragments, especially when the aim is to identify the specific cleavage of MMP-2 substrates. Due to these limitations, the production of MMP-2 in bacteria is of great interest and utility, since in this case this metalloprotein is free from other eukaryotic proteins.
Escherichia coli strains have been most often used for the production of recombinant proteins that do not require post-translational modifications for bioactivity.
MMP-2 has at least two types of well characterized post-translational modifications, namely disulfide bridges (Visse and Nagase, 2003) and phosphorylation (Sariahmetoglu et al., 2007). Therefore, bacterial expression of this MMP is challenging, since in this case there are several limitations regarding post-translational modifications that are made by eukaryotic cells. Genetic engineering has enabled expression of heterologous proteins with appropriate post-translational modifications in bacteria, which still remains as the most powerful and versatile system for protein expression (Baneyx and Mujacic, 2004). According to a recent review (Windsor and Steele, 2010) “numerous MMPs have been successfully expressed and purified from E. coli. These include stromelysin-1, stromelysin-2, stromelysin-3, matrilysin, elastase, collagenase-1, collagenase-3, neutrophil collagenase, and membrane type-1 MMP”. However, to our knowledge, full-length MMP-2 has not yet been expressed and purified from E. coli in a standard and reproducible manner.
Banyai et al. (1994) have expressed the three modules of the fibronectin type II of MMP-2 (FN-II) domain in E. coli. The recombinant proteins were isolated from inclusion bodies and refolded, and were then used to test the binding affinity of these domains to gelatin. Steffensen et al. (1995) have also expressed a 21-kDa fragment of MMP-2 consisting of three tandem fibronectin type II-like modules in E. coli and have purified it from inclusion bodies.
Ye et al. (1995) have expressed the catalytic domain of MMP-2 in E. coli, and the 19-kDa protein has been purified after in vitro refolding. Morrison et al. (2001) have studied the activation of MMP-2 involving membrane type-2 MMP and TIMP-2. While the MMP-2 hemopexin C domain (22 kDa) was expressed in E. coli, the hemopexin C domain deletion mutant of human pro-MMP-2 as well as the recombinant membrane type-2 MMP catalytic domain and recombinant TIMP-2 were expressed in Chinese hamster ovary cells.
Cheng et al. (2003) have expressed and purified the catalytic domain of human gelatinase A with and without the fibronectin-like insert in the E. coli strain BL21(DE3)/pLysS. Both proteins were functional after refolding from inclusion bodies and had molecular masses of 38 and 20 kDa, respectively. These authors showed that the absence of the fibronectin-like domain in MMP-2 resulted in decreased digestion rates for the tested substrates.
Each of the three fibronectin type II modules found in the catalytic domain of MMP-2 displays two disulfide bonds, totalling six disulfide bonds in this domain (Visse and Nagase, 2003). When disulfide bonds are not formed properly, proteins may become mis-folded, giving rise to accumulation of insoluble protein aggregates known as inclusion bodies (Burgess, 2009). Proteins are normally purified from such inclusion bodies under denaturing conditions, followed by refolding steps. However, the refolding process may not afford the same protein structure found in mammalian cells.
Interestingly, Peisley and Gooley (2007) have reported on a method for the expression of the isolated second type II fibronectin module from MMP-2 (FNII-2) with approximately 5 kDa fused with a polyhistidine-tag in E. coli. The thioredoxin reductase-deficient E. coli strain BL21trxB(DE3) was employed in this case, which allowed for formation of a disulfide bond. In this way, the correctly folded FNII-2 was obtained from the bacterial cytoplasmic lysate.
In this work a method has been tested in order to find out whether adequate amounts of functional, full-length MMP-2 can be achieved from the cytoplasmic lysate of bacteria in a reproducible way, aiming at MMP-2 purification under mild conditions.
2. Materials and methods
2.1. Subcloning of recombinant human MMP-2 (rhMMP-2)
The plasmid containing the cDNA encoding the sequence of the 72-kDa human gelatinase A (pGelA pBSSK−) was prepared as described previously (Fridman et al., 1992). After restriction digestion using EcoRI and SspI, the 3.1-kb fragment containing the DNA encoding MMP-2 was subcloned into the expression vector pET5a, which furnished the plasmid pET5a-MMP-2 (7.2 kb). The plasmid and the fragment were ligated with T4 DNA ligase. Ligation reactions were transformed into DH5-α cells, which were made competent by means of the CaCl2 method (Sambrook and Russell, 2001). The plasmid DNA was isolated using a Perfect Prep Plasmid Minikit (Eppendorf). The correct sequence was confirmed via DNA sequencing at the DNA Sequencing Facility of Departamento de Biologia Celular e Molecular e Bioagentes Patogênicos, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil.
