Abstract
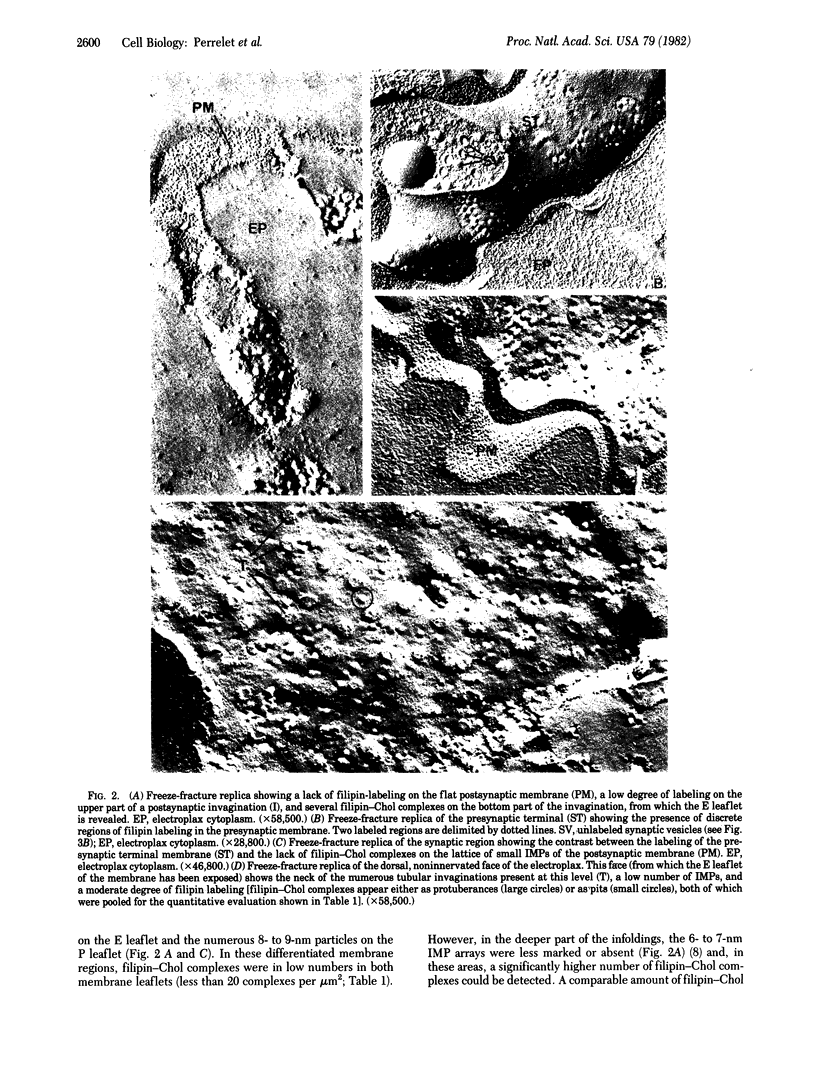
A cytochemical probe for cholesterol, the polyene antibiotic filipin, was applied to aldehyde-fixed samples of the electric organ of Torpedo marmorata to identify filipin-binding sites in the various membrane components of the organ and, hence, the probable cholesterol content at these levels. In both thin-sectioned and freeze-fractured samples, filipin-cholesterol complexes appeared numerous and homogeneously distributed on the Schwann cell plasma membrane. On the presynaptic membrane, filipin-cholesterol complexes occurred in patches alternating with unlabeled membrane segments. The postsynaptic, acetylcholine receptor-rich plasma membrane of the electroplax showed no or few filipin-cholesterol complexes in the flat region and upper part of the invaginations (both areas characterized by a lattice of small intramembrane particles); however, the membrane of the bottom part of the postsynaptic invaginations contained several complexes. The ventral, noninnervated plasma membrane of the electroplax showed a moderate, homogeneous filipin labeling. These data suggest that the distribution of cholesterol among membranes of the electroplax is not homogeneous and that the acetylcholine receptor-rich region of the postsynaptic membrane (as characterized by the lattice of small intramembrane particles) may contain little cholesterol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T., Baerwald R., Potter L. T. Postsynaptic membranes in the electric tissue of Narcine: II. A freeze-fracture study of nicotinic receptor molecules. Tissue Cell. 1977;9(4):595–608. doi: 10.1016/0040-8166(77)90029-5. [DOI] [PubMed] [Google Scholar]
- Andrews L. D., Cohen A. I. Freeze-fracture evidence for the presence of cholesterol in particle-free patches of basal disks and the plasma membrane of retinal rod outer segments of mice and frogs. J Cell Biol. 1979 Apr;81(1):215–228. doi: 10.1083/jcb.81.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman P. C., Nakajima Y. Membrane lipid heterogeneity associated with acetylcholine receptor particle aggregates in Xenopus embryonic muscle cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1278–1282. doi: 10.1073/pnas.78.2.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L. A morphological study of the cholinergic receptor protein from Torpedo marmorata in its membrane environment and in its detergent-extracted purified form. J Cell Sci. 1978 Feb;29:313–337. doi: 10.1242/jcs.29.1.313. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. I. Effects of black widow spider venom and Ca2+-free solutions on the structure of the active zone. J Cell Biol. 1979 Apr;81(1):163–177. doi: 10.1083/jcb.81.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. II. Effects of electrical stimulation and high potassium. J Cell Biol. 1979 Apr;81(1):178–192. doi: 10.1083/jcb.81.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi F., Conti-Tronconi B., Peluchetti D., Morgutti M. Effect of denervation on the organization of the postsynaptic membrane of the electric organ of Torpedo marmorata. Brain Res. 1975 Jun 6;90(1):133–118. doi: 10.1016/0006-8993(75)90687-3. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Akert K., Sandri C., Moor H. Ultrastructure of the "active zone" in the frog neuromuscular junction. Brain Res. 1973 Nov 23;62(2):373–380. doi: 10.1016/0006-8993(73)90699-9. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Friend D. S., Goerke J. Membrane sterol heterogeneity. Freeze-fracture detection with saponins and filipin. J Histochem Cytochem. 1979 Sep;27(9):1247–1260. doi: 10.1177/27.9.479568. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Bearer E. L. beta-Hydroxysterol distribution as determined by freeze-fracture cytochemistry. Histochem J. 1981 Jul;13(4):535–546. doi: 10.1007/BF01002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Salpeter S. R. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979 Jul;82(1):150–173. doi: 10.1083/jcb.82.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G., Gysin R., Flanagan S. D. Affinity partitioning of membranes. Evidence for discrete membrane domains containing cholinergic receptor. J Biol Chem. 1981 Sep 10;256(17):9126–9135. [PubMed] [Google Scholar]
