Abstract
The phenomenon of epithelial–mesenchymal transition (EMT) has gained attention in the field of cancer biology for its potential contribution to the progression of carcinomas. Tumor EMT is a phenotypic switch that promotes the acquisition of a fibroblastoid-like morphology by epithelial tumor cells, resulting in enhanced tumor cell motility and invasiveness, increased metastatic propensity and resistance to chemotherapy, radiation and certain small-molecule-targeted therapies. Tumor cells undergoing EMT are also known to increase the secretion of specific factors, including cytokines, chemokines and growth factors, which could play an important role in tumor progression. This review summarizes the current knowledge on the secretory properties of epithelial tumor cells that have undergone an EMT, with an emphasis on the potential role of the IL-8–IL-8 receptor axis on the induction and/or maintenance of tumor EMT and its ability to remodel the tumor microenvironment.
Keywords: brachyury, epithelial–mesenchymal transition, IL-8, metastasis, tumor microenvironment
The epithelial–mesenchymal transition (EMT) is a physiological process during embryogenesis that appears to be reinstated in adult tissues undergoing wound healing and tissue regeneration, or under certain pathological conditions such as fibrosis and cancer [1–3]. Tumor EMT involves a phenotypic switch that promotes the acquisition of a fibroblastoid-like morphology by epithelial tumor cells, reduces cell polarity and cell-to-cell contacts, and decreases the expression of epithelial markers, including E-cadherin and cytokeratins. Concomitantly, epithelial tumor cells undergoing EMT gain expression of mesenchymal-associated proteins, such as fibronectin and vimentin, and have enhanced cell motility, invasiveness and metastatic propensity in vivo [4,5]. Tumor EMT has also been shown to contribute to the acquisition of tumor resistance to chemotherapy, radiation [6–8] and certain small-molecule-targeted therapies [9], thus representing a major mechanism contributing to the progression of carcinomas.
Although evidence for the involvement of EMT in the progression of human carcinomas in vivo is limited so far, it has been observed that the loss of epithelial E-cadherin associates with tumor progression and metastasis in various tumor types [10–13]. In breast cancer, for example, reduction of the epithelial markers E-cadherin and cytokeratins, and upregulation of the mesenchymal markers vimentin and N-cadherin, have been shown to positively correlate with an aggressive tumor phenotype and a high rate of metastasis [14]. In recent reports, several groups have also demonstrated that induction of EMT in epithelial tumor cells results in the acquisition of features of tumor-initiating cells, also designated as cancer stem cells (CSCs) [2,15], including the ability to self-renew in vitro and in vivo, and enhanced resistance to cell death induced by various cytotoxic agents. This association between EMT and the acquisition of features similar to CSCs was also shown to take place in vivo. Residual breast tumor cell populations surviving following conventional treatments had an increased number of cells with markers of CSCs (CD44+, CD24− or CD24low) and mesenchymal features [16].
A significant area of research concerning the role of EMT in tumor progression has focused on the elucidation of the signaling events that trigger this phenotypic switch in carcinoma cells. Intrinsic signaling events, such as those seen with oncogenic Ras, have been shown to contribute to EMT in certain types of human carcinomas [4,17]; however, triggering tumor EMT is most often dependent on a variety of external signals provided by the tumor microenvironment, in the form of soluble growth factors, cytokines or components of the extracellular matrix (ECM) [18]. Well-established signals include those initiated by TGF-β, FGF, EGF and HGF, all of which have been shown to promote EMT in various tumor cell models [19,20]. The various cellular components of the tumor stroma, including cancer-associated fibroblasts, endothelial cells and immune cells, are potential sources of soluble factors capable of inducing EMT in neighboring cancer cells. Conversely, cancer cells can themselves produce cytokines, growth factors and other soluble mediators that may reprogram the surrounding stroma to promote tumor growth and dissemination. Furthermore, the soluble factors produced by tumor cells may act to induce and/or maintain EMT in neighboring tumor cells [21,22].
Amid the growing interest in understanding the role of EMT in tumor progression, only a few studies have focused on analyzing the pattern of secreted factors (the secretome) of tumor cells undergoing this phenotypic switch. For example, a proteomic analysis of the secretome of Madin–Darby canine kidney cells demonstrated that epithelial cells undergoing Ras-mediated EMT have the potential to actively remodel the extracellular compartment by reducing the expression of basement membrane constituents (collagen type IV and laminins) and augmenting the secretion of ECM constituents (osteonectin and collagen type I) and the extracellular proteases, including kallikreins and matrix metalloproteinases (MMPs) [23]. The identification of an ‘EMT-associated tumor secretome’ may not only lead to the identification of molecular markers of tumor EMT, but also to the identification of novel targets for interventions against tumor progression. This review summarizes the current knowledge on the secretory properties of mesenchymal-like tumor cells, with an emphasis on the potential role of the IL-8–IL-8 receptor (IL-8R) axis on the induction and/or maintenance of tumor EMT and in the remodeling of the tumor microenvironment.
