Abstract
BACKGROUND
Pancreatic enzyme supplementation is standard treatment for malabsorption due to chronic pancreatitis. The FDA recently required all manufacturers to submit New Drug Applications (NDAs) to continue to market these agents because published data demonstrated variation in formulation, bioavailability, and shelf-life while providing limited data about efficacy and safety.
AIM
To systematically review the design and results of randomized, parallel-design trials of pancreatic enzyme supplements in chronic pancreatitis patients with steatorrhea.
METHODS
A computer-assisted search of MEDLINE and EMBASE was performed to identify relevant studies. Two authors performed duplicate data extraction on study design, improvement in coefficient of fat absorption (CFA), diarrhea, and adverse events using pre-specified forms. Agreement between investigators for data extraction was greater than 95%.
RESULTS
Of 619 articles found through literature searching, 20 potentially relevant articles were identified and 4 manuscripts met inclusion criteria. No studies performed head-to-head comparisons of different supplements. Enzyme supplementation is more likely to improve CFA compared to placebo, but fat malaborption remained abnormal. Important differences in patient population, study endpoint, study design, pancreatic enzyme dosage, and measurement of CFA were present across trials which precluded comparison of different agents.
CONCLUSIONS
Enzyme supplementation improves CFA compared to placebo but may not abolish steatorrhea.
Keywords: Pancreas, Enzyme, Malabsorption
INTRODUCTION
Pancreatic enzyme supplements have been available for many years, but proof of efficacy and safety through the US Food and Drug Administration (FDA) New Drug Application (NDA) process was not required because these agents had been used for many years before institution of the NDA process. In April 2004, the FDA reported that “currently marketed pancreatic enzyme preparations differ in their composition, enzymatic activities, formulation, stability, and bioavailability. These differences have led to highly variable pancreatic enzyme preparation quality and therapeutic performances… such differences over time can lead to batch-to-batch inconsistency and to unacceptable variability in … quality and therapeutic performance”[1]. Therefore, the FDA determined that published data about pancreatic enzyme supplements was insufficient and that new randomized controlled trial (RCT) data would need to be submitted as part of NDAs for any marketed pancreatic enzyme supplements. These new data would need to demonstrate the efficacy and safety of these agents along with consistency in manufacturing and stability/shelf-life of these agents.
Although pancreatic enzyme supplementation is “standard of care” for malabsorption due to severe chronic pancreatitis, the efficacy and safety of these agents remains unclear. In order to assess efficacy and safety of these agents, broad questions about pancreatic exocrine insufficiency and specific questions about enzyme supplementation should be answered, and the FDA guidance [1] on pancreatic enzyme supplementation raised some of these questions. How should malabsorption be quantified and how should improvement in malabsorption be measured in clinical trials? What is the appropriate dose and timing of enzyme administration? Are porcine enzyme supplements stable over 12 months? What is the “shelf-life” of these agents? Is it necessary for enzyme supplements to consistently contain 100% of labeled claims for potency or will significant (+/− 65%) variation in the quantity of enzyme contained in each capsule impact efficacy and safety of these supplements? We propose to review published randomized controlled trial (RCT) data about pancreatic enzyme supplementation in chronic pancreatitis patients in order to address questions about the efficacy, safety, and stability of these agents.
No previous systematic review has qualitatively and quantitatively reviewed the study design and results of published RCTs on the efficacy and safety of these agents. Our goal is to perform this review. We focused our analysis on studies that met the recommended criteria in the FDA guidance [1] by limiting our analysis to parallel-design RCTs that used coefficient of fat absorption (CFA) to assess changes in malabsorption. We also sought to extract clinically relevant data about adverse events, quantity of pancreatic enzyme supplements included in each capsule, stability/shelf-life of capsules, and malabsorption symptoms such as diarrhea and weight loss. Through this review, we sought to validate the FDA's conclusion that published data about pancreatic enzyme supplements was insufficient and that new RCT data is needed. We also sought to determine the strengths and limitations of study design and results from published RCTs, identify gaps in the current literature, outline the design of additional studies which would clarify optimal therapy, and provide an up-to-date review of the existing RCT literature prior to publication of new RCTs required by the FDA.
MATERIALS AND METHODS
Literature Search
A computer-assisted search was conducted to identify potentially relevant publications in the following databases on December 7, 2007: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, OVID Cochrane Library, and the Centre for Reviews and Dissemination (CRD).
A search of the OVID MEDLINE database <1980 to November Week 2 2007> was performed using the following exploded (exp), medical subject heading (MeSH) and textwords: exp Chronic Pancreatitis/dt [Drug Therapy] OR exp Exocrine Pancreatic Insufficiency/dt [Drug Therapy] OR exp Pancreatitis OR exp Cystic Fibrosis OR exp Exocrine Pancreatic Insufficiency OR (pancreatitis or (pancrea$ adj2 insufficien$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] AND exp Enzymes/ OR (enzyme$ adj1 (pancrea$ or replace$ or supplement$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] OR (pancreatin or pancrease or pancrelipase or ultrase or cotazym or creon or kreon or theraclec or encron or protilase or lipase or hydrolase or exolipase or triglyceridase or ALTU-135).mp. [mp=title, original title, abstract, name of substance word, subject heading word]. This was then limited to humans, and a search filter designed to retrieve controlled clinical trials, systematic reviews, meta-analyses or randomized controlled trials was applied.