2.2. Protein expression
The pET5a-MMP-2 DNA was transformed into CaCl2-competent BL21(DE3)/pLysS using standard procedures (Sambrook et al., 1989). Single transformed colonies were picked with a sterile loop and streaked onto Luria-Bertani (LB) agar plates containing 100 μg/mL ampicillin and 34 μg/mL chlorolamphenicol. Isolated colonies were collected from the streaked plates and used to inoculate 5 mL LB medium containing the same concentration of antibiotics described above as well as 20% glucose. This procedure was undertaken at 37 °C and under shaking at 180 rpm overnight (this media will be termed starter culture hereafter). Five hundred microliters of the starter culture were employed for inoculation of 50 mL LB medium with the appropriate antibiotics, and the culture was incubated at 37 °C and submitted to shaking at 180 rpm for 2.5–3 h, until an OD600 of 0.5–0.7 was reached. After clones with higher protein expression had been selected, the expression levels were compared using different concentrations of isopropyl β-thiogalactopyranoside (IPTG) ranging from 0.05 to 1 mM as well as different induction times. In order to determine the best time to collect cells after induction, aliquots of the culture were harvested at 0, 2, 4, 8, 12, and 24 h. Cells were pelleted by centrifugation at 6000 × g for 10 min and stored at −80 °C until use. 1 mL of the supernatant was also stored and checked for the presence of MMP-2. No MMP-2 was found in the medium. Cells were subjected to six cycles of 15-s sonication, and an interval of 1 min was allowed between the cycles, with the cells placed on ice. The cell lysate was clarified by centrifugation at 10,000 × g for 10 min at 4 °C, to test whether 72-kDa MMP-2 was present in the supernatant (soluble in the bacterium cytoplasm) or in the pellet (most likely in inclusion bodies).
To assess the approximate amount, molecular weight, and activity of recombinant MMP-2, samples were assayed in gelatinzymograms and were also evaluated by means of fluorimetric gelatinase activity assay.
2.3. Sample preparation (sonication and centrifugation)
Cell pellets were thawed on ice and resuspended in 3 mL buffer containing protease inhibitors (50 mM Tris–HCl, pH 7.6, 0.15 M NaCl, 10 mM CaCl2, 0.005% Brij 35, 0.02% NaN3, 2 mM 1,10 ortho-phenanthroline, 2 mM phenylmethanesulfonylfluoride and 2 mM N-ethylmaleimide), termed TNCi. Lysozyme was added at a final concentration of 0.1 mg/mL, and the mixture was incubated at 4 °C for 30 min. 1,10 ortho-phenanthroline was omitted from the buffer used to prepare supernatants intended for fluorimetry. Cell suspension was then subjected to sonication and centrifugation as described above. Pellets and supernatants were harvested for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), gelatin zymography, and fluorimetric gelatinase activity assay.
2.4. Fluorimetric gelatinase assay
The gelatinolytic activity was measured at λex 495, λem 515 nm on a microplate spectrofluorometer (Gemini EM, Molecular Devices, Sunnyvale, CA, USA), using a gelatinase/collagenase activity kit (E12055, Molecular Probes, Oregon, USA). To this end, samples containing the protein at a concentration of 5 μg/mL per well were employed for determination by the Bradford method. Bovine serum albumin was utilized as protein standard (Sigma). Endpoint fluorescence was read after 120 min, and the results were compared to those of a standard curve prepared as recommended by the manufacturer (Castro et al., 2008).