Autocrine cytokine loops maintain tumor EMT
Several studies now indicate that, once EMT is initiated, the permanence of tumor cells in a mesenchymal status post-EMT is dependent on the existence of autocrine cytokine and/or growth factor loops that were initially responsible for the induction of EMT in the same cells (TABLE 1) [24,25]. TGF-β is a multifunctional cytokine regarded as being a tumor suppressor in normal epithelial cells and during early tumor growth, while acting as a potent tumor-promoting factor in late-stage tumors [26]. In late-stage or metastatic tumors, TGF-β promotes angiogenesis, recruits various cell types to the site of the tumor, including fibroblasts and immune cells, suppresses a functional anti-tumor immune response and induces tumor cell migration, invasion and EMT [27,28]. In multiple tumor cell models, it has also been demonstrated that, once tumor cells have undergone EMT, secretion of TGF-β is markedly upregulated [24,29,30]. Once secreted, TGF-β has the ability to function in an autocrine fashion, contributing to the maintenance of the mesenchymal and stem cell phenotypes of carcinomas that have passed through EMT [24,26]. This has been shown, for example, with Madin–Darby canine kidney cells, which were induced into EMT by the addition of TGF-β. This resulted in the establishment of an autocrine TGF-β signaling loop that was essential for sustaining the expression of the EMT regulator ZEB [25].
Table 1.
Cytokine loops that initiate/maintain tumor epithelial–mesenchymal transition.
| EMT-secreted factors | Function during tumor EMT |
|---|---|
| IL-8 | Induction of EMT |
| Induction of tumor stemness | |
| Maintenance of EMT by an autocrine IL-8 loop | |
| Angiogenesis | |
| Recruitment of TANs | |
|
| |
| IL-6 | Induction of EMT |
| Maintenance of EMT by an autocrine IL-6 loop | |
|
| |
| VEGF | Angiogenesis |
| Induction of EMT in epithelial tumor cells | |
|
| |
| TGF-β | Induction of EMT |
| Maintenance of EMT by an autocrine TGF-β loop | |
| Impairment of antitumor immune responses | |
|
| |
| MMPs | Degradation of ECM |
| Activation of growth factors | |
| Inactivation of protease inhibitors | |
| Induction of EMT | |
ECM: Extracellular matrix; EMT: Epithelial–mesenchymal transition; MMP: Matrix metalloproteinase; TAN: Tumor-associated neutrophil.
Similar results were observed with the secretion of the cytokine IL-6 [31] or VEGF [32] by tumor cells undergoing EMT. IL-6 is a pleiotropic cytokine involved in the differentiation and growth of hematopoietic stem cells, T cells and B cells, as well as the differentiation of other cell types that express IL-6R (CD126) and the coreceptor gp130 (CD130), including endothelial cells, osteoblasts and hepatocytes [33]. IL-6 is a well-known regulator of immune responses and a major contributor to the pathogenesis of various autoimmune and inflammatory diseases [34], and it has also been implicated as a potent growth factor for certain tumor cell types, including breast cancer cells [35]. Recent reports also demonstrate a role for IL-6 in tumor EMT [31]. MCF7 cells overexpressing Twist, for example, have been shown to upregulate the secretion of IL-6 and, at the same time, to activate STAT3, indicating the existence of a positive feedback loop involving autocrine-mediated IL-6 signaling events [31].
Tumor cells undergoing EMT are also known to secrete MMPs, which can degrade the structural components of the ECM to facilitate tumor cell migration, while simultaneously activating or inactivating growth factors or protease inhibitors, respectively [36]. The upregulation of MMPs has been associated with a variety of EMT processes, each case involving a subset of specific MMPs. For example, it has been demonstrated that the transcription factor SNAIL promotes EMT and simultaneously mobilizes the membrane-anchored MMPs, MT1-MMP and MT2-MMP, to allow breast cancer cells to transmigrate basement membrane barriers, initiate angiogenesis and intravasate into the circulation [37]. Interestingly, MMPs can also induce tumor EMT; treatment with MMP-3, for example, was shown to induce an epithelial–mesenchymal switch in mouse mammary epithelial cells [38,39]. Similarly, upregulation of MMP-28 expression in lung carcinoma A549 cells was shown to induce a TGF-β-dependent EMT by activating latent TGF-β complexes and ultimately inducing an EMT [40]. Altogether, these results indicate the importance of autocrine loops in maintaining the mesenchymal features of tumor cells that have undergone EMT.