The same search strategy was used to search the OVID Cochrane Library. Both the MEDLINE In-Process and Other Non-Indexed Citations, and the CRD databases were searched using textword combinations. A search of the EMBASE database <1980 to 2007 Week 49> was performed using search terms similar to those used in the MEDLINE search. Additional searches of the Digestive Diseases Week (DDW) abstracts from 2006–2007 were performed with the search terms “pancreatic enzymes” or “steatorrhea” or “chronic pancreatitis” or “cystic fibrosis”.
Study Selection Criteria
Study inclusion criteria were: (a) study design-RCT with parallel design; (b) study population-chronic pancreatitis based on TIGAR-O etiologic classification (Toxic-metabolic, Idiopathic, Genetic, Autoimmune, Recurrent and severe acute pancreatitis, and Obstructive) [2] with confirmed steatorrhea; (c) study intervention-oral placebo versus pancreatic extract preparations [uncoated, enteric coated microspheres (MSP), microspheres(MMSP), microtablets(MT)] (Table 1); (d) study endpoint-change in pancreatic malabsorption of fat based on coefficient of fat absorption (CFA; see Table 2) or coefficient of absorption (COA) of fat or fecal fat excretion (FFE) over a specific period of time. Also, we extracted data for the following endpoints: coefficients of nitrogen absorption (CNA), change in weight, nutritional measurements, clinical symptoms including diarrhea, and adverse events. Studies were excluded if: (a) etiology of malabsorption was non-pancreatic malabsorption due to bacterial overgrowth, small bowel mucosal disease, short gut or cholestatic liver disease or if patient had secondary pancreatic insufficiency due to pancreatic cancer/surgery.
Table 1.
Lipase conversion dose
| Correlation among 4 units of enzymatic activity for amylase, lipase and protease | ||||
|---|---|---|---|---|
| Enzyme | PhEur Unit | FIP unit | BP unit | USP units |
| Amylase | 1 | 1 | 1 | 4.15 |
| Lipase | 1 | 1 | 1 | 1 |
| Protease | 1 | 1 | 1 | 62.5 |
Adapted from [17]
Table 2.
Coefficient of Fat Absorption (CFA)
|
|
Since there are no gold-standard criteria for the diagnosis of chronic pancreatitis, studies were included if they used one of the following measures to identify chronic pancreatitis patients: individual imaging tests (calcification on imaging, ≥ 5 EUS criteria [3], and Cambridge criteria 2–3 [4] for ERCP, CT or US), direct pancreatic function testing, or multi-component diagnostic criteria (Ammann [5], Mayo [6], Japanese Pancreas Society [7]). The diagnosis of pancreatic malabsorption was considered based on findings of direct and/or indirect tests of pancreatic function. The criteria for the diagnosis of cystic fibrosis was based on the 1998 consensus[8].
Two investigators (A.W., M.D.) independently reviewed the titles and abstracts of all citations identified by the literature search. Potentially relevant studies were retrieved and the selection criteria applied. Agreement between investigators for selection of studies for the systematic review was greater than 95%, and disagreements were resolved by consensus. Studies published only as abstracts were included if they had sufficient information on study design, characteristics of participants, interventions and outcomes and if the first author of an abstract would provide full information, including final results.
Data Extraction and Assessment of Methodologic Quality of Individual Studies
Eligible articles were reviewed in a duplicate, independent manner by two investigators (A.W., B.W). For each study, the investigators recorded the study design, the inclusion and exclusion criteria, etiology of primary pancreatic insufficiency, diagnostic criteria for chronic pancreatitis, diagnostic criteria for pancreatic insufficiency, diagnostic criteria for cystic fibrosis, number of patients in each arm of study, age of patients, pancreatic enzyme used, dose of pancreatic enzyme, formulation of pancreatic enzyme, standardized lipase dose (units in USP see Table 2), timing of enzyme administration, concomitant use of other medications, use of a fecal fat balance study, duration of follow-up, quantification of fecal fat and its method of collection, results of primary endpoint, results of all secondary endpoints, and results of adverse event reporting.
There are no standardized criteria to assess the methodologic quality of RCTs about pancreatic enzyme supplementation, but there are validated criteria to assess the quality of RCTs about therapy [9]. These general criteria emphasize the importance of proper randomization, concealed allocation, double-blinding, and complete patient follow-up. Other appropriate methodologic criteria for any RCT include calculation of a sample size. With respect to pancreatic enzyme supplementation, it is crucial to confirm the presence of fat malabsorption based on CFA prior to enrolling patients into these studies. Also, since CFA measurements are dependent on the quantity of fat consumed (Table 2), dietary fat consumption during CFA measurements should be carefully controlled. This can be accomplished through multiple techniques including monitored dietary intake while in clinical research centers and use of stool dye markers to demarcate the beginning and end of a 72 hour fecal fat collection while a patient's diet is monitored. For this review, we assessed methodologic quality of individual RCTs with respect to use of randomization, concealed allocation, double-blinding, complete patient follow-up, confirmation of fat malabsorption prior to study enrollment, use of stool dye markers to demarcate beginning and end of a 72 hour fecal fat collection, and use of monitored dietary fat intake to confirm accurate measurement of CFA.
