Abstract
Obesity is an important risk factor for type 2 diabetes (T2D). Weight loss improves the major factors involved in the pathogenesis of T2D, namely insulin action and β-cell function, and is considered a primary therapy for obese patients who have T2D. Unfortunately, most patients with T2D fail to achieve successful weight loss and adequate glycemic control from medical therapy. In contrast, bariatric surgery causes marked weight loss and complete remission of T2D in most patients. Moreover, bariatric surgical procedures that divert nutrients away from the upper gastrointestinal tract are more successful in producing weight loss and remission of T2D than those that simply restrict stomach capacity. Although upper gastrointestinal tract bypass procedures alter the metabolic response to meal ingestion, by increasing early post-prandial plasma concentrations of glucagon-like peptide 1 and insulin, it is not clear whether these effects make an important contribution to long-term control of glycemia and T2D once substantial surgery-induced weight loss has occurred. Nonetheless, the effects of surgery on body weight and metabolic function indicate that bariatric surgery should be part of the standard therapy for T2D. More research is needed to advance our understanding of the physiological effects of different bariatric surgical procedures and possible weight loss-independent factors that improve metabolic function and contribute to the resolution of T2D.
Keywords: insulin sensitivity, beta-cell function, insulin secretion, roux-en-Y gastric bypass
The risk of developing type 2 diabetes mellitus (T2D) increases linearly with body mass index (BMI).1,2 Accordingly, the increase in the prevalence of obesity is likely responsible for the recent increase in T2D,3,4 which has become a major global health problem because of its high prevalence, causal relationship with serious medical complications and economic impact.
The pathogenesis of T2D involves both multi-organ insulin resistance and inadequate insulin secretion by pancreatic β-cells, leading to fasting and postprandial hyperglycemia. Normal glucose tolerance requires an appropriate integration of the metabolic response to an oral glucose challenge, specifically an appropriate increase in insulin secretion, insulin-mediated suppression of endogenous (primarily hepatic) glucose production, and insulin-mediated stimulation of glucose uptake by peripheral tissues (primarily skeletal muscle).5 All of these components are usually defective in patients who have T2D.6,7 Impaired fasting glucose and impaired glucose tolerance are involved in the transition from normal glucose tolerance to overt T2D, and are important independent risk factors for developing diabetes.8 Currently, the medications used to treat T2D are targeted toward improving β-cell function, increasing insulin sensitivity, or both. Despite an impressive armamentarium of available diabetes medications, 50% of patients with T2D fail to achieve adequate glycemic control, defined by the American Diabetes Association as a glycated hemoglobin (HbA1c) <7%, with medical therapy.9,10 In contrast, bariatric surgery results in complete resolution of T2D, usually defined as maintaining a normal fasting blood glucose concentration or HbA1c <7% after discontinuation of all diabetes medications, in most patients.11
Common bariatric surgical procedures and effects on body weight
Five bariatric surgical procedures are considered acceptable therapy for appropriate patients: 1) roux-en-Y gastric bypass (RYGB); 2) laparoscopic adjustable gastric banding (LAGB); 3) laparoscopic sleeve gastrectomy (LSG), and 4) bilopancreatic diversion (BPD) and 5) BPD with duodenal switch (DS) (Figure 1). Bariatric surgery causes weight loss by inducing a negative balance between energy intake (or absorption) and energy expenditure. The composite of data from a series of studies found that both resting and total daily energy expenditure decrease after bariatric surgery-induced weight loss, and that the decline in energy metabolism is appropriate for the change in body size and composition.12–15 Therefore, weight loss after RYGB, LAGB and LSG procedures is primarily due to a decrease in energy intake because there is little or no malabsorption after these procedures,16 and weight loss after BPD is due to a combination of decreased energy intake and malabsorption. However, weight loss efficacy is not the same among procedures. Data from a meta-analysis of more than 2,000 patients found that percent excess weight loss (i.e. total weight loss divided by baseline excess weight) at 2 years after surgery was 48%, 62% and 70% for LAGB, RYGB and BPD/BPD-DS, respectively.17 Weight loss achieved in patients treated with LSG is similar to those treated with RYGB.18–20
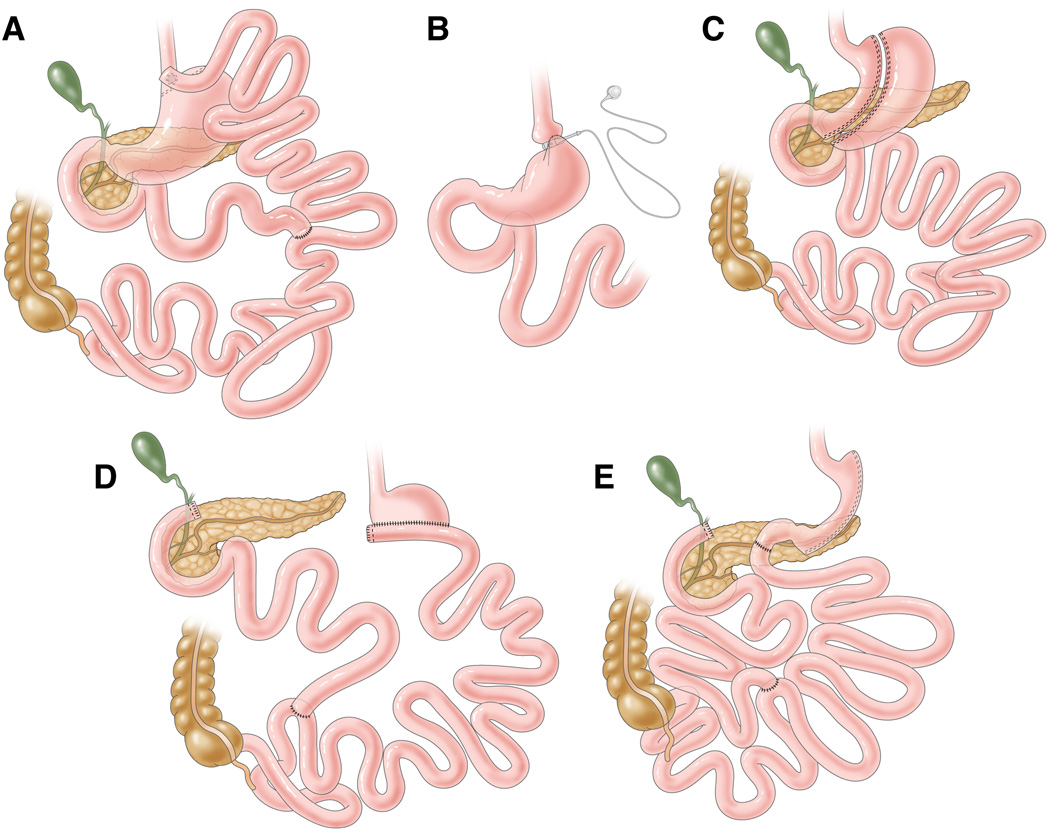
Figure 1.
Standard bariatric surgery procedures. (A) roux-en-Y gastric bypass, (B) laparoscopic adjustable gastric banding, (C) sleeve gastrectomy, (D) biliopancreatic diversion, (E) biliopancreatic diversion with duodenal switch. Roux-en-Y gastric bypass involves the creation of a small gastric pouch (<30 mL) that is connected to a segment of jejunum, which has been transected at 30–75 cm from the Ligament of Treitz, to form a Roux-en-Y limb. Bowel continuity is restored via an anastomosis between the “Roux” limb and the excluded biliopancreatic limb approximately 75–150 cm distal to the gastro-jejunostomy. Therefore, ingested food bypasses most of the stomach, the entire duodenum and a short segment of the jejunum. Laparoscopic adjustable gastric banding involves placing a silicone ring with an inflatable inner tube is placed around the upper stomach, just distal to the gastroesophageal junction. The inner tube is connected to a subcutaneous port, which is used to inject or withdraw saline to adjust the band diameter. Typically, six adjustments are made in the first year after band placement, as needed to enhance weight loss. Sleeve gastrectomy was originally intended as a first-stage procedure of BPD in high-risk patients, but has now become a stand-alone operation and is increasing in popularity. This procedure involves dividing the stomach along its vertical length in order to create a slender banana-shaped sleeve, and removing ~75% of the stomach. Biliopancreatic diversion involves a horizontal gastrectomy, leaving behind 200–500 mL of stomach, which is anastomosed to the small intestine, 250 cm from the ileocecal valve. The excluded biliopancreatic limb is anastomosed to the ileum, 50 cm from the ileocecal valve. The distal 50-cm common channel is where digestive secretions from the biliopancreatic limb mix with the ingested food delivered by the alimentary limb. Biliopancreatic diversion with duodenal switch involves constructing a 150–200 mL volume vertical sleeve gastrectomy with preservation of the pylorous and formation of a duodenal-ileal anastomosis. The excluded biliopancreatic limb is anastomosed to the ileum, 100 cm from the ileocecal valve, where digestive secretions and nutrients mix. These latter two procedures cause considerable malabsorption.
Effect of bariatric surgery on type 2 diabetes
The therapeutic superiority of bariatric surgery over medical therapy has been demonstrated in three 1-yr or 2-yr prospective randomized controlled trials.21–23 In one study, 73% of patients who had LAGB, while only 13% in the medical and lifestyle therapy group, achieved remission of T2D (defined as fasting plasma glucose concentration <126 mg/dL and HbA1c <6.2% without diabetes medications).21 In the second study, 42% of subjects who had RYGB surgery and 37% who had LSG, while only 12% of subjects treated with intensive medical therapy achieved diabetes remission (defined as HbA1c <6% with or without diabetes medications).22 In the third study, 95% of subjects who had BPD and 75% of those who had RYGB surgery (with the same weight loss), but no subjects randomized to conventional medical therapy, achieved remission of T2D (defined as fasting plasma glucose concentration <100 mg/dL and HbA1c <6.5% for at least 1 yr without diabetes medications).23 Data from a meta-analysis of 27 studies that evaluated medical outcomes after LSG found that diabetes resolved in 66% of patients.24 The results from most,18,19,22,25–27 but not all,20,28,29 studies that compared LSG with other therapeutic interventions found T2D remission rates after LSG were the same as those achieved after RYGB surgery,18,19,22,25–27 but greater than those achieved after conventional medical therapy,30,31 or LAGB.27,32 The clinical improvement in T2D after bariatric surgery results in full recovery of the cost of surgery within a few years, primarily because of the decreased need for medications used to treat diabetes and its associated complications.