IL-8 as an inducer of tumor EMT
Brachyury, tumor EMT & tumor EMT-secreted factors
The transcriptional control of EMT is dependent on the expression of a few transcriptional regulators, defined as ‘EMT transcription factors’, which are ultimately responsible for the regulation of the epithelial and mesenchymal genes modulated during the course of an EMT. While these transcription factors are normally involved in the control of EMT that takes place during embryogenesis, they are re-expressed by epithelial tumor cells undergoing this phenotypic switch. Examples of well-characterized EMT transcription factors include the zinc- finger proteins SNAIL, SLUG, ZEB1 and ZEB2 [41–44], and the helix-loop-helix transcription factor TWIST [45,46]. Recently, the authors’ group identified brachyury, a T-box transcription factor involved in the formation of the mesoderm during embryonic development [47], and a novel driver of EMT in human carcinomas [5]. The overexpression of brachyury in epithelial carcinoma cell lines has been shown to induce changes characteristic of an EMT, including increased tumor cell motility and invasiveness [5], while its inhibition in tumor cells having a more mesenchymal phenotype resulted in changes characteristic of a mesenchymal–epithelial transition, with loss of expression of mesenchymal markers, gain of epithelial markers, and concurrent loss of tumor cell motility and invasiveness. In vivo, it has been demonstrated that brachyury is required for the dissemination of lung carcinoma cells, as brachyury-silenced H460 cells showed a diminished ability to form spontaneous lung metastases in nude mice [5]. The potential role of brachyury in human cancer progression was also suggested by the observation that brachyury mRNA expression is predominant among late-stage lung tumor tissues, with a lower expression among stage I lung tumors [5].
Fernando et al. [48] recently demonstrated that the transition of human tumor cells from an epithelial to a mesenchymal-like phenotype, as a result of brachyury overexpression, is associated with the secretion of multiple cytokines, chemokines and growth factors that have previously been reported to promote tumor growth, motility, invasion and vascularization in various types of carcinomas [49–52]. Breast and pancreatic cancer cells were forced into EMT by overexpression of brachyury and culture supernatants evaluated for the expression of multiple cytokines, chemo kines and growth factors. Induction of tumor EMT was shown to enhance the secretion of a subset of inflammatory cytokines, various chemokines and angiogenic factors, which included IL-6 and IL-8, CXCL1, RANTES, OPG, VEGF, angiogenin and PLGF.
IL-8–IL-8R loops involved in EMT & tumor stemness
The chemokine IL-8, initially identified as a neutrophil-activating and chemotactic factor [53], plays multiple roles as a proinflammatory cytokine by mediating the activation and chemotaxis of various immune cell types, and can lead to chronic inflammatory conditions if aberrantly expressed [54]. Two major sources of this chemokine are monocytes and endothelial cells, which secrete IL-8 in response to various stimuli, such as exposure to IL-1 or TNF-α. In addition, other cell types, including fibroblasts, keratinocytes [55] and tumor cells, can secrete IL-8, particularly under stress conditions such as hypoxia or exposure to chemotherapy agents [56,57]. Once secreted, the activity of IL-8 depends on its binding to the IL-8Rs, IL-8R A (CXCR1) and IL-8RB (CXCR2), which are mainly expressed on neutrophils, monocytes, endothelial cells and some cancer cells [57,58]. In the context of a tumor, IL-8 is known to participate in cancer progression by promoting the angiogenic response of endothelial cells, the recruitment of neutrophils to the site of the tumor, and the proliferation, survival and migration of tumor cells [59,60]. It has been demonstrated that many types of human carcinomas, including breast, colon, cervical, gastric, lung and ovarian cancers, among others, express high levels of IL-8 relative to normal tissues [59]. In addition, multiple clinical studies in melanoma, as well as breast, ovarian, prostate and colon cancer have shown a direct correlation between serum IL-8 levels and disease progression [57]. In recent years, it has also been demonstrated that a link exists between IL-8, tumor EMT and tumor stemness. In colorectal cancer cell lines, for example, the induction of EMT via incubation with TGF-β [61] or via SNAIL overexpression [62] has been shown to induce the secretion of IL-8. Tumorspheres expanded from colorectal tumor tissues and cell lines were shown to contain cells with many features of CSCs, including chemo- and radio-resistance, expression of EMT markers and overexpression of the EMT regulator SNAIL. It was demonstrated that SNAIL directly activates the expression of IL-8 by binding to E3/E4 E-boxes located in the IL-8 promoter [62]. Blocking experiments showed that IL-8 expression is critical for SNAIL-induced EMT and tumor stemness, as blockade of IL-8 signaling resulted in decreased expression of the stem cell-associated genes SOX2, OCT4 and NANOG, and decreased formation of tumorspheres by SNAIL-overexpressing colorectal cancer cells [62].