Data Analysis
Due to vast differences in study design, study population, formulation and dosing of enzyme supplements, and definition of study endpoint, pooling of data into a meta-analysis or direct comparisons of different agents was not feasible. Therefore, results of individual RCTs are presented in tabular form.
RESULTS
Literature Search
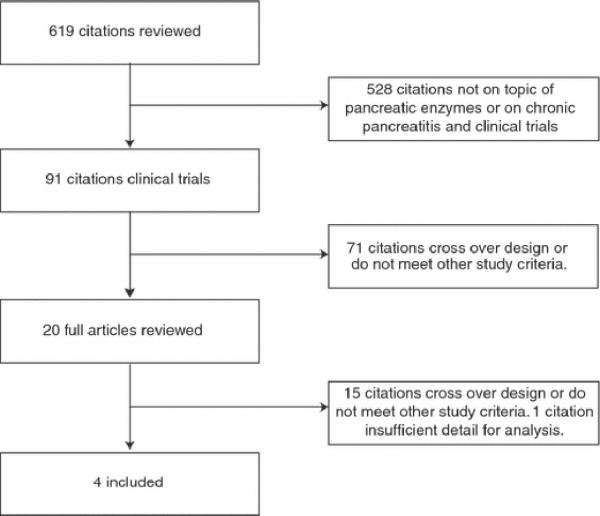
The MEDLINE search yielded 290 articles. The EMBASE search yielded 472 articles. DDW abstract reviews revealed no new articles (Figure 1). Manual searches of reference lists from potentially relevant papers identified 13 additional publications that were not detected using the computer-assisted strategy.
Figure 1.
Systematic review: pancreatic enzyme treatment of malabsorption associated with chronic pancreatitis
All citations were downloaded into Reference Manager® and then EndNote®, and duplicates were removed. 619 unique citations were obtained and the titles and abstracts of each citation were reviewed. Twenty relevant studies were identified, retrieved and completely reviewed. Sixteen studies did not meet study selection criteria due to lack of randomization, lack of placebo control, enrollment of patients with pancreatic cancer, pancreatic surgery, celiac disease or other small bowel malabsorptive disorder. One manuscript from a Croatian study could not be obtained despite repeated attempts to contact the author, and one additional abstract was not included because the abstract lacked sufficient information for inclusion and additional information could not be obtained despite contacting the first author.
Summary of RCT Results
Four studies met inclusion criteria (10–13), although these 4 studies enrolled different patient populations (Table 3) and administered different enzyme formulations and doses. None of the studies performed head-to-head comparisons of different pancreatic enzyme supplements, although different dosages of the same pancreatic enzyme supplement were assessed in one study [10]. All studies quantified steatorrhea by coefficient of fat absorption (Table 2). No studies assessed weight gain or weight loss with enzyme supplements. None of the RCTs included an assessment of the potency of porcine enzyme (i.e., variation in the quantity of enzyme contained in each capsule) or the stability/shelf-life of these agents. Study populations were relatively small (n = 29 and n = 26) in two studies [12–13] of chronic pancreatitis patients. Two studies used stool markers to demarcate treatment periods and to facilitate accurate 72 hour fecal fat collection measurements [10] [11]. Three studies [10–12]monitored dietary intake of fat in a controlled setting. All studies [10–13] were randomized, double-blind and had appropriate patient follow-up (Table 4).
Table 3.
Demographics of Study Population
| Article | Etiology | # of Patients | Gender (M/F) | Age(Years) |
|---|---|---|---|---|
|
| ||||
| Borowitz et al, 1983 [10] | Cystic Fibrosis | 129 ITT | ||
| 117mITT | 71/46 | |||
| 39 Group 1: | 28/11 | 21.3 (8) | ||
| 41 Group 2: | 22/19 | 22.2 (9.3) | ||
| 37 Group 3: | 21/16 | 20.9 (8.3) | ||
|
| ||||
| O'keefe et al, 2001 [12] | Alcohol | 29 | 28/1 | |
| 15 Active Tx. | 49.1(1.8) | |||
| 14 Controls | 57.8(2.1) | |||
|
| ||||
| Safdi et al, 2006 [13] | Chronic Panc. | 27 | 9/18 | |
| 26 Analyzed | ||||
| 12 Tx Group | 3/10 | 51.9(2.7) | ||
| 14 Controls | 6/8 | 51(3.0) | ||
|
| ||||
| Stern et al, 2000 [11] | Cystic Fibrosis | Adults: 36 | 22/14 | |
| 18 Tx Group | 10/8 | 23.3(1.2) | ||
| 18 Controls | 12/6 | 24.2(2.1) | ||
| Children: 38 | 18/20 | |||
| 18 Tx Group | 7/11 | 12.1(0.7) | ||
| 20 Controls | 11/9 | 12.8(0.6) | ||
Table 4.
