Abstract
This paper describes a new method for the catalytic aerobic oxygenation of unactivated sp3-C–H bonds. This transformation utilizes Pd(OAc)2 as a catalyst in conjunction with NaNO3 as a redox co-catalyst. Both oxime ether and pyridine derivatives are effective directing groups for these reactions. The oxygen incorporated into the product derives from the solvent (acetic acid). Preliminary results show that the addition of simple NaCl to the reaction mixture results in aerobic chlorination under analogous conditions.
Introduction
The development of metal-catalyzed methods for converting sp3-C–H bonds into C–O bonds using dioxygen as a terminal oxidant remains a grand challenge in organometallic chemistry.1,2 Methods for the selective aerobic oxygenation of unactivated 1° C–H bonds in the presence of weaker benzylic, allylic, 2°, or 3° C–H bonds remain particularly elusive.1,2 Over the past decade Pd-catalyzed ligand-directed C–H oxidation has emerged as a powerful approach to achieve sp3-C–H acetoxylation, alkoxylation, and hydroxylation.3 However, the oxidants used in these transformations are most typically reagents such as PhI(OAc)2, IOAc, or K2S2O8, which have the significant disadvantages of high cost, poor atom economy, the formation of stoichiometric byproducts, and/or moderate functional group tolerance.
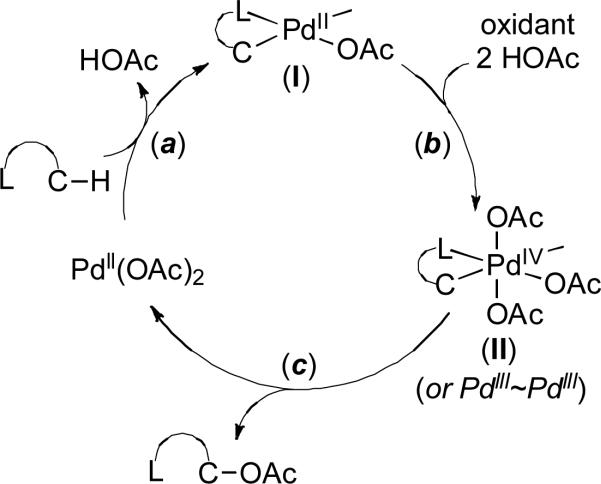
Mechanistic studies suggest that these reactions proceed via three key steps: (a) ligand-directed C–H activation to form cyclometalated PdII complexes of general structure I, (b) 2e– oxidation of I to form high valent Pd complex II, and (c) C–O bond-forming reductive elimination from II to release the oxygenated product (Figure 1).4 Importantly, at pH = 1, O2 has a comparable oxidation potential to PhI(OAc)2 ,5,6 suggesting that it should be thermodynamically capable of effecting the 2e– oxidation of palladacycle I to high valent Pd intermediate II. However, efforts to utilize dioxygen as a terminal oxidant in these transformations have generally been hampered by the slow kinetics of the aerobic oxidation of palladacycles.7,8 Recent elegant studies by Vedernikov2a,b and by Yu2c have shown that it is possible to address this challenge through careful selection of the supporting ligand and/or the substrate. However, these successful examples of aerobic Pd-catalyzed ligand-directed C–H oxygenation are far from a general solution, because they exhibit a narrow substrate scope. Most notably, neither is effective for promoting the C–H oxygenation of unactivated sp3-C–H bonds, which are arguably the most challenging substrates for these transformations.3
Figure 1.
Proposed mechanism for Pd-catalyzed ligand-directed C–H oxidation.
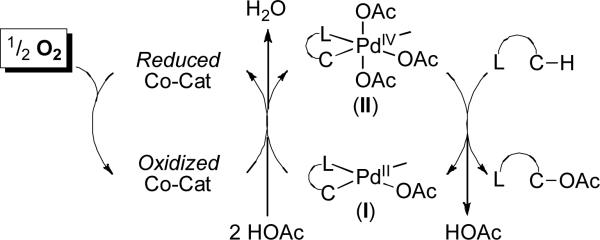
We reasoned that more general aerobic C–H oxidation reactions might be possible by using a redox co-catalyst9 that has high kinetic reactivity towards both O2 and palladacycles like I (Figure 2). This concept is inspired by the PdII/0-catalyzed Wacker oxidation, in which Cu(II)10 or polyoxometalates (POM's)11 are utilized as co-catalysts in conjunction with O2 as the terminal oxidant. A similar strategy has also been employed in some Pd-catalyzed benzene and methane oxidation reactions, most typically using POM or POM/NO2 as redox co-catalysts.12 The current manuscript uses this approach to achieve the Pd-catalyzed aerobic oxidation of unactivated sp3-C–H bonds in pyridine and oxime ether substrates using nitrate salts as redox co-catalysts. Both the scope and mechanism of these reactions are described, and their implications for expanding this method to diverse sp3-C–H functionalization reactions are discussed.