2.5. SDS-PAGE and gelatin zymography of MMP-2
For the zymography experiments, samples were diluted in sample buffer (4% SDS, 125 mM Tris–HCl; pH 6.8, 20% glycerol, and 0.001% bromophenol blue) and submitted to electrophoresis on a 12% SDS-PAGE gel or 12% SDS-PAGE co-polymerized with gelatin (0.1%) as the substrate (Castro et al., 2010). SDS-PAGE gels were stained with Coomassie Brilliant Blue G-250. Prior to staining, zymograms had been washed for 1 h at room temperature in 2 baths of a 2% Triton X-100 solution and then incubated at 37 °C for 16 h in Tris–HCl 50 mM, 10 mM CaCl2, pH 7.4. Gelatinolytic activities were detected as unstained bands against the background of the Coomassie blue-stained gelatin. A prestained protein ladder (PageRuler Plus Prestained Protein Ladder, Fermentas) was run in all the SDS-PAGE gels. Immunoblots were prepared by transferring the proteins from the SDS-PAGE gels to nitrocellulose membranes (Hybond ECL, Amersham Biosciences, Germany). These membranes were blocked in low-fat powder milk, followed by incubation of the membrane with the primary antibody at 1:1000 concentration (MAB3308, Chemicon, Temecula, CA, USA) and secondary antibody conjugated to horseradish peroxidase (AP160P, Chemicon, Temecula, CA, USA) at 1:2500 concentration. Membranes were developed using a chemiluminescent substrate kit (Immobilon Western, Millipore Corp., Billerica, MA, USA). Images were captured using the MF-ChemiBIS 1.6 system (DNR Bio-Imaging Systems, Jerusalem, Israel).
2.6. Purification of recombinant human MMP-2 (rhMMP-2)
After determination of the best time for MMP-2 expression (18 h at 18 °C under shaking at 180 rpm), a larger volume of culture (3 L) was expressed. The cells were then harvested by centrifugation and resuspended in 30 mL buffer. Sonication and centrifugation steps were performed as described above. The supernatant was loaded on a Gelatin Sepharose™ 4B (Amersham Biosciences) column pre-equilibrated with buffer. The column was washed with 2 volumes of buffer, and absorbed proteins were then eluted with the same buffer containing 5% DMSO (v/v). This method was based on the purification procedures described by Shimokawa and Nagase (2001). The fractions were concentrated in Amicon Ultra tubes (cutoff 50 kDa), and this procedure was also used to wash the DMSO off, via addition of ultrapure water. The fraction containing rhMMP-2 was lyophilized for 16 h and stored at −80 °C for further analysis. Prior to use in the different assays such as reverse zymography, aliquots were tested for activity and molecular mass as described above.
3. Results
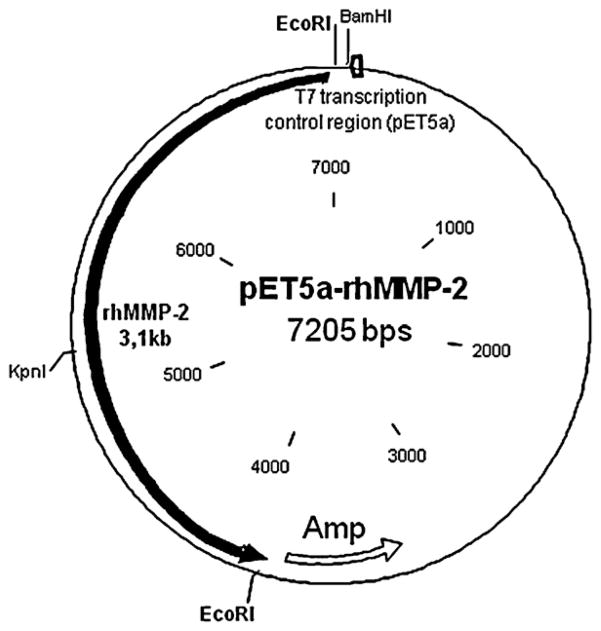
The expression vector that resulted from insertion of the MMP-2 cDNA into the pET vector (pET5a-MMP-2, with 7.2 kb) is shown in Fig. 1.
Fig. 1.
Expression vector map showing the major restriction enzyme cleavage sites, transcription control region, and antibiotic resistance information. After subcloning the resulting plasmid had 7.205 bps.
After clones with higher expression were selected, the expression levels were compared by using different concentrations of IPTG and different induction times. The best expression was induced by 100 μM IPTG (expression was considered high when high levels of gelatinolytic activity were detected by means of the fluorimetric gelatinase activity assay). After induction, incubation of cells at 18 °C rendered larger amounts of functional MMP-2, and this temperature was therefore used after induction, with shaking at 180 rpm.