Retrospective analyses of data from patients who have had RYGB surgery have identified specific characteristics that predict the response of T2D to surgery-induced weight loss.33–37 Several factors have been associated with treatment failure, including longer duration of T2D, inadequate postoperative weight loss, more severe diabetes requiring insulin therapy before surgery, and older age. In addition, early remission does not necessarily translate to long-term success, and relapse can occur. Data from the Swedish Obese Subjects (SOS) study, which evaluated the effect of bariatric surgery in 4,047 patients with T2D, found one-half of the 72% of subjects that achieved remission at 2 years after surgery remained in remission at 10 years.38 Another study conducted in 177 patients with T2D who had RYGB surgery, also found that nearly half of the 89% of patients who achieved an initial remission of T2D, experienced subsequent disease recurrence.37 The precise reasons for recurrence of diabetes are not known, but are likely related to recidivism of weight loss because relapse or worsening of T2D is associated with weight regain. In contrast, data from one study found 83% of patients who had RYGB surgery maintained weight loss and complete resolution of T2D at 14 years after surgery.39
An important confounding factor in interpreting the efficacy of bariatric surgery in treating T2D is the absence of a uniform definition of diabetes “remission" or “resolution,” and different criteria have been used in different studies. Remission has most often been defined as the withdrawal of all diabetes medications, in conjunction with a "normal" fasting plasma glucose concentration (ranging from <100 to <126 mg/dL), and/or a “normal” HbA1c (ranging from <6% to <7%).40 Obviously, differences in the definition of remission among studies will lead to differences in estimated remission rates.41,42 Nonetheless, remission of T2D can only occur by improving the major metabolic defects involved in the pathogenesis of T2D, namely insulin resistance and inadequate β-cell function.
Insulin Sensitivity
Assessment of insulin action
Insulin regulates a large number of metabolic functions across various organ systems. Therefore, reduced sensitivity to the action of insulin (i.e. insulin resistance) represents a suboptimal biological response of different metabolic pathways to normal circulating insulin concentrations.43 However, it has become common to define insulin sensitivity by its effects on glucose metabolism alone, particularly the ability of insulin to stimulate glucose disposal, which occurs primarily in skeletal muscle during hyperinsulinemia.
Different methods have been used to evaluate the effect of bariatric surgery on insulin sensitivity. These methods can be classified into tests that are based on: 1) basal plasma insulin and glucose concentrations, 2) the response to glucose or meal ingestion, 3) the response to intravenous glucose and insulin injection, and 4) the response to a continuous insulin infusion. Mathematical indices, based on the relationship between basal plasma glucose and insulin concentrations, are most commonly reported in bariatric surgery studies because they only require obtaining a fasting blood sample. The most commonly reported index is the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) score, which is the product of basal plasma insulin and glucose concentrations.44,45 However, static measures do not provide a robust assessment of insulin sensitivity because they are influenced by factors that are not related to insulin action, including impaired β-cell function, recent alterations in energy balance, and the absence of a standardized method for measuring plasma insulin concentration.46–51 More reliable assessments of insulin sensitivity are based on the response to a metabolic challenge. Measurement of plasma C-peptide, insulin and glucose concentrations after ingestion of an oral glucose load (oral glucose tolerance test, OGTT) or a mixed meal (mixed meal test) provides information on the total change in plasma concentrations as the area-under-the-curve (AUC) and allows for more intricate mathematical derivations to calculate an insulin sensitivity index.52–61 The intravenous glucose tolerance test (IVGTT) involves injecting a bolus of glucose followed by a bolus of insulin or an insulin secretagogue, and using the “minimal model” (mathematical model with the fewest parameters that provide a good fit to the plasma glucose and insulin/C-peptide concentration data) to estimate an insulin sensitivity index (Si).62–65 The insulin suppression test involves an intravenous infusion of somatostatin or a somatostatin analog to block pancreatic insulin secretion, followed by an infusion of insulin and glucose at constant rates for 3 hours.66,67 The measurement of steady-state plasma glucose concentration provides an index of insulin-mediated glucose uptake and is a marker of skeletal muscle insulin sensitivity. The insulin tolerance test involves modeling the plasma glucose response to a bolus injection of insulin to determine glucose disappearance rate.68–70 Finally, the hyperinsulinemic-euglycemic clamp (HEC) procedure involves a continuous infusion of insulin in conjunction with the infusion of dextrose at a variable rate to maintain a constant normal plasma glucose concentration (usually 90–100 mg/dL).71 The rate that glucose is infused to maintain euglycemia equals the rate that glucose is being removed from the circulation, primarily due to skeletal muscle glucose disposal. Therefore, the glucose infusion rate approximates the total rate of glucose disposal, but does not take into account the contribution from endogenous glucose production. Infusing an isotopically-labeled glucose tracer during the HEC procedure permits the assessment of total insulin-stimulated glucose uptake, derived from both endogenous glucose production and exogenous glucose infusion.72,73
Effect of bariatric surgery on insulin sensitivity
Calorie restriction and subsequent weight loss can have potent effects on insulin sensitivity. However, the effect of calorie restriction on insulin action in liver and skeletal muscle depends on the degree and duration of the energy deficit. Ingestion of a low-calorie (~1100 kcal/day) diet for only 48 h decreases intrahepatic triglyceride content and the HOMA-IR score, and increases hepatic insulin sensitivity, but does not affect skeletal muscle insulin action in obese subjects.74 Moreover, moderate diet-induced weight loss (6%–8%) improves hepatic, but not skeletal muscle, insulin sensitivity,75,76 whereas greater diet-induced weight loss is usually required to increase skeletal muscle insulin sensitivity.77 The results from these studies demonstrate that short-term calorie restriction has profound effects on liver metabolic function, but long-term calorie restriction with greater weight reduction is needed to affect skeletal muscle insulin action.
The effect of bariatric surgery-induced weight loss on insulin sensitivity has been evaluated in a large number of studies that used different methods to assess insulin action. Assessment of HOMA-IR score is the most commonly reported outcome measure. A recent meta-analysis of the results from 45 studies that evaluated HOMA-IR after RYGB, LAGB, LSG, and BPD surgeries found HOMA-IR score decreases within days and remains suppressed for more than 18 months after surgery (Figure 2).78 The results from most,50,79–86 but not all,87 studies demonstrate that short-term calorie restriction and minimal to moderate weight loss (≤10% of initial body weight) induced within days to weeks after RYGB surgery causes a similar decline in HOMA-IR score as a matched amount of weight loss induced by a low-calorie diet. In addition, data from most,81,88–92 but not all,93 studies have shown that the relative decrease in HOMA-IR score after surgery is about the same among different bariatric surgical procedures when matched for weight loss across procedures.
Figure 2.
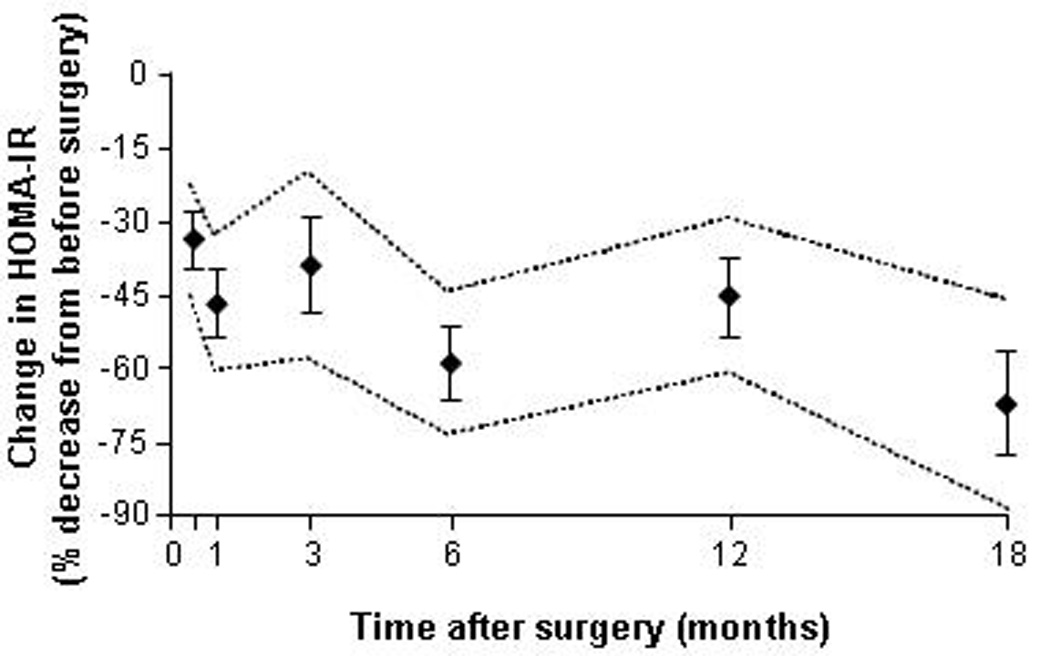
Changes in insulin sensitivity evaluated by using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) score at various time points after bariatric surgery (LAGB, RYGB, BPD and LSG). Data are weighed means±SE and dashed lines represent upper and lower 95% confidence intervals. Changes in HOMA-IR versus baseline (before surgery) are significant at all time points, P <0.0001. Data adapted from Rao et al.78
A series of studies evaluated the effect of bariatric surgery on insulin sensitivity based on the response to an oral glucose load or mixed meal challenge. The interpretation of the plasma glucose and insulin responses to glucose or meal ingestion is confounded after intestinal bypass procedures because of the rapid absorption of glucose and the subsequent spike in systemic glucose and insulin concentrations caused by the alteration in upper gastrointestinal (UGI) tract anatomy. The results from most studies show that marked weight loss (>15%) induced by any bariatric surgical procedure improves insulin sensitivity, determined by using mathematical modeling of plasma glucose and insulin or C-peptide responses to glucose or meal ingestion,94-99 or assessed as a decrease in the AUC for plasma glucose with or without a concomitant decrease in plasma insulin AUC.92,94,95,97,99–109 In addition, the long-term improvement in insulin sensitivity, evaluated by using mathematical modeling of OGTT data, correlates directly with weight loss.95 The effect of LAGB and RYGB on insulin sensitivity derived from oral glucose or meal ingestion when weight loss is ≤11% is unclear because of different results among studies.79,80,82,106,110,111 In contrast, a consistent decrease in glucose and insulin AUCs is observed early after BPD (1–4 weeks after surgery and 4%–6% weight loss).110,112–114
Data from studies that used the IVGTT to evaluate insulin sensitivity show significant improvements in Si in subjects who lost, on average, at least 17% of their initial body weight after bariatric surgery (Table 1).48,81,113,115–123 Although the effects of different surgical procedures on Si among subjects who lost less than 15% of their initial body weight are not entirely consistent, most studies show no significant change after LAGB or RYGB,48,81,115,117,122 but a marked improvement after BPD.113 We are aware of only one study that compared the effect of different surgical procedures on Si after the same amount of weight loss (~8% of initial body weight).81 In that study, a statistically significant improvement in Si was detected in nondiabetic subjects after LAGB surgery and in subjects with T2D after RYGB surgery, but not in nondiabetic subjects after RYGB. Another study found significant improvements in Si after RYGB and LAGB at 6 months after surgery, but no significant difference between surgical groups.117 The small numbers of subjects enrolled in the studies that used the IVGTT to assess insulin sensitivity (all had ≤15 subjects) and the variability in the dynamics of insulin action during an IVGTT124 affect the ability of these studies to detect significant effects of moderate weight loss or differences between surgical procedures on Si.