Figure 2.
Concept of redox co-catalysis for aerobic C–H oxidation
Results and Discussion
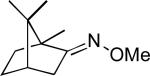
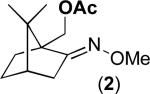
The Pd(OAc)2-catalyzed C–H acetoxylation of substrate 1 was selected as a test reaction, since it has been reported to proceed efficiently with oxidants like PhI(OAc)2 and K2S2O8.13 With air as the oxidant, this transformation provided only traces of product 2 (1% yield, Table 1, entry 1). Redox co-catalysts that are commonly used in PdII/0 catalysis (e.g., H5PMo10V2O40 (POM-V2) and Cu(OAc)2) proved ineffective (entries 2 and 3). Thus, we next explored nitrate and nitrite salts as precursors to more kinetically reactive NO2.14 Importantly, Campora has shown that NO2 can oxidize PdII to PdIV under mild conditions.15 Additionally, NO2 has been used as a redox co-catalyst in a wide variety of different transformations.9,10b,16 Gratifyingly, the use of NaNO2 or NaNO3 as the co-catalyst resulted in moderate yield (48 and 53%, respectively) of 2 (entries 4 and 5). Interestingly, while these reagents have previously been used for the Pd-catalyzed nitration of aromatics,17 the corresponding nitrated products were not observed in the current transformations.18,19 Under an N2 atmosphere, the NaNO3-mediated reaction proceeded in significantly lower yield (14%), corresponding to approximately 1 turnover of the redox co-catalyst (entry 6).
Table 1.
Optimization of redox co-catalyst
| ||||
|---|---|---|---|---|
| entry | co-cat | mol % co-cat | oxidant | yielda |
| 1 | none | 0 | Air | 1% |
| 2 | POM-V2b | 10 | Air | 0% |
| 3 | Cu(OAc)2 | 10 | air | 1% |
| 4 | NaNO2 | 10 | air | 48% |
| 5 | NaNO3 | 10 | air | 53% |
| 6 | NaNO3 | 10 | nonec | 14% |
| 7 | NaNO3 | 10 | O2 | 70% |
| 8 | NaNO3 | 25 | O2 | 91% |
| 9d | NaNO3 | 25 | O2 | >95% |
Yields determined by 1H NMR spectroscopic analysis and represent an average of 2 runs.
POM-V2 = H5PMo10V2O40.
N2 atmosphere.
110 °C.
Further optimization showed that moving from 1 atm of air to 1 atm of O2 in the Pd(OAc)2/NaNO3-catalyzed reaction resulted in a significant boost in yield to 70% of 2 (entry 7). Increasing the loading of NaNO3 to 25 mol % and the temperature to 110 °C led to further increases in yield. Under the final conditions (5 mol % of Pd(OAc)2, 25 mol % of NaNO3, and 1 atm O2 in AcOH/Ac2O at 110 °C for 18 h), 2 was formed in quantitative yield (entry 9).