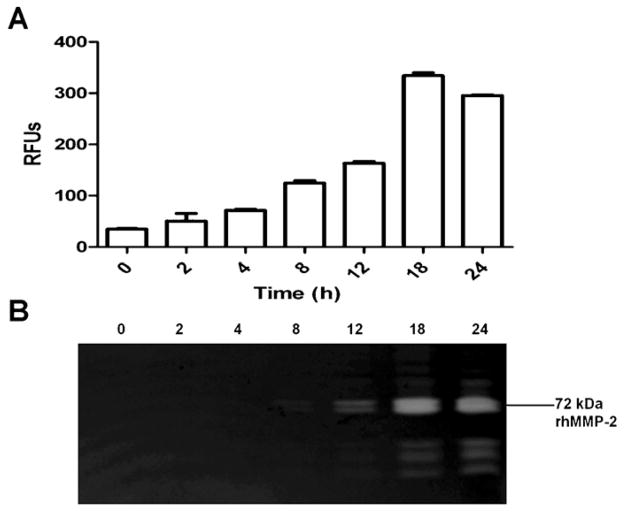
Zymographic and fluorimetric assays revealed the presence of the functional full-length (~72 kDa) MMP-2 in the supernatant of the bacterial cells after sonication. After IPTG induction, no MMP-2 was found in the medium or in the cell pellets employed during these assays (data not shown). No MMP-2 was found in uninduced cells (time 0, Fig. 2A and B). Some insoluble, non-functional MMP-2 was found in the cell pellets (data not shown), but this fraction was not further studied.
Fig. 2.
Expression of rhMMP-2 in BL21(DE3)/pLysS. Cytoplasmic supernatants were collected after different times of induction by IPTG. The rhMMP-2 activity was detected in the soluble fraction in neutral pH buffer after sonication of the bacterium pellet. Gelatinolytic activity was detected by a fluorimetric assay and is expressed as mean and S.D. (A) and in a gelatin zymogram (B). Recombinant protein expression was induced with 100 μM IPTG, and bacteria were harvested after different induction times, as indicated. Bacteria were grown at 18 °C and 180 rpm. RFUs: relative fluorescence units.
Fig. 2 displays the results of the experiment about induction time; only the supernatants were tested. The highest MMP-2 activity was observed after 18 and 24 h of induction in solution (Fig. 2A) and gel (Fig. 2B), respectively. The two techniques furnished the same results. These assays were carried out with different starting samples, at three different times. The zymographic analysis revealed a doublet around 72 kDa, compatible with the full-length MMP-2, as well as another lower molecular weight form. This doublet was by far the most prominent band observed in the zymogram. Other lower molecular forms observed are probably degradation products.
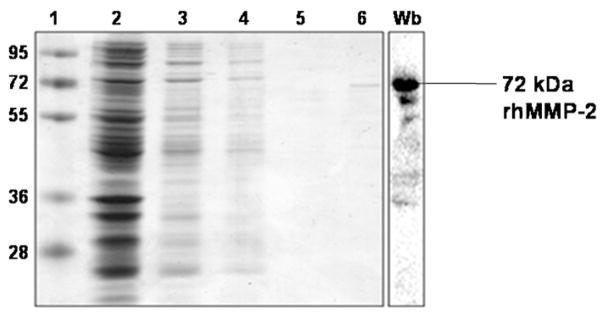
The rhMMP-2 doublet was not visible to the naked eye by ordinary SDS-PAGE before its chromatographic purification. This is indication that it was not overexpressed as compared to other bacterial proteins. After chromatography, one single 72-kDa MMP-2 form was observed by Coomassie Blue staining (Fig. 3; lane 6). Western blotting further confirmed the identity of MMP-2, and that the full-length 72 kDa was the major molecular form that was purified. This technique also revealed the presence of MMP-2 degradation products whose molecular weight did not coincide much with the lower molecular weight bands observed by zymography.
Fig. 3.