Table 1.
Effect of bariatric surgery-induced weight loss on insulin sensitivity assessed by using an intravenous glucose tolerance test (IVGTT) or the hyperinsulinemic-euglycemic clamp (HEC) procedure (values are percent change from before surgery values).
| LAGB/Gastroplasty | Roux-en-Y Gastric Bypass | Biliopancreatic Diversion | ||||
|---|---|---|---|---|---|---|
| Weight loss | IVGTT (Si) | HEC (GIR) | IVGTT (Si) | HEC (GIR) | IVGTT (Si) | HEC (GIR) |
| 3–14% | −3 (n=6)117 | −11 (n=15)117 | −26 (n=6)51 | 160 (n=9 T2D)113 | 85 (n=7 T2D)133 | |
| 63 (n=8)81 | 3 (n=27 wT2D)48 | −8 (n=7)51 | 96 (n=10 T2D)112 | |||
| 32 (n=15)115 | −3 (n=12)50 | 99 (n=10 T2D)112 | ||||
| 35 (n=11 T2D)117 | −3 (n=6 T2D)51 | 146 (n=9 T2D)113 | ||||
| 38 (n=9)81 | ||||||
| 58 (n=7 T2D)81 | ||||||
| 17–27% | 130 (n=5)117 | 66 (n=10)91 | 59 (n=18)117 | 23 (n=10)132 | 135 (n=8)132 | |
| 64 (n=27 wT2D)48 | 42 (n=17 wT2D)131 | |||||
| 66 (n=10 T2D)117 | 47 (n=10)91 | |||||
| 161 (n=15)115 | 67 (n=6)130 | |||||
| 84 (n=12)50 | ||||||
| 30–49% | 128 (n=12)116* | 150 (n=8)136* | 109 (n=7 T2D)122 | 46 (n=12)129 | 110 (n=7 T2D)133 | |
| 219 (n=8)122 | 74 (n=10)132 | 116 (n=8 T2D)134 | ||||
| 80 (n=13)128 | 146 (n=107 wT2D)135 | |||||
| 158 (n=17 wT2D)131 | 208 (n=8)132 | |||||
| 169 (n=13 T2D)129 | ||||||
LAGB = laparoscopic adjustable gastric banding; GIR = glucose infusion rate in µmol/kg fat-free mass·min; Si = insulin sensitivity index.
wT2D = study subjects included patients with type 2 diabetes; T2D = all study subjects had type 2 diabetes.
Vertical gastroplasty procedure.
Data from selected studies were not included because: 1) data from different surgical procedures were combined together,118,119 2) necessary data were missing,27,94,120,121,123,137 3) a hyperglycemic rather a euglycemic clamp procedure was used to assess insulin sensitivity,93 and 4) data from a study132 included in the table were reported also as part of other studies.96,138
Few studies have assessed insulin sensitivity after bariatric surgery by using the insulin suppression test or the insulin tolerance test. The results from these studies found no change in skeletal muscle insulin sensitivity (assessed as steady-state plasma glucose concentration or glucose disappearance rate) after minimal to moderate weight loss (<12%) induced by RYGB, but an improvement after marked weight loss (~30%).125–127
The HEC procedure has been used to evaluate skeletal muscle insulin sensitivity before and after weight loss induced by LAGB, RYGB, and BPD surgeries.27,50,51,93,94,96,112,113,128–138 The results from these studies demonstrate that skeletal muscle insulin sensitivity does not change after moderate (~10%) weight loss, 2–4 weeks after RYGB surgery,50,51 but increases markedly after greater (20%–40%) weight loss, 6–16 months after surgery.50,128–132,134,137 A similar improvement in insulin sensitivity (~150%) has been reported after marked weight loss (~33%) induced by vertical banded gastroplasty.136 In addition, we have found that 20% weight loss induced by either LAGB or RYGB causes nearly a two-fold increase in skeletal muscle insulin sensitivity, assessed by using the HEC procedure in conjunction with stable isotopically-labeled tracer infusion, but there was no difference between the two surgical groups.91 In contrast with the observations made after RYGB surgery, BPD improves skeletal muscle insulin sensitivity rapidly after minimal (<10%) weight loss.56,57,94,118,119,133–141 However, additional (up to 50%) weight loss causes only a small further increase in insulin-stimulated glucose disposal (Table 1 and Figure 3).50,51,91,112,113,128–136
Figure 3.
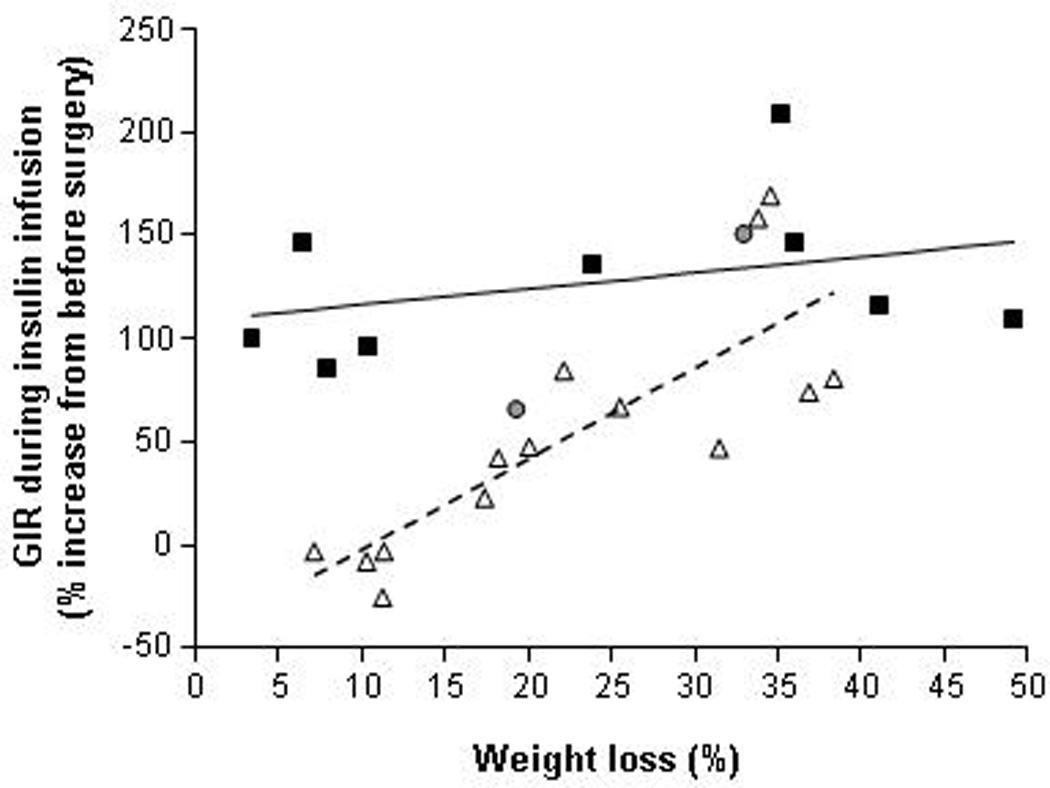
Relationship between percent weight loss and the change in insulin sensitivity, assessed as the relative increase in insulin-mediated glucose disposal (% change in glucose infusion rate [GIR] in µmol/kg fat-free mass per min) during a hyperinsulinemic-euglycemic clamp procedure (insulin infusion rate of 40–50 mU/m2 body surface area per minute) in subjects who had RYGB (white triangles), LAGB/gastroplasty (grey circles) or BPD (black squares) surgeries. Each data point represents the average change insulin sensitivity at a specific average percent weight loss within a study, calculated by using group mean values from published studies that reported adequate data to make these calculations.50,51,91,112,113,128–136 The linear regression lines for RYGB (dashed black) and BPD (continuous black) are shown.