Assessment of Methodologic Quality of Individual Studies
| Article | Randomized | Concealed Allocation | Double Blind | Complete patient follow-up | Sample Size calculation | CFA before study1 | Controlled timing of fecal fat collection2 | Monitoring fat intake during fecal fat collection.3 | Assessment of Symptom Improvement6 |
|---|---|---|---|---|---|---|---|---|---|
| Borowitz et al, 2006 [10] | Yes | Unkown | Yes | Yes | Yes | Yes-fecal elastase measurement | Yes, used FD&C blue 2 stool marker. | Yes | No |
| O'keefe et al, 2001 [12] | Yes | unknown | placebo group but unclear if investigators blinded | Yes | No | Yes | No | Yes | Yes |
| Safdi et al, 2006 [13] | Yes | Unkown | Yes | Yes | No | Yes | No4 | No | Yes |
| Stern et al, 2000 [11] | Yes | unknown | open-label run-in/ double-blind treatment phase | Yes | No | Yes | Yes, used food dye during double-blind treatment phase | Yes | Yes |
Confirmed fat malabsorption before study enrollment with CFA
Demonstrated appropriate timing of fecal fat collection through the use of stool markers to ensure that the patient monitored diet at the beginning and end of fecal fat collection.
Monitored in an inpatient setting
2 consecutive outpatient phases: a 2 week, single blind, placebo run-in phase (“wash out”) and a 2 week, double blind, treatment phase
Open label phase was to stabilize patient on a high fat diet (100g fat/day) and adjust pancrealipase dose
Assessment of symptom assessment includes stool frequency, stool consistency, abdominal discomfort, and/or global symptom improvement.
Enzyme supplementation improved fat absorption in all studies (Table 5) but the values for mean CFA, FFE, or fecal weight remained abnormal, indicating that steatorrhea was not abolished. This occurred independently of the degree of pancreatic exocrine insufficiency and the prandial enzyme lipase dose delivered (40,000 [12, 13], 100,000[10] or self-adjusted[11] USP units/meal), illustrating incomplete responses to non-uniform therapy. Three studies [11–13] showed that enzyme supplementation reduced stool frequency and improved stool consistency, but changes were statistically significant in only two of the studies [11, 13]. No significant differences in adverse events were identified in any study.
Table 5.
Study Design and Results of RCTs
| Article | Study Design | Key Inclusion (I)/Exclusion (E) Criteria | Tx Time | Formulation per capsule | Dose/Timing/Confounders (Lipase USP) Per Meal | End Point | Results | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Borowitz et al, 2006 [10] |
|
I: Adults
|
14 days | ALTU-135 (Bacterial cross- linked crystallized) | Groupl:5000 Group2:25000 Group3:100000 Meals and snacks. |
72 hr CFA (%) | 72 hr CFA (%) |
|||
| 1 n=39 | 2 n=41 | 3 n=37 | ||||||||
|
| ||||||||||
| Pre-Tx | 55±17.5 | 55.6±20.3 | 52.2±19.1 | |||||||
| On-Tx | 56.2±18.2 | 67±18.1 | 69.7±17.9 | |||||||
| No Diff | SS | SS | ||||||||
|
| ||||||||||
| O'keefe et al, 2001 [12] |
|
I: Age >18
E: Per text |
14 day |
Pancreatin (EC; MMSP) Lipase 10000 USP Amylase 33200 USP Proteases 37500 USP |
40000 Meals 20000 Snack |
CFA (%) | CFA (%) |
|||
| Control n=14 | Tx n=15 | |||||||||
|
| ||||||||||
| Before Tx | 60.28 ± 0.1 | 54.0 ± 9.7 | ND | |||||||
| After Tx | 80.8± 3.8 | SS | ||||||||
|
| ||||||||||
| 72hr FFE (g/day) | 72 hr FFE (g/day) |
|||||||||
| Control n=14 | Tx n=15 | |||||||||
|
| ||||||||||
| Before Tx | 44.3 ± 9.9 | 48.0 ± 10.6 | ND | |||||||
| After Tx | 20.3±4.3 | SS | ||||||||
|
| ||||||||||
| 72 hr Fecal Wt (g/day) | 72 hr Fecal Wt (g/day) |
|||||||||
| Control n=14 | Tx n=15 | |||||||||
|
| ||||||||||
| Before Tx | 379 ± 65 | 360 ± 64 | ND | |||||||
| After Tx | 267 ± 44 | SS | ||||||||
|
| ||||||||||
| Safdi et al, 2006 [13] |
|
I: Age> 18 years
E:Per text |
2 week washout 2 week treatment |
Creon 10 (EC; MMSP) Lipase 10000 USP Amylase 33200 USP Protease 37500 USP |
40000 Meals 20000 Snack |
72hr CFA (%) (SE) | 72 hr CFA (%) (SE) |
|||
| Control n=14 | Tx n=12 | |||||||||
|
| ||||||||||
| Before Tx | 55.9 (3.6) | 49.9 (8.8) | ND | |||||||
| After Tx | 68.0 (4.6) | 86.6 (2.7) | SS | |||||||
|
| ||||||||||
| Daily FFE (g/day) | Daily FFE (g/day) |
|||||||||
| Control n=14 | Tx n=12 | |||||||||
|
| ||||||||||
| Before Tx | 63.1 (7.2) | 75.1 (18.4) | ND | |||||||
| After Tx | 51.8 (9.4) | 18.6 (4.0) | SS | |||||||
|
| ||||||||||