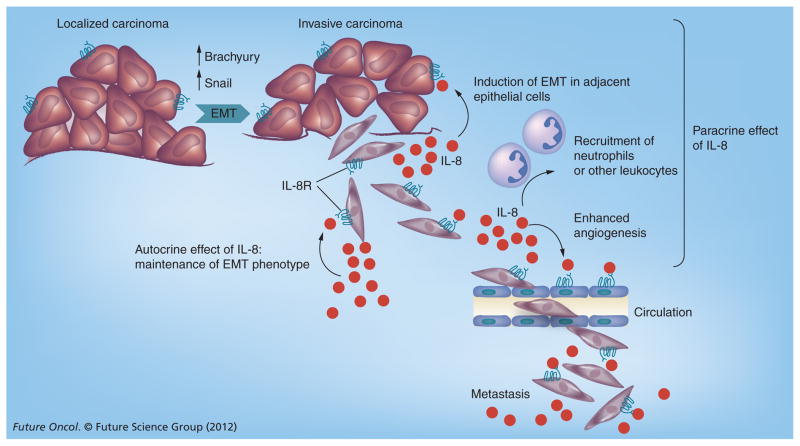
The existence of an autocrine positive loop between IL-8 and the EMT transcription factor brachyury in breast and lung cancer cell lines has also been demonstrated [48]. Upregulation of IL-8 and the IL-8Rs IL-8RA and IL-8RB was observed in epithelial tumor cells undergoing brachyury-mediated EMT. Antibody blockade of IL-8Rs markedly reduced fibronectin expression and invasiveness of brachyury-overexpressing breast cancer cells, providing evidence that the IL-8–IL-8R axis is essential for the maintenance of their mesenchymal, invasive phenotype (FIGURE 1) [48]. The IL-8–IL-8RA axis has also been shown to play a critical role in breast CSCs. Populations of CSCs, characterized by elevated activity of aldehyde dehydrogenase, have been shown to express elevated levels of IL-8RA and, in turn, the addition of purified IL-8 to epithelial breast cancer cells has been shown to increase the percentage of aldehyde dehydrogenase-positive cells, as well as to enhance the migration and invasiveness of CSCs in vitro [63]. Blockade of IL-8R activity via neutralizing antibodies or by utilizing the small-molecule inhibitor of IL-8R, repertaxin, decreased the breast CSC population both in vitro and in vivo [64], reinforcing the importance of the IL-8–IL-8R axis in breast CSCs. Additional studies conducted with primary colorectal cancer cells transfected with the stem cell-associated transcription factor OCT4 also showed that CSCs secrete higher levels of IL-8 and that neutralizing antibodies against IL-8 are able to inhibit tumorsphere formation along with the expression of the CSC markers CD133, CD44, SOX2, SNAIL and ABCG2, and decrease resistance to treatment with 5-fluorouracil [65]. Altogether, these studies emphasize the importance of IL-8 in carcinoma progression as an essential factor for the induction and maintenance of the mesenchymal and stem-like phenotype of aggressive, metastatic carcinoma cells.
Figure 1. Potential role of the IL-8–IL-8 receptor axis along tumor progression.
Tumor cells undergoing EMT have been shown to enhance the secretion of the chemokine IL-8 as well as to upregulate the expression of IL-8R. Secreted IL-8 could function in an autocrine loop to maintain the mesenchymal status of tumor cells that have passed through EMT, as well as in a paracrine fashion to induce adjacent epithelial tumor cells into EMT. The paracrine role of EMT may also involve its activity on endothelial cells to induce angiogenesis, as well as the recruitment of neutrophils and other leukocytes to the site of the tumor.
EMT: Epithelial–mesenchymal transition; IL-8R: IL-8 receptor.