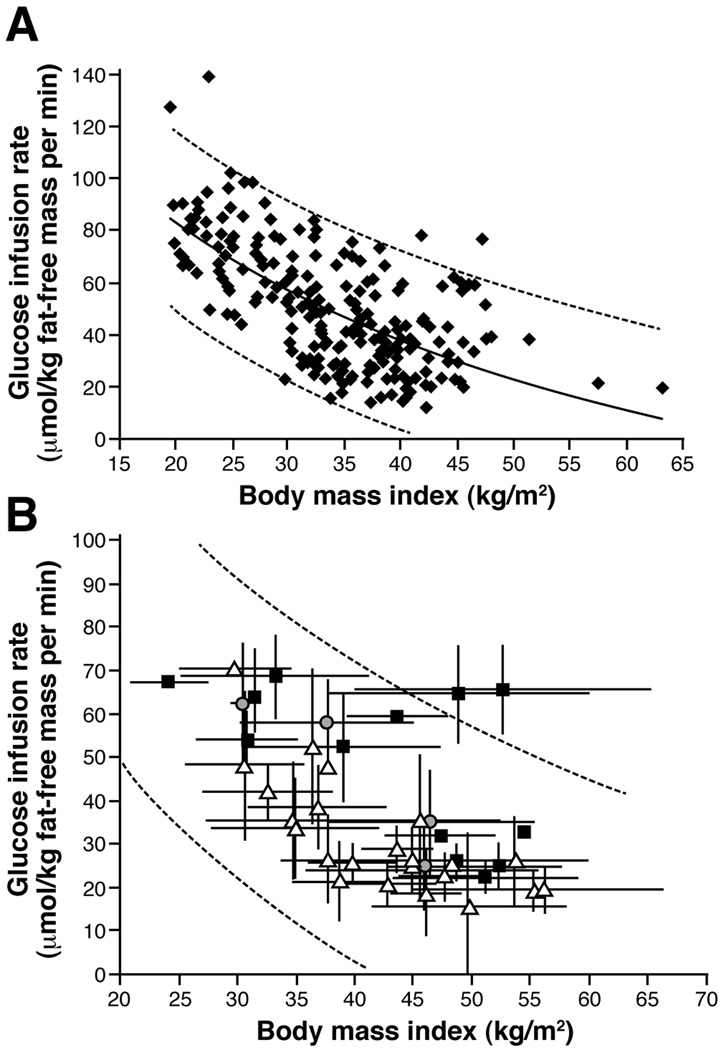
Although BMI is negatively correlated with insulin sensitivity, there is a large range in insulin sensitivity at any given BMI value.139,140 This variability makes it difficult to determine if the improvement in insulin sensitivity that occurs after bariatric surgery-induced weight loss is simply a function of weight loss itself or also involves weight loss-independent effects. Therefore, we determined the relationship between BMI and skeletal muscle insulin sensitivity, assessed by using the HEC procedure, in 220 lean, overweight and obese subjects (BMI ranging from 19.5 kg/m2 to 63.3 kg/m2; S. Klein, published and unpublished observations) in order to provide a range of values to evaluate the change in insulin sensitivity associated with weight loss induced by different bariatric surgical procedures. The glucose infusion rate (GIR) needed to maintain euglycemia during insulin infusion was negatively associated with BMI in a curvilinear fashion, but at any given BMI there was a 2- to 4- fold range in GIR (Figure 4A). The relationship between BMI and insulin sensitivity reported in patients before and after LAGB and RYGB surgeries is similar to the relationship between BMI and insulin sensitivity in our subjects who did not have surgery (Figure 4B),27,50,51,93,94,96,112,113,128–138 suggesting that weight loss, itself, is a primary determinant of the improvement in skeletal muscle insulin sensitivity. However, the pattern of rapid improvement in insulin sensitivity after BPD is different than that observed after RYGB and LAGB, which has led to the hypothesis that weight loss induced by BPD has greater effects on skeletal muscle insulin sensitivity than weight loss induced by diet therapy or RYGB.141
Figure 4.
(A) Relationship between body mass index and insulin sensitivity, assessed as insulin-mediated glucose infusion rate (GIR) during a hyperinsulinemic-euglycemic clamp procedure (insulin infused at a rate of 40–50 mU/m2 body surface area per minute) in 220 nondiabetic lean, overweight and obese subjects (171 women and 49 men, age 46 ± 12 years, BMI 34 ± 8 kg/m2 [means ± SD]) studied during the last 5 years in our laboratory. The logarithmic fit is shown with upper and lower 95% confidence limits (GIR = 282 − 66 × ln BMI; R2 = 43%; P = 2.6E-28). (B) Relationship between body mass index and insulin sensitivity, assessed as insulin-mediated glucose infusion rate (GIR) during a hyperinsulinemic-euglycemic clamp procedure (insulin infusion rate of 40–50 mU/m2 body surface area per minute). Data points represent group mean values and SDs before and after weight loss induced by RYGB (white triangles), LAGB/Gastroplasty (grey circles), and BPD (black squares), from studies that reported adequate data to make these calculations.50,51,91,112,113,128–136 Each study is represented by before and after surgery data points. Data points that do not have error bars were calculated by using group mean values when data were not reported in the desirable units but enough data were reported to allow for unit conversions. The upper and lower 95% confidence limits obtained in nondiabetic, non weight-reduced subjects (shown in Figure 4A) are shown (dashed lines) to provide a reference range of insulin sensitivity values at any given body mass index value.
In summary, static measures of insulin sensitivity, such as the HOMA-IR score, improve within days after bariatric surgery when weight loss is minimal. This improvement is also seen during early calorie restriction, presumably caused by a rapid decline in liver glycogen and hepatic glucose production, and an increase in hepatic insulin sensitivity.74,142 Greater weight loss (≥15% of initial body weight) after either RYGB or LAGB improves skeletal muscle insulin sensitivity; the improvement in muscle insulin sensitivity is primarily determined by the amount of weight loss itself. In contrast, BPD might have unique and unexplained effects on insulin action in muscle manifested by rapid improvement after minimal (<10%) weight loss but with little further improvement after greater weight loss is achieved. Nonetheless, marked weight loss (>30%) after LAGB, RYGB, and BPD surgeries results in similar improvements in skeletal muscle insulin sensitivity (Figures 3 and 4B). Few studies have evaluated the effect of LSG on insulin sensitivity. The limited available data indicate early (within the first week after surgery) reductions in the HOMA-IR score and glucose AUC in response to oral glucose or meal ingestion,90,105,143 and a later (12 months after surgery and greater weight loss) increase in insulin-mediated glucose-disposal after LSG to values similar to those observed after LAGB and RYGB.27,92
β-Cell Function
Normal β-cell function
The ability of pancreatic β-cells to secrete insulin in response to circulating glucose is critical for normal glucose homeostasis. This process involves a complex series of events that integrates nutritional, hormonal and neural factors. Insulin is first synthesized as preproinsulin which is cleaved to proinsulin, and then packaged in secretory granules where it is cleaved into equimolar amounts of insulin and C-peptide. These secretory granules fuse with the cell membrane, thereby releasing insulin, C-peptide, and proinsulin into the portal vein, and subsequently into the systemic circulation after metabolism by the liver. Therefore, portal vein insulin concentrations are usually ~2-fold higher than arterial concentrations.144,145 Although plasma insulin concentration is often used to assess β-cell function, the level of circulating insulin represents a summation of events that involve both β-cell (insulin secretion) and non-β-cell (hepatic insulin extraction and peripheral insulin clearance) metabolic pathways.
Plasma glucose is the major stimulator of insulin secretion. However, insulin concentration is greater after oral glucose ingestion than after intravenous glucose infusion, even when plasma glucose concentrations are the same,146 due to increased insulin secretion and decreased insulin clearance.147,148 This phenomenon, known as the “incretin effect,” is mediated, in part,149 by two gastrointestinal hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are released from gastrointestinal endocrine L cells and K cells, respectively.150
The metabolism of glucose in the β-cell stimulates insulin secretion. Glucose is transported from plasma into the β-cell by glucose transporters and is then metabolized to produce ATP, which triggers a cascade of cellular events that result in a biphasic secretion of insulin. The first phase of insulin secretion lasts only a few minutes, followed by a sustained second phase. The stimulation of insulin secretion during the first and second phases represents the release of insulin from at least two distinct pools of insulin secretory granules within the β-cell.151 The first phase represents the immediate release of a “readily releasable pool”, which usually accounts for less than 5% of total insulin secretory granules, whereas the second phase represents the slower release of a “reserve pool”. First phase insulin secretion is decreased in patients with impaired fasting glucose, whereas both first and second phases of insulin secretion are decreased in those with impaired glucose tolerance or T2D.8,152
Assessment of β-cell function
Several different techniques have been used in bariatric surgery studies to evaluate β-cell function (insulin secretion) in response to a dynamic metabolic perturbation, induced by glucose or meal ingestion, or intravenous glucose injection or infusion. The use of an intravenous injection or infusion of glucose does not provide a normal route and pattern of glucose delivery, which does not mimic the normal rise and fall in plasma glucose concentrations that occur after meal ingestion, and does not elicit a gastrointestinal incretin or neural response. This limitation is a particular concern in evaluating β-cell function after bariatric procedures that alter the anatomy of the UGI tract, because of the profound effect of surgery on the rate that ingested glucose is absorbed and delivered into the systemic circulation. Nonetheless, the insulin response after intravenous glucose administration does provide an estimate of glucose-dependent insulin secretion, because it circumvents other factors that are evoked by glucose or mixed meal ingestion.
The most common approach used to evaluate β-cell function involves measuring insulin concentrations at specific time points after an oral or intravenous glucose challenge or meal ingestion and integrating the AUCs to evaluate the insulin response. This approach permits an assessment of both the first (acute and early) and second (late) phases of insulin secretion. For example, the acute insulin response (AIR) to glucose is calculated as the insulin AUC early after intravenous glucose injection (usually during the first 10 minutes) and provides an index of first phase insulin secretion.153 The insulinogenic index,154 which is calculated as the increase in plasma insulin concentration 30 minutes after a metabolic perturbation divided by the corresponding increase in plasma glucose concentration, provides an index of early insulin secretion in relationship to the change in plasma glucose concentration. The hyperglycemic clamp procedure, in which plasma glucose concentration is raised to 125 mg/dl above basal levels by an exogenous glucose infusion, provides estimates of the first and second phases of insulin release by measuring the plasma insulin concentration responses to fixed hyperglycemia during the first 10 minutes of infusion and at later time points.71
Disposition Index
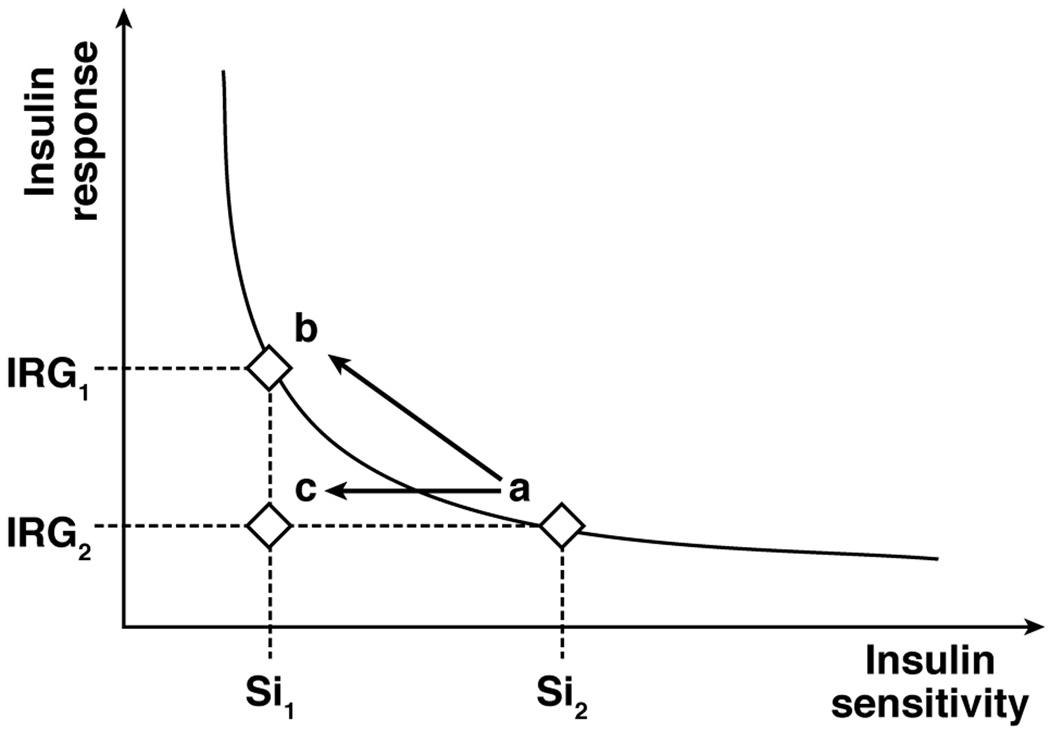
The observation that insulin secretion is related to insulin sensitivity in a hyperbolic fashion led to the concept of the disposition index (DI).153,155 The DI is calculated as the product of insulin sensitivity and the insulin secretory response to a glucose challenge, which provides an assessment of the appropriateness of the β-cell response in relationship to insulin sensitivity. For example, less insulin secretion is necessary to maintain normal glucose homeostasis in insulin-sensitive than in insulin-resistant subjects, and a compensatory increase in insulin secretion can maintain normal glucose homeostasis in insulin-resistant subjects (Figure 5). The DI concept was originally developed by using the IVGTT, so the classical DI is calculated as AIR × Si. However subsequent studies have used other estimates of insulin secretion and insulin sensitivity to calculate DI.