| Stern et al, 2000 [11] |
|
I: Age≥18 years
E: Per Text I:7–18 years Criteria as Above |
1–2 weeks open label 1 weeks double blinded |
Creon 20 (EC; MMSP) Lipase 20000 USP Amylase 66400 USP Protease 75000 USP |
Varying doses to optimize digestion. | 72hr CFA (%) (SEM) | 72 hr CFA (%) (SEM) |
|||
| Control n=18 | Tx n=18 | |||||||||
|
| ||||||||||
| Open Lbl | 87.8 (1.2) | 89.2 (1.1) | ND | |||||||
| DB Tx | 50.9 (7.3) | 87.2 (1.7) | SS | |||||||
|
| ||||||||||
| 72hr CFA (%) (SEM) | ||||||||||
| Control n=18 | Tx n=20 | |||||||||
|
| ||||||||||
| Open Lbl | 86.64 (1.02) | 87.36 (1.14) | ND | |||||||
| DB Tx | 52.15 (5.61) | 84.11 (2.22) | SS | |||||||
Results of Individual RCTs
Borowitz et al.[10] performed a randomized, double-blind, parallel dose-ranging study to determine the most efficacious dose of ALTU-135 (Trizytek™, Altus Pharmaceuticals, Cambridge, MA), a crystallized and cross-linked formulation of microbial pancreatic enzymes, on CFA in 130 CF patients from one of 26 CF Foundation-accredited centers. Eligible patients had severe pancreatic exocrine insufficiency, based on a low fecal elastase value (<100 mg/g), and successful collection of > 75% of stool samples for determining CFA. Patients on PPI were stratified. Enzyme dosing-regimens for lipase were classified as low (5000 USP units/meal), mid (25000 USP units/meal) and high (100000 USP units/meal) and taken with meals and snacks. Outpatient enzymes were discontinued upon admission to an inpatient facility to determine CFA during a high fat diet fecal balance study using a blue dye stool marker (FD&C #2) for indicating the initiation and end of an approximate 72-hour fecal fat collection. Upon discharge, subjects were randomized to one of three 14-day dosing regimens followed by completion of a second fecal fat balance study. Pair wise comparisons revealed that the mid- and highest dose groups had significantly greater mean CFA and CNA compared with low-dose groups (Table 4). Subjects with baseline CFA < 40% had a mean increase of 31% in CFA (p < 0.001). Specific data on improvement in diarrhea, weight gain and quality of life were not reported, although unpublished data on quality of life was collected the using the Cystic Fibrosis Questionnaire-Revised (CFQ-R). Adverse event reporting was detailed and treatment arms had no differences in serious adverse events or laboratory values. Approximately 85% of subjects reported some gastrointestinal adverse event during the trial, and a serious gastrointestinal adverse event was reported in four patients who withdrew from the trial.
O'Keefe et al. [12] studied 29 chronic pancreatitis patients using enteric coated, gastric acid resistant mini-microspheres containing lipase 10,000 USP U/capsule, amylase 33,200 USP U/capsule and proteases 37,500 USP U/capsule (Pancreatin, XX Pharmaceuticals, Anywhere, USA). Patients had chronic pancreatitis diagnosed by typical symptoms and radiographic evidence and had severe pancreatic exocrine insufficiency defined by reduced cholecystokinin-stimulated enzyme secretion or steatorrhea (>10g fat/day). All patients had a fat-balanced diet during the study's three phases: a 7-day study to assess malabsorption (Phase 1) followed by a 7-day run-in period on pancreatic enzyme therapy (Phase 2) and then a randomized, parallel-group 2-week treatment phase with pancreatic enzymes or placebo (Phase 3). Treatment with enzymes or placebo was four capsules with meals and two capsules with snacks. The enzyme treated group (lipase 40,000 USP units/meal) had a greater mean CFA (80.8% vs. 54.0%; p=0.002) and lower FFE (p = 0.003) and fecal nitrogen excretion (p < 0.004). Severity of abdominal pain, abdominal distention, flatulence, stool frequency and stool consistency were assessed with an unspecified scale but no numerical data was provided. The treatment arms had no significant differences in these symptoms, although stool frequency and stool consistency trended toward improvement in the enzyme treatment group. In patients with insulin-dependent diabetes, one patient developed hyperglycemic ketoacidosis after resuming enzymes and two patients experienced symptomatic hypoglycemia after discontinuing enzyme therapy. The authors cautioned that enzyme supplementation may disturb glucose control in insulin-dependent diabetes but provided no other adverse events data.