An interesting observation in the studies conducted by Fernando et al. was that culture supernatants from brachyury-overexpressing mesenchymal tumor cells were able to induce other epithelial cancer cells, including breast MCF7 and T47D luminal cancer cells, to undergo an EMT characterized by enhanced expression of brachyury, SNAIL and SLUG [48]. IL-8R-blocking studies showed that this EMT-inducing effect was at least in part due to the EMT-inducing activity of the chemokine IL-8. The role of IL-8 in tumor EMT was also demonstrated by directly exposing breast epithelial tumor cells to purified, recombinant human IL-8 in vitro, a treatment that significantly reduced the expression of epithelial E-cadherin and increased fibronectin expression in MCF7 and T47D luminal breast cancer cells [48].
Similar to IL-8, the addition of purified, recombinant IL-6 was sufficient to promote an EMT phenotype in breast cancer cells, resulting in increased tumor invasiveness in vitro and a high rate of tumor cell proliferation in vivo [31]. These results suggest that soluble factors secreted by tumor cells undergoing EMT could function in a paracrine mode to induce adjacent epithelial tumor cells to undergo this phenotypic switch (TABLE 1).
Potential effects of IL-8 on the tumor microenvironment
As depicted in FIGURE 1, IL-8 released by tumor cells undergoing EMT could play several roles in the context of tumor progression by: maintaining the mesenchymal, invasive phenotype of tumor cells that have undergone EMT via an autocrine loop; exerting a paracrine effect on adjacent epithelial tumor cells in order to induce EMT; enhancing angiogenesis and potentially attracting immune cells to the site of the tumor, thus creating an inflammatory environment that could further favor tumor dissemination; and metastasis.
Among the cellular targets of IL-8 are the endothelial cells, which can be induced to proliferate and migrate in response to IL-8 signaling, therefore resulting in neovascularization [66]. In a study with non-small-cell lung carcinoma cells forced into EMT via SNAIL overexpression, the enhanced secretion of the chemokine IL-8 led to enhanced angiogenesis and tumor growth in vivo [67]. The SNAIL-mediated increase in tumor burden was efficiently abrogated with anti-IL-8RB neutralizing antibodies, a result that indicated the fundamental role of IL-8 in SNAIL-mediated tumor progression [67]. These results also demonstrated that tumor cells undergoing EMT have the potential to directly affect their surrounding stroma via the secretion of soluble factors. A similar example was reported with MCF7 breast cancer cells undergoing EMT via Twist overexpression, which grew as highly vascularized tumors in vivo as a result of their increased secretion of the angiogenic factor VEGF [68].
In addition to its effects on endothelial cells, IL-8 is also known to be a strong chemotactic factor for neutrophils [59]. The enhanced secretion of IL-8 by tumor cells undergoing EMT could also lead to enhanced recruitment of neutrophils, which, in turn, have been shown to exert various protumorigenic and prometastatic functions. For example, in a study conducted with a K-RAS-mutated lung adenocarcinoma model, enhanced secretion of the chemokines MIP-2 and CXCL1, murine homologs of IL-8, was linked to the recruitment of neutrophils to the site of the tumor [69]. Tumor-associated neutrophils, in turn, have been shown to assist in tumor progression by different means. For example, tumor-associated neutrophils have been shown to directly facilitate the escape of melanoma tumor cells from the circulation into lung tissues via ICAM-1-mediated binding of tumor cells to the surface of neutrophils [70]. Moreover, tumor progression can be assisted by tumor-associated neutrophils via the secretion of multiple proteinases, including MMPs, which could remodel the ECM and favor tumor migration [71]. Although several studies have shown enhanced secretion of IL-8 in the context of tumor EMT, so far there have been no studies specifically investigating the leukocytic infiltrates of tumors characterized by EMT in contrast to those with a fully epithelial phenotype.
Tumor cells undergoing EMT are not the only source of IL-8 in the microenvironment of a progressive tumor. As previously mentioned, various cellular components of the tumor stroma, including fibroblasts, endothelial cells and immune cells, can secrete IL-8 in response to various stress factors. Of particular interest are reports demonstrating that fibroblasts undergoing chemotherapy- or radiation-induced DNA damage and senescence, but not nonsenescent fibroblasts, are able to induce EMT and invasiveness in epithelial cancer cells. It was shown that this effect was mediated via the secretion of biologically active proteins by senescent fibroblasts, designated as the senescence-associated secretory phenotype, and, in particular, via secretion of IL-6 and IL-8 [49]. These observations indicate that the tumor stroma has the potential to promote tumor progression via secretion of multiple factors involved in intercellular signaling, acting in a paracrine fashion on the epithelial tumor compartment. As a result, IL-8 released by the stroma could directly influence tumor cell proliferation [72], migration, invasion and EMT [48,61,73], and, as more recently shown, could help tumor cells to evade stress-induced apoptosis [74].