Figure 5.
The disposition index (DI) is the product of insulin sensitivity (Si) and the insulin response to glucose (IRG). In subjects with normal fasting plasma glucose concentration and normal glucose tolerance, Si is related to IRG in a hyperbolic fashion. In obese subjects with normal glucose tolerance, an increase in the β-cell response to a glucose challenge compensates for decreased insulin sensitivity and maintains normal glucose homeostasis (a → b; no change in DI). However, an inadequate β-cell response to a decrease in insulin sensitivity results in impaired glucose homeostasis and eventually type 2 diabetes (a → c; reduction in DI).
Effect of bariatric surgery on β-cell function
Intestinal bypass procedures and LSG increase the rate of delivery of ingested glucose into the systemic circulation causing a spike in plasma glucose concentrations,90,156 which influences the rapidity and magnitude of the insulin secretory response to glucose or mixed meal ingestion. The alteration in glucose absorption rate complicates the interpretation of the surgical effect on β-cell function when evaluated by using an oral glucose or meal challenge.
Most,94,95,99,101–104,107,109 but not all,97,105,106,108 studies found significant reductions in total postprandial (following glucose or mixed meal ingestion) insulin AUC after marked weight loss (>15%) induced by any type of bariatric surgery procedure. After minimal to moderate weight loss (<10%), which occurs early after surgery, the total insulin response to glucose or mixed meal ingestion is usually decreased after BPD,110,112–114 but not after LAGB,110 vertical banded gastroplasty,108 or RYGB,50,79,90,106,111 whereas a decrease90 or no change105 has been observed 1 week after LSG (5–6% weight loss). In addition, the shape of the insulin response curve is different after RYGB and LSG than after LAGB, with a faster rise in insulin concentration after oral glucose or meal ingestion, a greater insulin peak, and a steeper decline thereafter.
It is difficult to determine the precise effect of different bariatric surgical procedures on other important aspects of β-cell function, particularly early insulin secretion (within the first 30 minutes after stimulation by glucose) and the DI in response to a perturbation in circulating glucose, because of considerable heterogeneity of results among studies, which is likely related to differences in patient populations, surgery-induced weight loss, and the methods used to assess the insulin response and the DI. Nonetheless, a careful evaluation of the published studies suggests the following summary of effects with each surgical procedure: 1) LAGB surgery does not significantly affect early insulin secretion in most studies81,98,117 but increases the DI after at least moderate weight loss;81,91,98 2) RYGB surgery does not significantly affect early insulin secretion but increases the DI after marked weight loss;48,81,91,93,106,117,121,122,129,157 3) BPD surgery either does not alter or decreases early insulin secretion in patients who do not have diabetes but increases early insulin secretion in patients with T2D,120,123,158 and increases the DI; and 4) LSG surgery does not affect early insulin secretion in people without diabetes but increases early insulin secretion in patients with T2D,105,143 without reported data on the DI.
Weight loss-independent therapeutic effects of intestinal bypass surgery
There is a growing perception that bariatric surgical procedures that divert ingested nutrients away from the UGI tract have weight-loss independent therapeutic effects on glycemic control.40,159 This notion is based primarily on: 1) the early postoperative effects of RYGB surgery on glycemic control; 2) the long-term efficacy of different surgical procedures on T2D resolution; 3) the effect of duodenal-jejunal bypass surgery, which bypasses the UGI tract but causes minimal weight loss; 4) the hormonal response to glucose or mixed meal ingestion; and 5) UGI tract bypass in rodent models. However, it is difficult to make definitive conclusions from these studies because of inconsistent data among studies and limitations in study design.
Early postoperative effects of RYGB surgery on glycemic control
Glycemic control improves rapidly after RYGB surgery in patients with T2D, before large changes in body weight have occurred.39,79,84,94,111–113,120,160–166 In fact, 30%–100% of patients who have had RYGB or BPD are able to discontinue all diabetes medications and maintain a normal fasting blood glucose concentration within a few days after surgery, and only a 1%–2% weight loss.84,112,165 However, patients who have RYGB surgery experience a sudden and marked reduction in energy intake, from ~3000–6000 kcal/day to ~200–300 kcal/day,12,80 making it difficult to separate the effect of calorie restriction from intestinal bypass on the improvement in glycemic control. Short-term calorie-restriction improves glucose homeostasis and can reduce the need for diabetes medications, because of a decrease in both the ingestion of carbohydrate calories and hepatic glucose production. The decline in plasma glucose concentration and HOMA-IR score observed in patients several days after RYGB surgery is similar to those observed in control subjects given the same diet.80 The liver is particularly sensitive to short-term changes in energy balance. Consuming a low-calorie diet (~1100 kcal/day) for 2–4 days decreases basal hepatic glucose production rate and increases insulin-mediated suppression of hepatic glucose production,74,167,168 which is likely an important contributor to the rapid decline in HOMA-IR score observed after RYGB surgery.84 In patients with T2D, consuming a very-low-calorie diet (≤800 kcal/day) results in lower fasting plasma glucose and insulin concentrations and decreased HOMA-IR score within 4–10 days80,85,169 and remission of T2D within 8 weeks.86 Therefore, the rapid improvement in glycemic control after RYGB surgery could simply be a normal response to marked caloric restriction.
Effect of intestinal bypass procedures on T2D
The remission rate of T2D is not the same among surgical procedures. The results from a meta-analysis involving ~8,000 patients with T2D found that the rate of diabetes resolution was much greater in patients who had surgical procedures that involved anatomical diversion of the UGI tract (BPD and RYGB: 95% and 80% resolution rates, respectively) than those that simply restricted the stomach (LAGB: 57% resolution rate).11,17 However, the interpretation of these data is confounded by differences in weight loss among procedures.11,17 We are aware of only one study that compared the effect of RYGB with LAGB on T2D resolution after patients in both groups lost the same amount of weight.87 At 2 years after surgery, when each group had lost ~30% of their initial body weight, 72% of patients who had RYGB, but only 17% of patients who had LAGB, achieved remission of T2D. Although it is unclear why patients who had LAGB did so poorly in that study, these results suggest an extraordinary weight loss-independent therapeutic effect of RYGB, which requires further confirmation.
Effect of duodenal-jejunal bypass surgery
Duodenal-jejunal bypass (DJB) surgery involves creating a duodenojejunostomy, without restriction or exclusion of the stomach. This procedure prevents ingested nutrients from direct contact with the duodenum and proximal jejunum, but causes minimal or no weight loss. Case reports of three patients who achieved diabetes remission without weight loss after DJB surgery support the notion of weight loss-independent effects of UGI tract bypass.170,171 However, data from clinical studies containing larger cohorts of patients found DJB surgery did not result in a high rate of T2D remission. The composite of results from four studies containing a total of 61 patients, found that although many patients had improved glycemic control, assessed by fasting blood glucose and HbA1c values, only 5% achieved remission of T2D, defined as discontinuation of all diabetes medications, HbA1c <6.5%, and fasting blood glucose <126 mg/dL.172–175 In addition, DJB surgery generally caused about a 7% decrease in body weight in the first 6 months after surgery, which could have contributed to improved glycemic control. The remission rate in a fifth study cannot be determined from the reported data, but body weight decreased by 8%, HbA1c improved to a range of 5.8%–7.9%, and diabetes medications were completely discontinued in 90% of patients at 6 months after surgery.176 The results from these studies suggest that UGI tract bypass has beneficial effects on glycemic control, but the marked difference in diabetes remission rates between patients who have had DJB and those who have had RYGB surgery suggests that marked weight loss, gastric exclusion, and/or other physiological factors associated with UGI tract bypass surgeries have important therapeutic effects.
Hormonal response to oral glucose or mixed meal ingestion
Patients who have had bariatric UGI tract bypass procedures demonstrate a large early postprandial increase in plasma GLP-1 and insulin concentrations,111,177–181 which is not observed after LAGB181 or diet induced weight loss.79 In general, peak active GLP-1 and insulin concentrations are about two-fold greater after, than before, bypass surgery. However, despite the greater early increase in plasma insulin, the postprandial peak in plasma glucose concentration is usually greater after RYGB than LAGB or low-calorie diet induced weight loss, and the change in postprandial glucose AUC is often similar after weight loss induced by RYGB, LAGB or a low-calorie diet.80,93,107 Therefore, the altered hormonal response to glucose or meal ingestion does not result in a greater improvement in postprandial glycemic control than weight loss itself. It is unlikely that alterations in the secretion of other gastrointestinal hormones that are known to affect glucose homeostasis are important contributors to the improvement in glycemic control observed after UGI tract bypass surgery. The effects of bariatric surgery on the secretion of GIP and glucagon after glucose or meal ingestion are not clear, because of marked differences in results among studies demonstrating an increase, decrease or no change in postprandial plasma GIP111,162,181–183 and glucagon79,83,181,182,184,185 concentrations after RYGB surgery.