Safdi et al. [13] conducted a multi-center randomized placebo-controlled trial examining the effect of Creon 10 (Solvay Pharmaceutical, Marietta, GA) which are enteric-coated, delayed release, mini-microsphere pancrelipase capsules (lipase 10,000 USP, Amylase 33,200 USP, Protease 37,500 USP) on steatorrhea and symptom scores in 27 patients with chronic pancreatitis. The authors did not specify criteria for diagnosing chronic pancreatitis, but eligibility for enrollment required a ≥ 12-month history of severe pancreatic exocrine insufficiency, prior enzyme supplementation of ≥ 6-months with satisfactory symptom control and a mean CFA < 80% or fecal fat values > 10 g/d during the two-week run-in phase (when all patients received placebo). During the run-in and treatment phases, all patients had a diet of ≥100 g fat daily for 6 days and had stool samples collected during the last 72 hours of this period. Only 27 of 64 patients met enrollment criteria for the two week double blind treatment phase, when they took four capsules with meals and two with snacks. Enzyme treated patients had greater mean CFA (86.6% vs. 68.0%; p=0.0185) and significantly reduced FFE. Stool frequency and stool consistency were quantified by the patient and global symptom improvement was assessed by both the physician and the patient. The enzyme treatment group had reduced stool frequency, improved stool consistency and greater physician-assessed global symptom improvement. Adverse event reporting was detailed and there were no serious adverse events, no patient withdrawals from the trial due to adverse events and no differences in abnormal lab values between the treatment and placebo groups.
Stern et al.[11] studied the effect of Creon 20 (Solvay Pharmaceutical, Marietta, GA) which are enteric-coated, delayed release, mini-microsphere pancrelipase capsules (lipase 20,000 USP, Amylase 66,400 USP, Protease 75,000 USP) on fat absorption in 38 pediatric and 36 adult CF patients with steatorrhea. Eligible patients had CF diagnosed by sweat chloride testing and clinical symptoms of steatorrhea and had a mean CFA < 80% or fecal fat values > 10 g/d. During the open-label phase of the study, all patients self-adjusted the dose of enzymes to optimize digestion while maintaining a high-fat (100 g/day) diet. During the double-blind treatment phase, patients received the same diet with 5–7 days of the self-adjusted dose of enzyme supplement or placebo. Enzyme treatment increased mean CFA in adult (87.2% vs. 50.9%; p<0.001) and pediatric (84.1% vs. 52.2%; p<0.001) patients. Stool frequency and stool consistency were quantified by the patient and global symptom improvement was assessed by the physician. The enzyme treatment group had reduced stool frequency,improved stool consistency and greater physician-assessed global symptom improvement. Adverse event reporting was detailed and there were fewer treatment-emergent adverse events in the enzyme treatment arm, no GI serious adverse events, and one patient withdrawal from the trial due to GI adverse events and no differences in abnormal lab values between groups.
DISCUSSION
The FDA has noted variability in composition, enzymatic activities, formulation, stability, and bioavailability of available pancreatic enzyme supplements and stated that these differences have led to highly variable pancreatic enzyme preparation quality and therapeutic performances[1]. Therefore, the FDA determined that published data about pancreatic enzyme supplements was insufficient, new randomized controlled trial (RCT) data would need to be submitted as part of NDAs for any marketed pancreatic enzyme supplements, and new data would need to demonstrate the efficacy and safety of these agents, consistency in manufacturing, and stability/shelf-life of these agents. Therefore, we performed a systematic review to validate the FDA's conclusion that published data about pancreatic enzyme supplements was insufficient. Through this review, we also sought to determine the strengths and limitations of study design and results from published parallel-design RCTs, identify gaps in the current literature, outline the design of additional studies which would clarify optimal therapy, and provide an up-to-date review of the existing parallel-design RCT literature prior to publication of new RCTs required by the FDA.
Our systematic review found that only four well-designed, parallel-group, placebo-controlled RCTs have been published. In these trials, enzyme supplementation improves coefficient of fat malabsorption (CFA) compared to placebo, but fat malabsorption remained despite enzyme supplementation, indicating that steatorrhea was not abolished. Stool frequency and stool consistency improved with enzyme supplementation, but no studies assessed the impact of enzyme supplementation on weight gain because the trials were too brief. No significant differences in adverse events were identified between placebo-treated groups and enzyme-treated groups, but the size of these four RCTs (n = 246 total patients) limit conclusions about the safety and tolerability of these agents. No studies performed a head-to-head comparison of different pancreatic enzyme supplements and the available studies had important differences in study design. Therefore, direct comparisons about the efficacy and safety of different agents cannot be performed. Overall, insufficient data is available to determine optimal treatment to maximize fat absorption, improve symptoms or minimize adverse events. Considering that these supplements are a cornerstone of treatment for patients with chronic pancreatitis and steatorrhea, it is surprising that so little published RCT data supports their safety and efficacy and the FDAs conclusion that published data is insufficient appears to be appropriate.