Conclusion
Multiple studies have now demonstrated that tumor cells undergoing EMT acquire the capacity to secrete a milieu of cytokines, chemokines and growth factors that could potentiate tumor dissemination by modulating the tumor microenvironment. Although most of these studies have been conducted with tumor cell lines in vitro, the findings may have implications for cancer therapy. Pharmacological inhibition of these cytokine regulatory loops appears to be a rational approach for improving interventions against tumor progression. For example, blockade of the IL-8 signaling loop in solid tumors might favor clinical outcome by suppressing tumor growth, angiogenesis and the EMT-promoting activity of the IL-8–IL-8R axis. It is important to point out that the acquisition of a mesenchymal-like phenotype by carcinoma cells is postulated to be a transient and, perhaps, focalized process that may involve a few cells at the tumor–stroma interface. However, the transient character of EMT is still a topic of debate as some studies have supported the idea that mesenchymal-like tumor cells are required to switch back to their epithelial phenotype once they have reached the site of metastasis [75,76], while others have shown that EMT-associated molecules, such as Twist, remain upregulated at the site of metastatic prostate cancer lesions [46]. This potentially transient nature of EMT raises the question of when an intervention against IL-8 signaling could be adequate to block or reverse the appearance of tumor EMT. In this regard, the expression of IL-8 is known to be elevated in tumors compared with normal tissues, but few data are available on the expression of IL-8 in relation to tumor stage, or its levels in primary versus metastatic lesions. To better understand what type of tumors and which stages would benefit the most from anti-IL-8 interventions, further studies are needed to comparatively characterize IL-8 expression and features of EMT at various stages of human tumor development. The identification of additional regulators of EMT and, in particular, their validation as modulators of EMT in vivo may ultimately lead to combinatorial strategies that could more efficiently prevent tumor metastasis.
Future perspective
Owing to the relevant role of EMT in the progression of carcinomas, future antitumor interventions that specifically target tumor cells undergoing EMT could be envisioned to interfere with metastatic disease. The elimination of tumor cells that exhibit a mesenchymal phenotype could potentially be achieved by blocking the signaling pathways that trigger and/or maintain tumor EMT. In particular, blockade of the IL-8–IL-8R axis appears to be an attractive strategy to disrupt the autocrine positive feedback loop between EMT and IL-8 while simultaneously decreasing the paracrine signals that mesenchymal tumor cells could exert on their surrounding environment. Secretion of IL-8 is also a feature of the tumor stroma, and blockade of IL-8 signal ing could be fundamental in lessening the tumor-promoting signals originated on stromal fibroblasts, monocytes, neutrophils and endothelial cells in response to stressful environments, including hypoxia, acidosis or genotoxic damage. Supporting this strategy, several preclinical studies have already demonstrated the ability of neutralizing antibodies to the IL-8Rs, a humanized antibody against IL-8 (ABX-IL-8) and the small-molecule inhibitor repertaxin to inhibit angiogenesis, tumor growth and metastasis in xenograft tumor models [64,77,78].
A similar strategy to inhibit EMT via blockade of the signaling pathways that trigger this phenotypic conversion is exemplified in a recent report by Reka et al. [79], in which PPAR-γ synthetic ligands were used for inhibition of TGF-β-induced EMT in lung cancer cells. Activation of PPAR-γ was achieved by utilizing thiozolidinediones, compounds commonly utilized as insulin-sensitizing agents for the treatment of Type 2 diabetes, which inhibited TGF-β-induced EMT features, including migration, invasion and secretion of MMPs, and decreased the development of experimental lung metastasis in vivo.
An alternative strategy to directly eradicate tumor cells undergoing EMT is the use of cancer vaccine approaches that target essential regulators of the process such as the EMT transcription factors. In this context, the T-box transcription factor brachyury fulfills two major requirements for a molecule to be used as a target for vaccine approaches: brachyury is highly tumor-specific, being expressed in various human carcinomas but absent in most human normal adult tissues [5,80]; and brachyury-specific cytotoxic T lymphocytes can be expanded from the blood of cancer patients against an epitope of the brachyury protein [80]. Moreover, brachyury-specific T cells can lyze tumor cells that express the brachyury protein, indicating that a vaccine approach is a viable option for the generation of a long-lasting immune response against this EMT regulator. Based on these observations, a brachyury- based cancer vaccine is currently undergoing Phase I clinical evaluation in patients with carcinomas. As multiple preclinical and clinical studies are demonstrating the feasibility and benefit of employing combinatorial therapies for the treatment of cancer, it can be anticipated that the elimination of metastatic tumor cells via targeting of EMT may rely on the combination of the approaches described above.