Studies conducted in rodent models
A series of studies, conducted in different rodent models (e.g. obese Zucker, Sprague Dawley, Long-Evans, and Osborne Mendel rats186–193 and C57BL6, C57BI6 and Lepr(db) mice194,195) have evaluated the effects of RYGB surgery on glucose homeostasis. The results from these studies demonstrate that, compared with sham-operated or pair-fed animals, RYGB surgery caused a greater improvement in one or more of the following outcome measures: basal plasma glucose and insulin concentrations, HOMA-IR score, glucose AUC after glucose ingestion, liver insulin sensitivity and skeletal muscle insulin sensitivity.186–188,190,193–195 However, greater body weight and body fat in sham-operated or pair-fed control animals than the RYGB group187–189,191–195 confound the interpretation of these results. It is difficult to match body weight across treatment groups despite the use of pair-feeding, because energy expenditure is markedly increased after RYGB surgery in rodent models.196–199
The effect of bypassing the UGI tract alone (DJB surgery) on glycemic control in rodents is complex because it depends on the animal model being studied. In Goto-Kakizaki rats, a non-obese model of polygenic T2D, DJB reduced fasting plasma glucose concentrations, increased insulin secretion in response to a mixed meal, improved oral glucose tolerance, and decreased the HOMA-IR score despite weight stability.200–206 In addition, DJB surgery in non-obese streptozotocin-induced diabetic rats reduced plasma glucose and endogenous glucose production within 2 days of surgery, independent of changes in body weight.207 Beneficial metabolic effects of DJB compared to sham operated animals have also been found in diabetic Zucker rats, but conclusions regarding weight loss-independent effects are confounded by differences in body weight between groups.208 In contrast, DJB surgery in diet-induced obese Long Evans and Wistar rats, did not affect fasting blood glucose or insulin concentrations, oral glucose tolerance, plasma glucose response to insulin injection, endogenous glucose production rate, hepatic insulin sensitivity, or skeletal muscle insulin sensitivity.209
Conclusions and future research directions
Bariatric surgery is the most effective available therapy for T2D. Most patients with T2D who are treated with RYGB surgery achieve remission of their diabetes, defined as maintaining normal or near normal blood glucose concentrations and HbA1c without the use of diabetes medications. Remission rates as high as 95% have been reported after the BPD-DS procedure, but this operation is not widely used because of a high rate of postoperative nutritional abnormalities. In addition, even those who do not achieve diabetes remission usually experience a marked improvement in glycemic control. Accordingly, bariatric surgery should be considered a part of standard therapy for T2D, but only a fraction of eligible patients with T2D undergo bariatric surgery. Although UGI tract bypass procedures and even LSG cause marked alterations in the metabolic response to meal ingestion, manifested primarily by an increase in early postprandial plasma GLP-1 and insulin concentrations, the importance of these changes in improving glycemic control and ameliorating T2D after substantial surgery-induced weight loss has occurred is not clear. For example, the improvement in β-cell function and skeletal muscle insulin sensitivity is the same after 20% weight loss induced by either LAGB or RYGB.91 However, the marked improvement in β-cell function and insulin sensitivity after minimal weight loss in patients who have had BPD is distinct from other surgical procedures. The notion of weight-loss independent therapeutic effects of this operation is further supported by the recent observation that glycemic control and remission of T2D was greater in patients who had BPD than those who had RYGB surgery, despite the same weight loss in both groups.23
The currently approved indication for bariatric surgery in patients who have T2D is a BMI of 35 kg/m2 or greater, which was established in 1991 by a consensus conference sponsored by the National Institutes of Health.210 However, this guideline was developed before: i) minimally invasive techniques were used for bariatric surgery; ii) improved technical expertise reduced perioperative risks; iii) LAGB and LSG procedures were introduced into the mainstream and more data were available to evaluate BPD; and iv) data from long-term observational studies and randomized clinical trials were available to assess the effect of bariatric surgery on T2D. Furthermore, it might be inappropriate for one BMI value to be the gate to bariatric surgery, because it ignores the influence of race and ethnicity, body composition, and age on the relationship between BMI and metabolic risk.211 Therefore, a re-evaluation of the indications for bariatric surgery in patients with T2D, based on the latest available evidence and risk-benefit assessment, is needed. We believe the use of BMI cut-off values to determine eligibility for surgery is not an optimal approach. Instead, strategies should be developed and ultimately tested to provide the most appropriate therapeutic medical and surgical approaches to: i) prevent the development of diabetes in high-risk patients, ii) achieve optimal glycemic control, iii) preserve β-cell function, iv) improve insulin sensitivity, and v) reduce the risk of developing serious diabetes complications. In addition, clinical studies are needed to: i) determine how to identify patients who will not adequately benefit from bariatric surgery; ii) determine the effectiveness of specific bariatric surgical procedures in patients with T2D who have failed to achieve adequate glycemic control with aggressive medical therapy; and iii) determine the risk-benefit relationship of surgical therapy in special populations, such as adolescents and those with a BMI <35 kg/m2.
The profound effects of bariatric surgery on body weight and T2D expose the limitations of our understanding of human behavior and metabolic physiology, but underscore the need to address several fundamental research questions: i) Why does bariatric surgery cause weight loss, and do the mechanisms responsible for weight loss differ among procedures?; ii) Why does bariatric surgery-induced weight loss cause such remarkable effects on metabolic function?; iii) Are there weight loss-independent effects of UGI tract bypass surgery or LSG that improve metabolic function, and are these effects important in improving glycemic control once marked weight loss is achieved?; iv) Do RYGB, BPD and LSG have different effects on β-cell function in patients with T2D than in those with normal oral glucose tolerance?; and v) Do specific surgical procedures cause unique physiological perturbations (e.g. decreased branched-chain amino acids,82 increased bile acids,212 and altered gut microbiome213) that improve metabolic function? The use of bariatric surgery as a model to answer these questions will likely provide new insights into the mechanisms responsible for the regulation of body weight and glucose homeostasis.
Acknowledgments
Grant support: National Institutes of Health grants DK 37948, DK 56341 (Nutrition Obesity Research Center), and UL1 RR024992 (Clinical and Translational Science Award).
Abbreviations
- T2D
type 2 diabetes mellitus
- BMI
body mass index
- HbA1c
glycated hemoglobin
- RYGB
roux-en-Y gastric bypass
- LAGB
laparoscopic adjustable gastric banding
- LSG
laparoscopic sleeve gastrectomy
- BPD
bilopancreatic diversion
- BPD-DS
BPD with duodenal switch
- HOMA-IR
homeostasis model assessment of insulin resistance
- IVGTT
intravenous glucose tolerance test
- Si
insulin sensitivity index derived from the IVGTT
- HEC
hyperinsulinemic-euglycemic clamp
- AUC
area under the concentration-versus-time curve
- OGTT
oral glucose tolerance test
- GIR
glucose infusion rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: D.B. reviewed the pertinent literature, collated and analyzed data and wrote the manuscript; F.M. reviewed the pertinent literature, collated and analyzed data and wrote the manuscript. S.K. designed the review outline, obtained funding, reviewed the data, and wrote the manuscript.
Disclosures: SK serves on a Scientific Advisory Board for Ethicon Endo-Surgery
REFERENCES
- 1.Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 6.Mitrakou A, Kelley D, Veneman T, et al. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes. 1990;39:1381–1390. doi: 10.2337/diab.39.11.1381. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988;37:79–85. doi: 10.1016/0026-0495(88)90033-9. [DOI] [PubMed] [Google Scholar]
- 8.Kanat M, Mari A, Norton L, et al. Distinct beta-Cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes. 2012;61:447–453. doi: 10.2337/db11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resnick HE, Foster GL, Bardsley J, et al. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes - 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Das SK, Roberts SB, McCrory MA, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 13.Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti G, Mingrone G, Marcoccia S, et al. Body composition and energy expenditure after weight loss following bariatric surgery. J Am Coll Nutr. 2000;19:270–274. doi: 10.1080/07315724.2000.10718926. [DOI] [PubMed] [Google Scholar]
- 15.Coupaye M, Bouillot JL, Coussieu C, et al. One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obes Surg. 2005;15:827–833. doi: 10.1381/0960892054222768. [DOI] [PubMed] [Google Scholar]
- 16.Odstrcil EA, Martinez JG, Santa Ana CA, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 17.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 18.Boza C, Gamboa C, Salinas J, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: a case-control study and 3 years of follow-up. Surg Obes Relat Dis. 2012;8:243–249. doi: 10.1016/j.soard.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Kehagias I, Karamanakos SN, Argentou M, et al. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg. 2011;21:1650–1656. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
- 20.Chouillard EK, Karaa A, Elkhoury M, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity: case-control study. Surg Obes Relat Dis. 2011;7:500–505. doi: 10.1016/j.soard.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 22.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 24.Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707–713. doi: 10.1016/j.soard.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Bayham BE, Greenway FL, Bellanger DE, et al. Early resolution of type 2 diabetes seen after Roux-en-Y gastric bypass and vertical sleeve gastrectomy. Diabetes Technol Ther. 2012;14:30–34. doi: 10.1089/dia.2011.0151. [DOI] [PubMed] [Google Scholar]
- 26.Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012 doi: 10.1007/s00464-012-2166-y. in press. [DOI] [PubMed] [Google Scholar]
- 27.Abbatini F, Rizzello M, Casella G, et al. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24:1005–1010. doi: 10.1007/s00464-009-0715-9. [DOI] [PubMed] [Google Scholar]
- 28.Lee WJ, Chen CY, Chong K, et al. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:683–690. doi: 10.1016/j.soard.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143–148. doi: 10.1001/archsurg.2010.326. [DOI] [PubMed] [Google Scholar]