Our review allowed us to identify methodologic issues which may complicate the study of pancreatic enzyme supplementation. First, diagnosis of pancreatic exocrine insufficiency in chronic pancreatitis patients is challenging because there are no universally accepted diagnostic criteria, and patients typically don't develop severe exocrine pancreatic insufficiency for years after diagnosis of alcohol-induced chronic pancreatitis: 8–22% at time of diagnosis, 44–48% after 13–26 years and 91–100% after 14–36 years [Also include reference 5][6]. This is a crucial issue because many patients labeled with pancreatic exocrine insufficiency due to alcohol-induced chronic pancreatitis and treated with supplements may not actually have steatorrhea. In the study by Safdi et al.[13], all 64 patients who entered the run-in phase had previously received supplements for pancreatic insufficiency, but only 27 had fat malabsorption after careful testing. This illustrates the importance of using 72 fecal fat collection as an inclusion criteria in future trials in order to confirm steatorrhea. Conversely, CF patients are probably a more homogenous population because diagnostic criteria are clearly defined and 80% of patients have severe pancreatic exocrine insufficiency at birth. Second, the technique for performing 72 fecal fat collections may vary considerably, and quantification of steatorrhea may be altered by differences in stool collection and the quantity of fat in diets. One possible, albeit costly, solution would be to admit patients to clinical research centers where dietary fat intake can be controlled and the timing of the fecal fat collection during monitored dietary fat intake can be confirmed by use of a stool dye marker (e.g., FD&C, Blue #2). Third, most RCTs of pancreatic enzyme supplementation are only 14 days long which is not adequate time to assess the impact of supplements on weight gain. These trials may also be too brief to fully assess the safety and tolerability of these supplements, although long-term, open-label safety trials would also meet this need. Finally, there are no head-to-head comparisons of different enzyme supplements, and, due to important differences in study design, it is not appropriate to compare CFAs of different supplements across studies. Given these limitations, it is difficult to identify the optimal agent/dose of pancreatic enzyme supplementation.
In order to identify the optimal agent/dose of pancreatic enzyme supplements, pancreatic enzymes should contain a stable and quantifiable dose of pancreatic enzyme. This has been recommended in the FDAs guidance [1], but none of the trials in our systematic review specifically assessed the quantity or stability of porcine pancreatic enzyme in study capsules. Producing capsules with a stable and quantifiable dose of porcine pancreatic enzyme may be quite important for pediatric cystic fibrosis patients, who commonly receive individualized dosing of pancreatic enzyme supplements. With growth failure, pediatricians may prescribe dosages of pancreatic enzyme supplements that are higher than recommended (> 10,000 units of lipase per kilogram) and higher doses of pancreatic enzyme are associated with a 10-fold increase in the risk of fibrosing colonopathy which frequently requires colectomy [14]. This issue could be minimized if the quantity and stability of porcine enzyme supplement in capsules were standardized. Unfortunately, the FDA Guidance emphasized that the dosage and stability of porcine pancreatic enzymes varies considerably [14]. Furthermore, “since high doses of pancreatic enzymes have been associated with safety problems, the finished product should be formulated to 100 percent of the label-claimed lipase enzyme activity” [1]. In contrast to this recommendation, past manufacturing practices have allowed “averages” where 65% more enzyme is provided in a capsule than is described on a label in order to extend the shelf-life of porcine pancreatic enzyme supplements. This mismatch between enzyme package label and actual quantities of active porcine enzyme presents an additional challenge to individualizing therapy. Given these issues, the FDA Guidance states that “due to the inherent lability that has been observed in pancreatic enzyme preparations, stability data through 12 months at recommended storage temperature…should be provided” and also recommends verification of the labeled quantity of porcine enzyme in capsules. Again, no RCTs in our review studied these issues, although future RCTs should study these issues as secondary endpoints in their RCTs and seek to determine if these variations impact efficacy and safety of supplements.
This review is limited by several factors. First, because there is minimal published data, our conclusions about the efficacy of pancreatic enzyme supplements for fat malabsorption are limited. Second, we do not have access to the results of on-going RCTs of different enzyme supplements. These RCTs will provide the foundation for NDA submissions to the FDA in order to gain marketing approval. Nevetheless, we think it is important to systematically review the current RCT data about these treatments because it will serve as a foundation to assess the study design, efficacy, and safety of on-going RCTs. Third, we excluded randomized cross-over studies from our review. This is almost certainly the most controversial aspect of our systematic review. The use of randomized cross-over studies is problematic because important intra-subject variability has been demonstrated during test-retest studies in the same patient [15, 16]. However, it could be argued that it is unclear if intra-subject variability of pancreatic exocrine function is greater than inter-subject variability and that cross-over studies provide helpful data. However, we designed this systematic review to determine if adequate data was available to confirm the efficacy and safety of pancreatic enzyme supplements based upon the FDA's standards for study design, and the use of cross-over studies was strongly discouraged in the FDA's “Guidance for Industry Exocrine Pancreatic Insufficiency Drug Products-Submitting NDAs” [1]. In this document, the FDA advised against using cross-over trials when stating “patients should first be stabilized on existing therapy to establish baseline conditions… if baseline conditions are not re-established between treatment periods [in a cross-over study], or if treatment in one period carries over into the subsequent period, the results likely will not be interpretable.” Proper establishment of baseline conditions would probably require standardized quantification of steatorrhea with use of dye markers (e.g., FD&C, Blue #2) to identify the beginning and end of the 72 hour stool collection and monitored dietary fat intake during and in-between treatment phases. The vast majority of randomized cross-over studies do not meet these criteria and would not be acceptable for inclusion in a NDA submissions to the FDA. Therefore, we feel justified in excluding cross-over studies from the current systematic review, although it may be appropriate to perform a separate systematic review of cross-over trials in the future to fully assess all randomized trial data of pancreatic enzyme supplements.