Gelatin affinity purification of rhMMP-2 produced from BL21(DE3)/pLysS. The rhMMP-2 was purified on a gelatin-sepharose column as described in Section 2, samples from each step were analyzed in a 12% SDS-PAGE gel, and proteins were stained with Coomassie Blue. Lane 1, molecular weight markers (in kDa); lane 2, BL21(DE3)/pLysS whole pellet; lane 3, soluble fraction after cells were sonicated; lane 4, unbound fraction; lane 5, wash fraction; lane 6, relatively pure rhMMP-2 (72 kDa) eluted with 5% DMSO. Western blotting (Wb) confirmed the identity of the rhMMP-2 and revealed the presence of some possible degradation products of the full-length protein.
After lyophilization, a final yield of approximately 0.12 mg rhMMP-2/L of LB medium was obtained.
4. Discussion
This study provides evidence that it is feasible to express the full-length rhMMP-2 in bacteria and purify high amounts of this protease to homogeneity under mild conditions. The final purified MMP-2 product is mostly constituted by the 72-kDa MMP-2 form, which displayed functional activity in different assays.
The use of genetically modified bacteria described herein was essential for attainment of the results. Different bacterial strains were tested, and the best results regarding MMP-2 activity were obtained when BL21(DE3)/pLysS was transformed. This strain is deficient in 2 proteases, namely lon and omp-t (Terpe, 2006), and is therefore suitable for expression of proteins that are toxic to bacteria, which is probably the case of MMP-2. BL21(DE3)/pLysS had been previously employed by Cheng et al. (2003), and following high expression levels, the catalytic domain of MMP-2 had been refolded. The aim of the present study was not to achieve MMP-2 overexpression, since high expression levels in bacteria sometimes lead to formation of inclusion bodies, which has already been described in earlier studies. The low level expression seems to have created adequate conditions for MMP-2 to remain soluble in the bacterium cytoplasm. The rhMMP-2 produced in this way conserved its full length up to the end of the purification process (using protease inhibitors and low temperature throughout the study), and displayed activity in two activity assays. The possibility to purify MMP-2 under mild conditions in a gelatin-sepharose has many advantages over the need of refolding it after its recovery from inclusion bodies. Firstly, the chromatographic steps are easier and faster as compared to the refolding process. Secondly, the ability of MMP-2 to bind to gelatin culminates in purification of functional MMP-2 molecules, since only MMP-2 molecules with adequately folded FN-II domains can bind to gelatin columns. Moreover, in this purification process there is no need for employing a tag that would later have to be removed by proteinases, or that could adversely affect folding of the recombinant protein.
In summary, the method described here involves fewer steps and reduced inducing time, is less expensive, and is less prone to contamination with other proteinases and MMP inhibitors as compared to expression of rhMMP-2 in eukaryotic tissue cultures. This protocol will simplify the preparation of full-length rhMMP-2 from bacteria and will facilitate studies on the gamut of biological actions of this important proteinase.
Acknowledgments
This study was funded by the State of Sao Paulo Research Foundation (Fundação de Amparo a Pesquisa do Estado de Sao Paulo, FAPESP). We thank Dr. Jose Moacyr Marin for allocation of equipment and technical discussions.
Abbreviations
- MMPs
matrix metalloproteinases
- TIMP
tissue inhibitor of met-alloproteinase
- FN-II
fibronectin type II
References
- Ali MA, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R. Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion njury. Circulation. 2010;122:2039–2047. doi: 10.1161/CIRCULATIONAHA.109.930222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 2010;52:410–428. doi: 10.1016/j.pcad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22:1399–1408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- Banyai L, Tordai H, Patthy L. The gelatin-binding site of human 72 kDa type IV collagenase (gelatinase A) Biochem J. 1994;298 (Pt 2):403–407. doi: 10.1042/bj2980403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RR. Refolding solubilized inclusion body proteins. Methods Enzymol. 2009;463:259–282. doi: 10.1016/S0076-6879(09)63017-2. [DOI] [PubMed] [Google Scholar]