- 30.Leonetti F, Capoccia D, Coccia F, et al. Obesity, type 2 diabetes mellitus, and other comorbidities: A prospective cohort study of laparoscopic sleeve gastrectomy vs medical treatment. Arch Surg. 2012 doi: 10.1001/archsurg.2012.222. in press. [DOI] [PubMed] [Google Scholar]
- 31.Abbatini F, Capoccia D, Casella G, et al. Type 2 diabetes in obese patients with body mass index of 30–35 kg/m2: sleeve gastrectomy versus medical treatment. Surg Obes Relat Dis. 2012;8:20–24. doi: 10.1016/j.soard.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Inabnet WB, 3rd, Winegar DA, Sherif B, et al. Early outcomes of bariatric surgery in patients with metabolic syndrome: an analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2012;214:550–556. doi: 10.1016/j.jamcollsurg.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Dixon JB, Dixon AF, O'Brien PE. Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese. Homeostatic model assessment. Diabet Med. 2003;20:127–134. doi: 10.1046/j.1464-5491.2003.00889.x. [DOI] [PubMed] [Google Scholar]
- 34.Dixon JB. Obesity and diabetes: the impact of bariatric surgery on type-2 diabetes. World J Surg. 2009;33:2014–2021. doi: 10.1007/s00268-009-0062-y. [DOI] [PubMed] [Google Scholar]
- 35.Kadera BE, Lum K, Grant J, et al. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis. 2009;5:305–309. doi: 10.1016/j.soard.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Hamza N, Abbas MH, Darwish A, et al. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surg Obes Relat Dis. 2011;7:691–696. doi: 10.1016/j.soard.2010.03.292. [DOI] [PubMed] [Google Scholar]
- 37.Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254–259. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 39.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- 41.Blackstone R, Bunt JC, Cortes MC, et al. Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surg Obes Relat Dis. 2012 doi: 10.1016/j.soard.2012.05.005. in press. [DOI] [PubMed] [Google Scholar]
- 42.Pournaras DJ, Aasheim ET, Sovik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg. 2012;99:100–103. doi: 10.1002/bjs.7704. [DOI] [PubMed] [Google Scholar]
- 43.Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978;27:1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- 44.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 45.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 46.Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 47.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 48.Lin E, Phillips LS, Ziegler TR, et al. Increases in adiponectin predict improved liver, but not peripheral, insulin sensitivity in severely obese women during weight loss. Diabetes. 2007;56:735–742. doi: 10.2337/db06-1161. [DOI] [PubMed] [Google Scholar]
- 49.van Dielen FM, Nijhuis J, Rensen SS, et al. Early insulin sensitivity after restrictive bariatric surgery, inconsistency between HOMA-IR and steady-state plasma glucose levels. Surg Obes Relat Dis. 2010;6:340–344. doi: 10.1016/j.soard.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lima MM, Pareja JC, Alegre SM, et al. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3871–3875. doi: 10.1210/jc.2010-0085. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 53.Gutt M, Davis CL, Spitzer SB, et al. Validation of the insulin sensitivity index (ISI0,120): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 54.Avignon A, Boegner C, Mariano-Goulart D, et al. Assessment of insulin sensitivity from plasma insulin and glucose in the fasting or post oral glucose-load state. Int J Obes Relat Metab Disord. 1999;23:512–517. doi: 10.1038/sj.ijo.0800864. [DOI] [PubMed] [Google Scholar]
- 55.Levine R, Haft DE. Carbohydrate homeostasis. N Engl J Med. 1970;283:237–246. doi: 10.1056/NEJM197007302830506. [DOI] [PubMed] [Google Scholar]
- 56.Soonthornpun S, Setasuban W, Thamprasit A, et al. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab. 2003;88:1019–1023. doi: 10.1210/jc.2002-021127. [DOI] [PubMed] [Google Scholar]
- 57.Maki KC, Kelley KM, Lawless AL, et al. Validation of insulin sensitivity and secretion indices derived from the liquid meal tolerance test. Diabetes Technol Ther. 2011;13:661–666. doi: 10.1089/dia.2010.0240. [DOI] [PubMed] [Google Scholar]
- 58.Selimoglu H, Duran C, Kiyici S, et al. Comparison of composite whole body insulin sensitivity index derived from mixed meal test and oral glucose tolerance test in insulin resistant obese subjects. Endocrine. 2009;36:299–304. doi: 10.1007/s12020-009-9213-z. [DOI] [PubMed] [Google Scholar]
- 59.Breda E, Cavaghan MK, Toffolo G, et al. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50:150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 60.Dalla Man C, Campioni M, Polonsky KS, et al. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54:3265–3273. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 61.Mari A, Schmitz O, Gastaldelli A, et al. Meal and oral glucose tests for assessment of beta -cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab. 2002;283:E1159–E1166. doi: 10.1152/ajpendo.00093.2002. [DOI] [PubMed] [Google Scholar]
- 62.Bergman RN, Ider YZ, Bowden CR, et al. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 63.Finegood DT, Hramiak IM, Dupre J. A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes. J Clin Endocrinol Metab. 1990;70:1538–1549. doi: 10.1210/jcem-70-6-1538. [DOI] [PubMed] [Google Scholar]
- 64.Saad MF, Steil GM, Kades WW, et al. Differences between the tolbutamide-boosted and the insulin-modified minimal model protocols. Diabetes. 1997;46:1167–1171. doi: 10.2337/diab.46.7.1167. [DOI] [PubMed] [Google Scholar]
- 65.Boston RC, Stefanovski D, Moate PJ, et al. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 66.Shen SW, Reaven GM, Farquhar JW. Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest. 1970;49:2151–2160. doi: 10.1172/JCI106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harano Y, Hidaka H, Takatsuki K, et al. Glucose, insulin, and somatostatin infusion for the determination of insulin sensitivity in vivo. Metabolism. 1978;27:1449–1452. doi: 10.1016/0026-0495(78)90091-4. [DOI] [PubMed] [Google Scholar]
- 68.Greenwood FC, Landon J, Stamp TC. The plasma sugar, free fatty acid, cortisol, and growth hormone response to insulin. I. In control subjects. J Clin Invest. 1966;45:429–436. doi: 10.1172/JCI105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akinmokun A, Selby PL, Ramaiya K, et al. The short insulin tolerance test for determination of insulin sensitivity: a comparison with the euglycaemic clamp. Diabet Med. 1992;9:432–437. doi: 10.1111/j.1464-5491.1992.tb01813.x. [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez-Ortiz LJ, Martinez-Abundis E, Gonzalez-Ortiz M. A new model to fit glucose concentration during the insulin tolerance test improving the predictive capability to estimate insulin sensitivity. Nutr Metab Cardiovasc Dis. 2006;16:78–79. doi: 10.1016/j.numecd.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 71.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 72.Korenblat KM, Fabbrini E, Mohammed BS, et al. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 74.Kirk E, Reeds DN, Finck BN, et al. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato F, Tamura Y, Watada H, et al. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab. 2007;92:3326–3329. doi: 10.1210/jc.2006-2384. [DOI] [PubMed] [Google Scholar]
- 77.Niskanen L, Uusitupa M, Sarlund H, et al. The effects of weight loss on insulin sensitivity, skeletal muscle composition and capillary density in obese non-diabetic subjects. Int J Obes Relat Metab Disord. 1996;20:154–160. [PubMed] [Google Scholar]
- 78.Rao RS, Yanagisawa R, Kini S. Insulin resistance and bariatric surgery. Obes Rev. 2012;13:316–328. doi: 10.1111/j.1467-789X.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 79.Laferrere B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plum L, Ahmed L, Febres G, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 2011;19:2149–2157. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laferrere B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–E131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 84.Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 85.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1985;61:917–925. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 86.Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–971. doi: 10.1097/SLA.0b013e3181efc49a. [DOI] [PubMed] [Google Scholar]
- 88.Ballantyne GH, Farkas D, Laker S, et al. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16:1189–1197. doi: 10.1381/096089206778392158. [DOI] [PubMed] [Google Scholar]
- 89.Lee WJ, Lee YC, Ser KH, et al. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–1125. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 90.Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 91.Bradley D, Conte C, Eagon JC, et al. Does Roux-en Y gastric bypass surgery have independent effects on β-cell function and insulin sensitivity after marked weight loss? [abstract] ADA. 2012 [Google Scholar]
- 92.Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic roux-en-y gastric bypass and sleeve gastrectomy: A randomized, prospective trial. Obes Surg. 2012;22:740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–471. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49:2136–2143. doi: 10.1007/s00125-006-0337-x. [DOI] [PubMed] [Google Scholar]
- 95.Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:E1372–E1379. doi: 10.1210/jc.2011-0446. [DOI] [PubMed] [Google Scholar]
- 96.Camastra S, Manco M, Mari A, et al. β-Cell function in morbidly obese subjects during free living: long-term effects of weight loss. Diabetes. 2005;54:2382–2389. doi: 10.2337/diabetes.54.8.2382. [DOI] [PubMed] [Google Scholar]
- 97.De Paula AL, Stival AR, Halpern A, et al. Improvement in insulin sensitivity and beta-cell function following ileal interposition with sleeve gastrectomy in type 2 diabetic patients: potential mechanisms. J Gastrointest Surg. 2011;15:1344–1353. doi: 10.1007/s11605-011-1550-6. [DOI] [PubMed] [Google Scholar]
- 98.Sesti G, Folli F, Perego L, et al. Effects of weight loss in metabolically healthy obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. PLoS One. 2011;6:e17737. doi: 10.1371/journal.pone.0017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiellini C, Iaconelli A, Familiari P, et al. Study of the effects of transoral gastroplasty on insulin sensitivity and secretion in obese subjects. Nutr Metab Cardiovasc Dis. 2010;20:202–207. doi: 10.1016/j.numecd.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 100.Bendezu R, Wieland RG, Green SG, et al. Certain metabolic consequences of jejunoileal bypass. Am J Clin Nutr. 1976;29:366–370. doi: 10.1093/ajcn/29.4.366. [DOI] [PubMed] [Google Scholar]
- 101.Bosello O, Armellini F, Pelloso M, et al. Glucose tolerance in jejunoileal bypass for morbid obesity: a fifteen month follow-up. Diabete Metab. 1978;4:159–162. [PubMed] [Google Scholar]
- 102.Sirinek KR, O'Dorisio TM, Hill D, et al. Hyperinsulinism, glucose-dependent insulinotropic polypeptide, and the enteroinsular axis in morbidly obese patients before and after gastric bypass. Surgery. 1986;100:781–787. [PubMed] [Google Scholar]