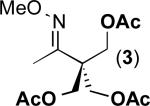
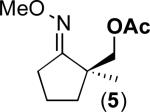
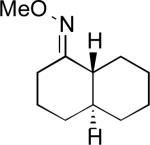
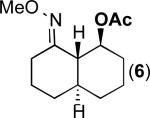
We next evaluated the substrate scope of this transformation. As shown in Table 2, a variety of substrates containing both oxime ether and pyridine directing groups underwent aerobic Pd(OAc)2/NaNO3-catalyzed sp3-C–H acetoxylation.20 Substrates containing 1° sp3-C–H sites β to the directing group generally underwent smooth and high yielding C–H oxygenation. In contrast, the functionalization of a 2° C–H bond to form 6 proceeded in only modest 41% isolated yield, even with 1 equiv of NaNO3.21 In substrates containing tert-butyl substituents proximal to the directing group, triacetoxylated products predominated (for example, entries 2 and 6-9). Importantly, ligand-directed aerobic oxidation at unactivated 1° sp3-C–H sites β to the directing group proceeded selectively, even in the presence of much weaker (but not proximal) benzylic C–H bonds (entry 9). In some instances, increased loading of NaNO3 (up to 1 equiv per C–H bond being functionalized) was necessary to achieve high yields (e.g., entries 5 and 10); however, the low cost of this oxidant relative to something like PhI(OAc)2 still makes this a synthetically useful procedure.22
Table 2.
Substrate scope for C–H oxygenation
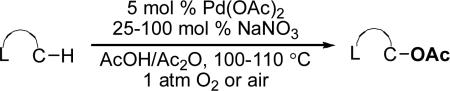
| |||
|---|---|---|---|
| entry | substrate | product | isolated yield |
| 1 |
|
|
80% |
| 2 |
|
|
83% |
| 3 |
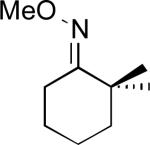
|
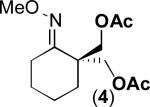
|
60% |
| 4 |
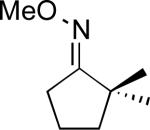
|
|
51% |
| 5 |
|
|
41% |
| 6a |
|
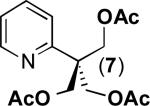
|
79% |
| 7a |
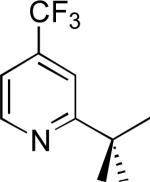
|
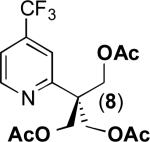
|
78% |
| 8a |
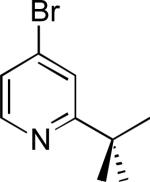
|
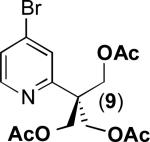
|
77% |
| 9 |
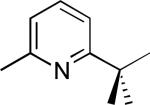
|
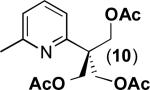
|
68% |
| 10 |
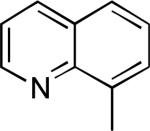
|
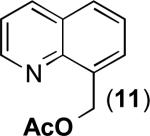
|
80% |
4 Å molecular sieves added.
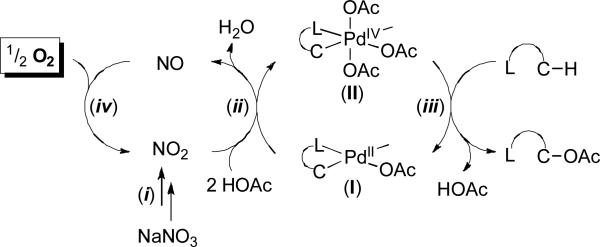
We hypothesize that these transformations proceed via the series of catalytic cycles shown in Figure 3. Step i involves the decomposition of nitrate to NO2. This process is well-precedented,14 and has been exploited to access NOX redox co-catalysts in a number of other aerobic oxidation reactions.16 Next the NO2 and 2 equiv of AcOH react with cyclopalladated intermediate I to generate high oxidation state Pd compound II along with NO and H2O (step ii).15 Carbon-oxygen bond-forming reductive elimination (step iii) then releases the C–H oxidation product, while the NO is oxidized by O2 to regenerate NO2 (step iv).16
Figure 3.
Proposed catalytic cycle. The complete supporting ligand set on Pd is unclear at this time, and nitrate and/or nitrite may serve as ligands during catalysis.
To directly probe for the intermediacy of NO, we conducted the catalytic reactions in the presence of 2,6-di-tert-butyl-4-methylphenol (BHT). NO is known to react rapidly with BHT to afford 2,6-di-tert-butyl-4-methyl-4-nitrosocyclohexa-2,5-dienone (TBMND),23 and the consumption of NO in this manner would be expected to have a detrimental effect on the C–H acetoxylation reaction. As anticipated, the addition of 25 mol % of BHT to the catalytic oxidation of 1 resulted in a significant reduction in the yield of 2, from >95% to 12% under otherwise analogous conditions (eq. 1).24 When the NaNO3/O2 oxidation reaction of 1 was assayed after 30 min, 1H NMR spectroscopic analysis showed resonances consistent with the formation of TBMND (in 26% yield based on BHT).25 Together, these two experiments provide preliminary support for the generation of NO as a key intermediate in this transformation.