Executive summary.
Autocrine cytokine loops maintain tumor epithelial–mesenchymal transition
Tumor cells undergoing epithelial–mesenchymal transition (EMT) can profoundly modulate their microenvironment via enhanced secretion of multiple soluble factors, including IL-8, IL-6, TGF-β, VEGF and matrix metalloproteinases, among others.
Once EMT has been initiated, tumor cells can sustain their mesenchymal phenotype by establishing autocrine loops that involve tumor-secreted cytokines and/or growth factors and concurrent upregulation of their cognate receptors.
IL-8 as an inducer of tumor EMT
-
Brachyury, tumor EMT and EMT-secreted factors:
Brachyury, a highly tumor-specific T-box transcription factor, is a regulator of EMT in human carcinomas;
Brachyury-induced tumor EMT is characterized by enhanced IL-8–IL-8 receptor (IL-8R) signaling, which is essential for the maintenance of the mesenchymal phenotype of brachyury-overexpressing human tumor cells.
-
IL-8–IL-8R loops involved in EMT and tumor stemness:
IL-8–IL-8R loops maintain the mesenchymal phenotype of tumor cells undergoing EMT;
Blockade of IL-8–IL-8R signaling decreases expression of stem cell-associated markers and tumorsphere formation of cancer stem cells;
IL-8 induces EMT in epithelial tumor cells.
Potential effects of IL-8 on the tumor microenvironment
IL-8 induces angiogenesis and recruitment of neutrophils to the site of the tumor.
Stroma-derived IL-8 can induce EMT in epithelial tumor cells.
Future perspective
Pharmacological inhibition of cytokine regulatory loops may improve anticancer interventions by reversing the metastatic phenotype of tumor cells and reducing paracrine signals on the tumor microenvironment.
Blockade of IL-8 signaling in solid tumors might counter tumor progression by decreasing angiogenesis, tumor growth and the EMT-promoting activity of the IL-8–IL-8R axis.
A cancer vaccine against the EMT regulator brachyury is currently undergoing Phase I clinical evaluation for the treatment of human carcinomas.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was funded by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪.Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. A comprehensive review of the role of the epithelial–mesenchymal transition (EMT) in normal and pathological conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 3.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 5▪.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor brachyury promotes epithelial–mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533–544. doi: 10.1172/JCI38379. Describes the T-box transcription factor as a regulator of EMT in human carcinomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurrey NK, Jalgaonkar SP, Joglekar AV, et al. Snail and Slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 8.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65(20):9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 10.Guarino M, Rubino B, Ballabio G. The role of epithelial–mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 11.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13(23):7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 12.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 13.Sawada K, Mitra AK, Radjabi AR, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68(7):2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial–mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68(4):989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 15▪▪.Mani SA, Guo W, Liao MJ, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. First paper describing a link between tumor EMT and the acquisition of characteristics associated with cancer stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. Describes the association between cancer stem cells and tumor EMT in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Ngo VN, Marani M, et al. Critical role for transcriptional repressor Snail2 in transformation by oncogenic RAS in colorectal carcinoma cells. Oncogene. 2010;29(33):4658–4670. doi: 10.1038/onc.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabbah M, Emami S, Redeuilh G, et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11(4–5):123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Moustakas A, Heldin CH. Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 21.Nannuru KC, Singh RK. Tumor–stromal interactions in bone metastasis. Curr Osteoporos Rep. 2010;8(2):105–113. doi: 10.1007/s11914-010-0011-6. [DOI] [PubMed] [Google Scholar]
- 22.Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathias RA, Wang B, Ji H, et al. Secretome-based proteomic profiling of Ras-transformed MDCK cells reveals extracellular modulators of epithelial–mesenchymal transition. J Proteome Res. 2009;8(6):2827–2837. doi: 10.1021/pr8010974. [DOI] [PubMed] [Google Scholar]
- 24.Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory PA, Bracken CP, Smith E, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial–mesenchymal transition. Mol Biol Cell. 2011;22(10):1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8(23):1243–1252. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- 27.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 28.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 29.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15(3):195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Jechlinger M, Grunert S, Tamir IH, et al. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22(46):7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial–mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Moreno O, Lecanda J, Green JE, et al. VEGF elicits epithelial–mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Exp Cell Res. 2009;316(4):554–567. doi: 10.1016/j.yexcr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 34.Rose-John S, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11(5):613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 35.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21(13):3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 36.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6(10):764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 37.