- Marsh D., Barrantes F. J. Immobilized lipid in acetylcholine receptor-rich membranes from Torpedo marmorata. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4329–4333. doi: 10.1073/pnas.75.9.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Perrelet A., Vassalli P., Orci L. Absence of filipin-sterol complexes from large coated pits on the surface of culture cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6391–6395. doi: 10.1073/pnas.76.12.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Bridgman P. C. Absence of filipin-sterol complexes from the membranes of active zones and acetylcholine receptor aggregates at frog neuromuscular junctions. J Cell Biol. 1981 Feb;88(2):453–458. doi: 10.1083/jcb.88.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. D., Gibson R. E., Sumikawa K. The nicotinic acetylcholine receptor from Torpedo electroplax. Prog Brain Res. 1979;49:279–291. doi: 10.1016/S0079-6123(08)64640-3. [DOI] [PubMed] [Google Scholar]
- Orci L., Montesano R., Meda P., Malaisse-Lagae F., Brown D., Perrelet A., Vassalli P. Heterogeneous distribution of filipin--cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):293–297. doi: 10.1073/pnas.78.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Perrelet A., Dunant Y. A peculiar substructure in the postsynaptic membrane of Torpedo electroplax. Proc Natl Acad Sci U S A. 1974 Feb;71(2):307–310. doi: 10.1073/pnas.71.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Sandri C., Akert K., Moor H. Structure and ultrastructure of the frog motor endplate. A freeze-etching study. Cell Tissue Res. 1974 Jun 24;149(4):437–455. doi: 10.1007/BF00223024. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Demel R. A., Sobel A., Van Deenen L. L., Changeux J. P. Interaction of the acetylcholine (nicotinic) receptor protein from Torpedo marmorata electric organ with monolayers of pure lipids. Eur J Biochem. 1978 Apr;85(1):27–42. doi: 10.1111/j.1432-1033.1978.tb12209.x. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Ellisman M. H. Studies of excitable membranes. I. Macromolecular specializations of the neuromuscular junction and the nonjunctional sarcolemma. J Cell Biol. 1974 Nov;63(2 Pt 1):567–586. doi: 10.1083/jcb.63.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Karnovsky M. J. Evaluation of the polyene antibiotic filipin as a cytochemical probe for membrane cholesterol. J Histochem Cytochem. 1980 Feb;28(2):161–168. doi: 10.1177/28.2.6766487. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Synaptic membrane structure in Torpedo electric organ. J Neurocytol. 1975 Dec;4(6):697–712. doi: 10.1007/BF01181631. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealock R. Identification of regions of high acetylcholine receptor density in tannic acid-fixed postsynaptic membranes from electric tissue. Brain Res. 1980 Oct 20;199(2):267–281. doi: 10.1016/0006-8993(80)90689-7. [DOI] [PubMed] [Google Scholar]
- Sealock R., Kavookjian A. Postsynaptic distribution of acetylcholine receptors in electroplax of the torpedine ray, Narcine brasiliensis. Brain Res. 1980 May 19;190(1):81–93. doi: 10.1016/0006-8993(80)91161-0. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Branton D. Reconstitution of intramembrane particles in recombinants of erythrocyte protein band 3 and lipid: effects of spectrin-actin association. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3891–3895. doi: 10.1073/pnas.73.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]