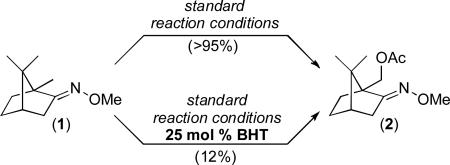 |
(1) |
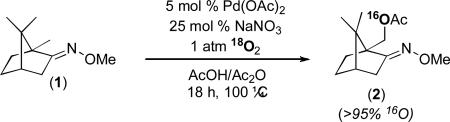
Another important feature of the mechanism proposed in Figure 3 is that the oxygen atom in the product derives from acetic acid and not from O2. To test this, we performed the reaction of substrate 1 in the presence of 1 atm of 18O2. Consistent with the proposed mechanism, HRMS analysis of the isolated product showed that <5% of the 18O label was incorporated into 2 under these conditions (eq. 2).26
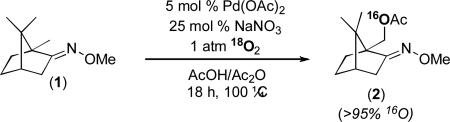 |
(2) |
This observation suggests that the use of different nucleophilic solvents and/or additives should enable the incorporation of other functional groups under aerobic oxidation conditions. To test this possibility, we examined the Pd(OAc)2/NaNO3-catalyzed aerobic oxidation of 2-tert-butylpyridine in propionic acid. Gratifyingly, this transformation provided 80% yield of the corresponding oxygenated product 12 (eq. 3).
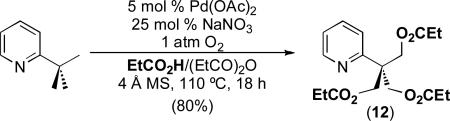 |
(3) |
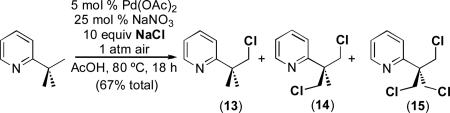
A second preliminary experiment showed that the addition of 10 equiv of NaCl to the Pd(OAc)2/NaNO3-catalyzed aerobic oxidation of 2-tert-butylpyridine produces a mixture of chlorinated products 13, 14, and 15 (eq. 4). While the latter reaction has thus far proven challenging to optimize further, this result provides extremely promising precedent that redox cocatalysis can be utilized to achieve diverse aerobic C–H functionalization reactions.
 |
(4) |
Conclusions
In summary, this paper demonstrates the use of a combination of Pd(OAc)2 and NaNO3 or NaNO2 to catalyze the aerobic C–H oxygenation of unactivated sp3-C–H bonds. Preliminary mechanistic and synthetic studies suggest that this approach could enable more general C–H functionalization reactions with dioxygen as the terminal oxidant. Investigations aimed at understanding the mechanism, decreasing catalyst loadings, and further increasing the scope of these reactions are currently underway in our laboratory. More generally, since commercial Pd(OAc)2 is frequently contaminated with nitrite salts,19 we suggest that redox co-catalysis by NOx may play a previously unappreciated role in a variety of other ‘Pd(OAc)2’-catalyzed aerobic oxidation reactions.
Supplementary Material
Acknowledgements
We thank the NIH (GM073836) for support of this research. KJS thanks the ACS Division of Organic Chemistry/Eli Lilly, Novartis, and Rackham Graduate School for graduate fellowships. We also thank Sharon Neufeldt for conducting the reactions in reference 24.