- Castro MM, Rizzi E, Figueiredo-Lopes L, Fernandes K, Bendhack LM, Pitol DL, Gerlach RF, Tanus-Santos JE. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198:320–331. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus-Santos JE, Gerlach RF. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010;29:194–201. doi: 10.1016/j.matbio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Chen JM, Aimes RT, Ward GR, Youngleib GL, Quigley JP. Isolation and characterization of a 70-kDa metalloprotease (gelatinase) that is elevated in Rous sarcoma virus-transformed chicken embryo fibroblasts. J Biol Chem. 1991;266:5113–5121. [PubMed] [Google Scholar]
- Cheng D, Shen Q, Nan F, Qian Z, Ye QZ. Purification and characterization of catalytic domains of gelatinase A with or without fibronectin insert for high-throughput inhibitor screening. Protein Expr Purif. 2003;27:63–74. doi: 10.1016/s1046-5928(02)00530-2. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- Fridman R, Fuerst TR, Bird RE, Hoyhtya M, Oelkuct M, Kraus S, Komarek D, Liotta LA, Berman ML, Stetler-Stevenson WG. Domain structure of human 72-kDa gelatinase/type IV collagenase. Characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J Biol Chem. 1992;267:15398–15405. [PubMed] [Google Scholar]
- Gao CQ, Sawicki G, Suarez-Pinzon WL, Csont T, Wozniak M, Ferdinandy P, Schulz R. Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res. 2003;57:426–433. doi: 10.1016/s0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- Ito A, Yamada M, Sato T, Sanekata K, Sato H, Seiki M, Nagase H, Mori Y. Calmodulin antagonists increase the expression of membrane-type-1 matrix metalloproteinase in human uterine cervical fibroblasts. Eur J Biochem. 1998;251:353–358. doi: 10.1046/j.1432-1327.1998.2510353.x. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Binner S, Nagase H. Steps involved in activation of the complex of pro-matrix metalloproteinase 2 (progelatinase A) and tissue inhibitor of metal-loproteinases (TIMP)-2 by 4-aminophenylmercuric acetate. Biochem J. 1995;308 (Pt 2):645–651. doi: 10.1042/bj3080645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18:690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- Morodomi T, Ogata Y, Sasaguri Y, Morimatsu M, Nagase H. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochem J. 1992;285 (Pt 2):603–611. doi: 10.1042/bj2850603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CJ, Butler GS, Bigg HF, Roberts CR, Soloway PD, Overall CM. Cellular activation of MMP-2 (gelatinase A) by MT2-MMP occurs via a TIMP-2-independent pathway. J Biol Chem. 2001;276:47402–47410. doi: 10.1074/jbc.M108643200. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley AA, Gooley PR. High-level expression of a soluble and functional fibronectin type II domain from MMP-2 in the Escherichia coli cytoplasm for solution NMR studies. Protein Expr Purif. 2007;53:124–131. doi: 10.1016/j.pep.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor Laboratory Press; New York: 1989. [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual, third ed. Cold Spring Harbor Laboratory Press; New York: 2001. [Google Scholar]
- Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, Holmes C, Berthiaume LG, Holt A, Sawicki G, Schulz R. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW. Release of gelatinase A during platelet activation mediates aggregation. Nature. 1997;386:616–619. doi: 10.1038/386616a0. [DOI] [PubMed] [Google Scholar]
- Shimokawa K, Nagase H. Purification of MMPs and TIMPs. In: Clark I, editor. Methods in Molecular Biology – Matrix Metalloproteinase Protocols. Humana Press Inc; Totowa: 2001. pp. 275–304. [DOI] [PubMed] [Google Scholar]
- Steffensen B, Chen Z, Pal S, Mikhailova M, Su J, Wang Y, Xu X. Fragmentation of fibronectin by inherent autolytic and matrix metalloproteinase activities. Matrix Biol. 2011;30:34–42. doi: 10.1016/j.matbio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen B, Wallon UM, Overall CM. Extracellular matrix binding properties of recombinant fibronectin type II-like modules of human 72-kDa gelatinase/type IV collagenase high affinity binding to native type I collagen but not native type IV collagen. J Biol Chem. 1995;270:11555–11566. doi: 10.1074/jbc.270.19.11555. [DOI] [PubMed] [Google Scholar]
- Terpe K. Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2006;72:211–222. doi: 10.1007/s00253-006-0465-8. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Windsor LJ, Steele DL. Expression of recombinant matrix metalloproteinases in Escherichia coli. Methods Mol Biol. 2010;622:67–81. doi: 10.1007/978-1-60327-299-5_4. [DOI] [PubMed] [Google Scholar]
- Ye QZ, Johnson LL, Yu AE, Hupe D. Reconstructed 19 kDa catalytic domain of gelatinase A is an active proteinase. Biochemistry. 1995;34:4702–4708. doi: 10.1021/bi00014a026. [DOI] [PubMed] [Google Scholar]