In conclusion, our systematic review indicates that only four well-designed, parallel-group, placebo-controlled RCTs have been published about the efficacy and safety of pancreatic enzyme supplementation in chronic pancreatitis patients with steatorrhea. The published trials demonstrate that enzyme supplementation improves CFA compared to placebo, but fat malaborption is still present after enzyme supplementation. Stool frequency and consistency also improve with supplementation, but no data on weight gain/loss is provided and minimal data on adverse events is available. None of these trials provide data about stability of porcine enzyme preparations over 12 months, bioavailability, or batch-to-batch consistency in quantity of enzyme supplement per capsule which may also impact the efficacy and safety of the supplements. Important differences in patient population, pancreatic enzyme dosage, and quantification of steatorrhea were present across trials and no head-to-head trials of different enzyme supplements have been performed. Therefore, direct comparisons about the efficacy of different agents cannot be performed and insufficient data is available to determine optimal pancreatic enzyme supplementation.
Acknowledgments
Financial Support: The writing of this manuscript was supported by an unrestricted grant from Altus Pharmaceuticals. The authors are solely responsible for all aspects of this systematic review, including data analysis and writing of the manuscript.
Dr. DiMagno is supported by KO8 DK073298 (M.J.D.).
REFERENCES
- 1.Tang C, Biemond I, Lamers CB. Cholecystokinin receptors in human pancreas and gallbladder muscle: a comparative study [see comments] Gastroenterology. 1996;111(6):1621–6. doi: 10.1016/s0016-5085(96)70025-2. [DOI] [PubMed] [Google Scholar]
- 2.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 3.Raimondo M, Wallace MB. Diagnosis of early chronic pancreatitis by endoscopic ultrasound. Are we there yet? JOP. 2004;5(1):1–7. [PubMed] [Google Scholar]
- 4.Axon AT, et al. Pancreatography in chronic pancreatitis: international definitions. Gut. 1984;25(10):1107–12. doi: 10.1136/gut.25.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullhaupt B, Truninger K, Ammann R. Impact of etiology on the painful early stage of chronic pancreatitis: a long-term prospective study. Z Gastroenterol. 2005;43(12):1293–301. doi: 10.1055/s-2005-858733. [DOI] [PubMed] [Google Scholar]
- 6.Layer P, et al. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107(5):1481–7. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 7.Homma T, Harada H, Koizumi M. Diagnostic criteria for chronic pancreatitis by the Japan Pancreas Society. Pancreas. 1997;15(1):14–5. doi: 10.1097/00006676-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998;132(4):589–95. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 9.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Borowitz D, et al. Study of a novel pancreatic enzyme replacement therapy in pancreatic insufficient subjects with cystic fibrosis. J Pediatr. 2006;149(5):658–662. doi: 10.1016/j.jpeds.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Stern RC, et al. A comparison of the efficacy and tolerance of pancrelipase and placebo in the treatment of steatorrhea in cystic fibrosis patients with clinical exocrine pancreatic insufficiency. Am J Gastroenterol. 2000;95(8):1932–8. doi: 10.1111/j.1572-0241.2000.02244.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe SJ, Cariem AK, Levy M. The exacerbation of pancreatic endocrine dysfunction by potent pancreatic exocrine supplements in patients with chronic pancreatitis. J Clin Gastroenterol. 2001;32(4):319–23. doi: 10.1097/00004836-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Safdi M, et al. The effects of oral pancreatic enzymes (Creon 10 capsule) on steatorrhea: a multicenter, placebo-controlled, parallel group trial in subjects with chronic pancreatitis. Pancreas. 2006;33(2):156–62. doi: 10.1097/01.mpa.0000226884.32957.5e. [DOI] [PubMed] [Google Scholar]
- 14.FitzSimmons SC, et al. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336(18):1283–9. doi: 10.1056/NEJM199705013361803. [DOI] [PubMed] [Google Scholar]
- 15.Francisco MP, et al. Ranitidine and omeprazole as adjuvant therapy to pancrelipase to improve fat absorption in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(1):79–83. doi: 10.1097/00005176-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Borowitz D, Konstan MW, O'Rourke . Coefficient of Fat and Nitrogen Absorption in Healthy Subjects and Individuals with Cystic Fibrosis. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Layer P, Keller J, Lankisch PG. Pancreatic enzyme replacement therapy. Current Gastroenterology Reports. 2001;3(2):101–8. doi: 10.1007/s11894-001-0005-8. [DOI] [PubMed] [Google Scholar]