Footnotes
† Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Notes and references
- 1.a Shi Z, Zhang C, Tang C, Jiao N. Chem. Soc. Rev. 2012;41:3381. doi: 10.1039/c2cs15224j. [DOI] [PubMed] [Google Scholar]; b Zhou M, Crabtree RH. Chem. Soc. Rev. 2011;40:1875. doi: 10.1039/c0cs00099j. [DOI] [PubMed] [Google Scholar]; c Newhouse T, Baran PS. Angew. Chem. Int. Ed. 2011;50:3362. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Alonso DA, Najera C, Pastor IM, Yus MC. Chem. Eur. J. 2010;16:5274. doi: 10.1002/chem.201000470. [DOI] [PubMed] [Google Scholar]; e Punniyamurthy T, Rout L. Coord. Chem. Rev. 2008;252:134. [Google Scholar]
- 2.a Vedernikov AN. Acc. Chem. Res. 2012;45:803. doi: 10.1021/ar200191k. [DOI] [PubMed] [Google Scholar]; b Zhang Y-H, Yu J-Q. J. Am. Chem. Soc. 2009;131:14654. doi: 10.1021/ja907198n. [DOI] [PubMed] [Google Scholar]; c Zhang J, Khaskin E, Anderson NP, Zavalij PY, Vedernikov AN. Chem. Commun. 2008:3625. doi: 10.1039/b803156h. [DOI] [PubMed] [Google Scholar]
- 3.a Li H, Li B-J, Shi Z-J. Catal. Sci. Technol. 2011;1:191. [Google Scholar]; b Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654. doi: 10.1002/chem.200902374. [DOI] [PubMed] [Google Scholar]
- 4.a Powers DC, Ritter T. Acc. Chem. Res. 2012;45:840. doi: 10.1021/ar2001974. [DOI] [PubMed] [Google Scholar]; b Hickman AJ, Sanford MS. Nature. 2012;484:177. doi: 10.1038/nature11008. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Muniz K. Angew. Chem., Int. Ed. 2009;48:9412. doi: 10.1002/anie.200903671. [DOI] [PubMed] [Google Scholar]; e Canty AJ. Dalton Trans. 2009:10409. doi: 10.1039/b914080h. [DOI] [PubMed] [Google Scholar]
- 5.Oxtoby DW, Gillis HP, Nachtrieb NH. Principles of Modern Chemistry. 5th Ed. Thomson, Brooks, Cole; London: 2002. p. A42. [Google Scholar]
- 6.Giffard M, Mabon G, Leclair E, Mercier N, Allain M, Gorgues A, Molinie P, Neilands O, Krief P, Khodorkovsky V. J. Am. Chem. Soc. 2001;123:3852. doi: 10.1021/ja0058839. [DOI] [PubMed] [Google Scholar]
- 7.See ref. 4a-d and Ryabov AD. Chem. Rev. 1990;90:403.
- 8.For examples of the stoichiometric aerobic oxidation of PdII complexes to form detectable high valent Pd intermediates, see: Khusnutdinova JR, Qu F, Zhang Y, Rath NP, Mirica LM. Organometallics. 2012;31:4627.Khusnutdinova JR, Rath NP, Mirica LM. J. Am. Chem. Soc. 2012;134:2414. doi: 10.1021/ja210841f.Chuang GJ, Wang W, Lee E, Ritter T. J. Am. Chem. Soc. 2011;133:1760. doi: 10.1021/ja108396k.
- 9.Piera J, Backvall J-E. Angew. Chem. Int. Ed. 2008;47:3506. doi: 10.1002/anie.200700604. [DOI] [PubMed] [Google Scholar]
- 10.a Keith JA, Henry PM. Angew. Chem. Int. Ed. 2009;48:9038. doi: 10.1002/anie.200902194. [DOI] [PubMed] [Google Scholar]; b Cornell CN, Sigman MS. Inorg. Chem. 2007;46:1903. doi: 10.1021/ic061858d. [DOI] [PubMed] [Google Scholar]; c Takacs JM, Jiang X-T. Curr. Org. Chem. 2003;7:369. [Google Scholar]; d Negishi E-I, editor. Handbook of Organopalladium Chemistry for Organic Synthesis. Vol. 2. Wiley; New York: 2002. [Google Scholar]
- 11.Neumann R, Khenkin AM. Chem. Commun. 2006:2529. doi: 10.1039/b600711m. [DOI] [PubMed] [Google Scholar]
- 12.For examples, see: Yuan J, Wang L, Wang Y. Ind. Eng. Chem. Res. 2011;50:6513.An Z, Pan X, Liu X, Han X, Bao H. J. Am. Chem. Soc. 2006;128:16028. doi: 10.1021/ja0647912.Liu Y, Murata K, Inaba M. J. Mol. Catal. A. 2006;256:247.Burton HA, Kozhevnikov IV. J. Mol. Catal. A. 2002;185:285.Passoni LC, Cruz AT, Buffon R, Schuchardt U. J. Mol. Catal. A. 1997;120:117.Eberson L, Jonsson E. Acta Chem. Scand, B. 1974;28:771.