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci USA. 2009;106(48):20318–20323. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139(7):1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Przybylo JA, Radisky DC. Matrix metalloproteinase-induced epithelial–mesenchymal transition: tumor progression at Snail’s pace. Int J Biochem Cell Biol. 2007;39(6):1082–1088. doi: 10.1016/j.biocel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci. 2006;119(Pt 18):3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 41.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 42.Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21(20):3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 43.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 44.Shih JY, Tsai MF, Chang TH, et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11(22):8070–8078. doi: 10.1158/1078-0432.CCR-05-0687. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Kwok WK, Ling MT, Lee TW, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65(12):5153–5162. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990;343(6259):617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- 48▪▪.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial–mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71(15):5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. First paper describing the ability of IL-8 signaling to induce and maintain brachyury-induced EMT in human breast carcinoma cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luboshits G, Shina S, Kaplan O, et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59(18):4681–4687. [PubMed] [Google Scholar]
- 51.Dannenmann C, Shabani N, Friese K, Jeschke U, Mylonas I, Bruning A. The metastasis-associated gene MTA1 is upregulated in advanced ovarian cancer, represses ERbeta, and enhances expression of oncogenic cytokine GRO. Cancer Biol Ther. 2008;7(9):1460–1467. doi: 10.4161/cbt.7.9.6427. [DOI] [PubMed] [Google Scholar]
- 52.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21(14):1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) J Immunol. 1987;139(3):788–793. [PubMed] [Google Scholar]
- 54.Skov L, Beurskens FJ, Zachariae CO, et al. IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplantar pustulosis. J Immunol. 2008;181(1):669–679. doi: 10.4049/jimmunol.181.1.669. [DOI] [PubMed] [Google Scholar]
- 55.Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 56.Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;186(8):1201–1212. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 58.Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86(3):529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- 59.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 60.Gabellini C, Trisciuoglio D, Desideri M, et al. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45(14):2618–2627. doi: 10.1016/j.ejca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Bates RC, DeLeo MJ, 3rd, Mercurio AM. The epithelial–mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299(2):315–324. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 62.Hwang WL, Yang MH, Tsai ML, et al. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology. 2011;141(1):279–291. doi: 10.1053/j.gastro.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 63▪▪.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. First paper describing the ability of IL-8 signaling to induce a cancer stem cell phenotype in human breast carcinoma cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪▪.Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120(2):485–497. doi: 10.1172/JCI39397. Demonstrates the potential of treatment strategies targeting the IL-8–IL-8 receptor axis to target tumor cells with a cancer stem cell phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang CJ, Chien Y, Lu KH, et al. Oct4-related cytokine effects regulate tumorigenic properties of colorectal cancer cells. Biochem Biophys Res Commun. 2011;415(2):245–251. doi: 10.1016/j.bbrc.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 66.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 67.Yanagawa J, Walser TC, Zhu LX, et al. Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15(22):6820–6829. doi: 10.1158/1078-0432.CCR-09-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mironchik Y, Winnard PT, Jr, Vesuna F, et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65(23):10801–10809. doi: 10.1158/0008-5472.CAN-05-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji H, Houghton AM, Mariani TJ, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25(14):2105–2112. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 70.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70(14):6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 72.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151(5):2667–2675. [PubMed] [Google Scholar]
- 73.De Larco JE, Wuertz BR, Rosner KA, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158(2):639–646. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maxwell PJ, Gallagher R, Seaton A, et al. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26(52):7333–7345. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 75.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66(23):11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 76.Bukholm IK, Nesland JM, Borresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients [see comments] J Pathol. 2000;190(1):15–19. doi: 10.1002/(SICI)1096-9896(200001)190:1<15::AID-PATH489>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 77.Mian BM, Dinney CP, Bermejo CE, et al. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9(8):3167–3175. [PubMed] [Google Scholar]
- 78.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 79.Reka AK, Kurapati H, Narala VR, et al. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial–mesenchymal transition. Mol Cancer Ther. 2010;9(12):3221–3232. doi: 10.1158/1535-7163.MCT-10-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80▪.Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. Describes the potential use of immunotherapy to target tumor cells undergoing EMT via cancer vaccine approaches against the T-box transcription factor brachyury. [DOI] [PubMed] [Google Scholar]