- 13.a Desai LV, Malik HA, Sanford MS. Org. Lett. 2006;8:1141. doi: 10.1021/ol0530272. [DOI] [PubMed] [Google Scholar]; b Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 14.a Theimann M, Scheibler E, Wiegand KW. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2005. Nitric Acid, Nitrous Acid, and Nitrogen Oxides. [Google Scholar]; b Laue W, Theimann M, Scheibler E, Wiegand KW. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2005. Nitrates and Nitrites. [Google Scholar]
- 15.Campora J, Palma P, del Rio D, Carmona E, Graiff C, Tiripicchio A. Organometallics. 2003;22:3345. [Google Scholar]
- 16.For recent examples where either NO2– or NO3– salts were used as precursors to NO2 in an aerobic oxidation reaction, see: Wang L, Li J, Yang H, Lv Y, Gao S. J. Org. Chem. 2012;77:790. doi: 10.1021/jo202301s.Shibuya M, Osada Y, Sasano Y, Tomizawa M, Iwabuchi Y. J. Am. Chem. Soc. 2011;133:6497. doi: 10.1021/ja110940c.Zhang G, Wen X, Wang Y, Mo W, Ding C. J. Org. Chem. 2011;76:4665. doi: 10.1021/jo102571e.Liu R, Liang X, Dong C, Hu X. J. Am. Chem. Soc. 2004;126:4112. doi: 10.1021/ja031765k.
- 17.For examples, see: Itahara T, Ebihara R, Kawasaki K. Bull. Chem. Soc. Jpn. 1983;56:2171.Norman ROC, Parr WJE, Thomas CB. J. Chem. Soc. Perkin Trans. I. 1974:369.Henry PM. J. Org. Chem. 1971;36:1886.
- 18.Nitrated compounds were not observed based on 1H NMR spectroscopic analysis of the crude reaction mixtures.
- 19.This lack of nitrated products may be due to a preference for the O-versus N-linkage isomer of Pd-bound NOX under the reaction conditions. For a recent discussion of cyclometalated Pd(NO2) complexes, see: Bajwa SE, Storr TE, Hatcher LE, Williams TJ, Baumann CG, Whitwood AC, Allan DR, Teat SJ, Raithby PR, Fairlamb IJS. Chem. Sci. 2012;3:1656.
- 20.Several other sp3-C–H substrates were examined (e.g. butan-2-one-O-methyl oxime, butan-2-one-O-acetyl oxime, and 2-t-butyl-4,4-dimethyl-4,5-dihydrooxazole) that did not undergo high yielding C–H acetoxylation under the current optimal conditions.
- 21.Neufeldt SR, Sanford MS. Acc. Chem. Res. 2012;45:636. doi: 10.1021/ar300014f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A limitation of these reaction conditions is that they are poorly effective for functionalization of sp2-C–H substrates (which have previously been viewed as less ‘challenging’ substrates for Pd C–H oxidation reactions).21 For example, subjecting 2-phenylpyridine to the reaction conditions resulted in no reaction (>95% recovered starting material). Likewise, acetophenone O-methyl oxime showed limited reactivity under the optimized reaction conditions, providing 29% of the monoacetoxylated product (with 58% recovered starting material).
- 23.Janzen EG, Wilcox AL, Manoharan V. J. Org. Chem. 1993;58:3597. [Google Scholar]
- 24.The addition of BHT had a much smaller effect on the C–H acetoxylation of 1 with PhI(OAc)2 (87% yield without BHT and 58% yield with 25 mol % of BHT). This suggests that the results in eq 1 are not solely due to detrimental interactions between BHT and Pd intermediates during catalysis.
- 25.TBMND is unstable under the reaction conditions, which likely accounts for the less-than-quantitative yield.
- 26.Gruber CC, Oberdorfer G, Voss CV, Kremsner JM, Kappe CO, Kroutil W. J. Org. Chem. 2007;72:5778. doi: 10.1021/jo070831o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.