- 103.Karayiannakis AJ, Syrigos KN, Zbar A, et al. The effect of vertical banded gastroplasty on glucose-induced beta-endorphin response. J Surg Res. 1998;80:123–128. doi: 10.1006/jsre.1998.5466. [DOI] [PubMed] [Google Scholar]
- 104.Kopp HP, Kopp CW, Festa A, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23:1042–1047. doi: 10.1161/01.ATV.0000073313.16135.21. [DOI] [PubMed] [Google Scholar]
- 105.Lee WJ, Ser KH, Chong K, et al. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147:664–669. doi: 10.1016/j.surg.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 106.Umeda LM, Silva EA, Carneiro G, et al. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21:896–901. doi: 10.1007/s11695-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 107.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valverde I, Puente J, Martin-Duce A, et al. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–397. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 109.de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg. 2009;19:313–320. doi: 10.1007/s11695-008-9678-5. [DOI] [PubMed] [Google Scholar]
- 110.Pontiroli AE, Gniuli D, Mingrone G. Early effects of gastric banding (LGB) and of biliopancreatic diversion (BPD) on insulin sensitivity and on glucose and insulin response after OGTT. Obes Surg. 2010;20:474–479. doi: 10.1007/s11695-010-0076-4. [DOI] [PubMed] [Google Scholar]
- 111.Laferrere B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 113.Salinari S, Bertuzzi A, Asnaghi S, et al. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salinari S, Bertuzzi A, Iaconelli A, et al. Twenty-four hour insulin secretion and beta cell NEFA oxidation in type 2 diabetic, morbidly obese patients before and after bariatric surgery. Diabetologia. 2008;51:1276–1284. doi: 10.1007/s00125-008-1007-y. [DOI] [PubMed] [Google Scholar]
- 115.Gletsu N, Lin E, Khaitan L, et al. Changes in C-reactive protein predict insulin sensitivity in severely obese individuals after weight loss surgery. J Gastrointest Surg. 2005;9:1119–1126. doi: 10.1016/j.gassur.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 116.Letiexhe MR, Scheen AJ, Gerard PL, et al. Insulin secretion, clearance and action before and after gastroplasty in severely obese subjects. Int J Obes Relat Metab Disord. 1994;18:295–300. [PubMed] [Google Scholar]
- 117.Lin E, Davis SS, Srinivasan J, et al. Dual mechanism for type-2 diabetes resolution after Roux-en-Y gastric bypass. Am Surg. 2009;75:498–502. [PMC free article] [PubMed] [Google Scholar]
- 118.Garcia-Fuentes E, Garcia-Almeida JM, Garcia-Arnes J, et al. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg. 2006;16:1179–1188. doi: 10.1381/096089206778392383. [DOI] [PubMed] [Google Scholar]
- 119.Herbst CA, Hughes TA, Gwynne JT, et al. Gastric bariatric operation in insulin-treated adults. Surgery. 1984;95:209–214. [PubMed] [Google Scholar]
- 120.Briatore L, Salani B, Andraghetti G, et al. Restoration of acute insulin response in T2DM subjects 1 month after biliopancreatic diversion. Obesity (Silver Spring) 2008;16:77–81. doi: 10.1038/oby.2007.9. [DOI] [PubMed] [Google Scholar]
- 121.Morinigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 122.Lin E, Liang Z, Frediani J, et al. Improvement in β-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab. 2010;299:E706–E712. doi: 10.1152/ajpendo.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, et al. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 124.Pillonetto G, Caumo A, Cobelli C. Dynamic insulin sensitivity index: importance in diabetes. Am J Physiol Endocrinol Metab. 2010;298:E440–E448. doi: 10.1152/ajpendo.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Foo J, Krebs J, Hayes MT, et al. Studies in insulin resistance following very low calorie diet and/or gastric bypass surgery. Obes Surg. 2011;21:1914–1920. doi: 10.1007/s11695-011-0527-6. [DOI] [PubMed] [Google Scholar]
- 126.Geloneze B, Tambascia MA, Pareja JC, et al. The insulin tolerance test in morbidly obese patients undergoing bariatric surgery. Obes Res. 2001;9:763–769. doi: 10.1038/oby.2001.105. [DOI] [PubMed] [Google Scholar]
- 127.Nijhuis J, van Dielen FM, Schaper NC, et al. Short-term overfeeding induces insulin resistance in weight-stable patients after bariatric surgery. Obes Surg. 2008;18:300–305. doi: 10.1007/s11695-007-9306-9. [DOI] [PubMed] [Google Scholar]
- 128.Pereira JA, Lazarin MA, Pareja JC, et al. Insulin resistance in nondiabetic morbidly obese patients: effect of bariatric surgery. Obes Res. 2003;11:1495–1501. doi: 10.1038/oby.2003.200. [DOI] [PubMed] [Google Scholar]
- 129.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093–2102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 130.Promintzer-Schifferl M, Prager G, Anderwald C, et al. Effects of gastric bypass surgery on insulin resistance and insulin secretion in nondiabetic obese patients. Obesity (Silver Spring) 2011;19:1420–1426. doi: 10.1038/oby.2011.92. [DOI] [PubMed] [Google Scholar]
- 131.Gastaldi G, Russell A, Golay A, et al. Upregulation of peroxisome proliferator-activated receptor gamma coactivator gene (PGC1A) during weight loss is related to insulin sensitivity but not to energy expenditure. Diabetologia. 2007;50:2348–2355. doi: 10.1007/s00125-007-0782-1. [DOI] [PubMed] [Google Scholar]
- 132.Muscelli E, Mingrone G, Camastra S, et al. Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med. 2005;118:51–57. doi: 10.1016/j.amjmed.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 133.Mingrone G, DeGaetano A, Greco AV, et al. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia. 1997;40:599–605. doi: 10.1007/s001250050721. [DOI] [PubMed] [Google Scholar]
- 134.Rosa G, Mingrone G, Manco M, et al. Molecular mechanisms of diabetes reversibility after bariatric surgery. Int J Obes (Lond) 2007;31:1429–1436. doi: 10.1038/sj.ijo.0803630. [DOI] [PubMed] [Google Scholar]
- 135.Valera-Mora ME, Simeoni B, Gagliardi L, et al. Predictors of weight loss and reversal of comorbidities in malabsorptive bariatric surgery. Am J Clin Nutr. 2005;81:1292–1297. doi: 10.1093/ajcn/81.6.1292. [DOI] [PubMed] [Google Scholar]
- 136.Jimenez J, Zuniga-Guajardo S, Zinman B, et al. Effects of weight loss in massive obesity on insulin and C-peptide dynamics: sequential changes in insulin production, clearance, and sensitivity. J Clin Endocrinol Metab. 1987;64:661–668. doi: 10.1210/jcem-64-4-661. [DOI] [PubMed] [Google Scholar]
- 137.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 139.Farin HM, Abbasi F, Reaven GM. Body mass index and waist circumference both contribute to differences in insulin-mediated glucose disposal in nondiabetic adults. Am J Clin Nutr. 2006;83:47–51. doi: 10.1093/ajcn/83.1.47. [DOI] [PubMed] [Google Scholar]
- 140.Ferrannini E, Natali A, Bell P, et al. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care. 2009;32:514–520. doi: 10.2337/dc08-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rothman DL, Magnusson I, Katz LD, et al. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254:573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 143.Basso N, Capoccia D, Rizzello M, et al. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg Endosc. 2011;25:3540–3550. doi: 10.1007/s00464-011-1755-5. [DOI] [PubMed] [Google Scholar]
- 144.Staehr P, Hother-Nielsen O, Levin K, et al. Assessment of hepatic insulin action in obese type 2 diabetic patients. Diabetes. 2001;50:1363–1370. doi: 10.2337/diabetes.50.6.1363. [DOI] [PubMed] [Google Scholar]
- 145.Magkos F, Fabbrini E, Patterson BW, et al. Portal vein and systemic adiponectin concentrations are closely linked with hepatic glucose and lipoprotein kinetics in extremely obese subjects. Metabolism. 2011;60:1641–1648. doi: 10.1016/j.metabol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perley M, Kipnis DM. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes. 1966;15:867–874. doi: 10.2337/diab.15.12.867. [DOI] [PubMed] [Google Scholar]
- 147.Nauck M, Stockmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 148.Shuster LT, Go VL, Rizza RA, et al. Incretin effect due to increased secretion and decreased clearance of insulin in normal humans. Diabetes. 1988;37:200–203. doi: 10.2337/diab.37.2.200. [DOI] [PubMed] [Google Scholar]
- 149.Meier JJ, Holst JJ, Schmidt WE, et al. Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab. 2007;293:E849–E856. doi: 10.1152/ajpendo.00289.2007. [DOI] [PubMed] [Google Scholar]
- 150.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 151.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bacha F, Gungor N, Lee S, et al. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32:100–105. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seltzer HS, Allen EW, Herron AL, Jr, et al. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46:323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bergman RN, Ader M, Huecking K, et al. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–S220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 156.Roslin MS, Dudiy Y, Weiskopf J, et al. Comparison between RYGB, DS, and VSG effect on glucose homeostasis. Obes Surg. 2012 doi: 10.1007/s11695-012-0686-0. in press. [DOI] [PubMed] [Google Scholar]
- 157.Hofso D, Jenssen T, Bollerslev J, et al. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2011;164:231–238. doi: 10.1530/EJE-10-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Briatore L, Salani B, Andraghetti G, et al. Beta-cell function improvement after biliopancreatic diversion in subjects with type 2 diabetes and morbid obesity. Obesity (Silver Spring) 2010;18:932–936. doi: 10.1038/oby.2010.28. [DOI] [PubMed] [Google Scholar]
- 159.Sandoval D. Bariatric surgeries: beyond restriction and malabsorption. Int J Obes (Lond) 2011;35(Suppl 3):S45–S49. doi: 10.1038/ijo.2011.148. [DOI] [PubMed] [Google Scholar]
- 160.Chiellini C, Rubino F, Castagneto M, et al. The effect of bilio-pancreatic diversion on type 2 diabetes in patients with BMI <35 kg/m2. Diabetologia. 2009;52:1027–1030. doi: 10.1007/s00125-009-1333-8. [DOI] [PubMed] [Google Scholar]
- 161.Pories WJ, Caro JF, Flickinger EG, et al. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg. 1987;206:316–323. doi: 10.1097/00000658-198709000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smith BR, Hinojosa MW, Reavis KM, et al. Remission of diabetes after laparoscopic gastric bypass. Am Surg. 2008;74:948–952. [PubMed] [Google Scholar]
- 164.Scopinaro N, Papadia F, Camerini G, et al. A comparison of a personal series of biliopancreatic diversion and literature data on gastric bypass help to explain the mechanisms of resolution of type 2 diabetes by the two operations. Obes Surg. 2008;18:1035–1038. doi: 10.1007/s11695-008-9531-x. [DOI] [PubMed] [Google Scholar]
- 165.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alam I, Stephens JW, Fielding A, et al. Temporal changes in glucose and insulin homeostasis after biliopancreatic diversion and laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2011 doi: 10.1016/j.soard.2011.10.018. in press. [DOI] [PubMed] [Google Scholar]
- 167.Markovic TP, Jenkins AB, Campbell LV, et al. The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care. 1998;21:687–694. doi: 10.2337/diacare.21.5.687. [DOI] [PubMed] [Google Scholar]
- 168.Jazet IM, Pijl H, Frolich M, et al. Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism. 2005;54:705–712. doi: 10.1016/j.metabol.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 169.Kelley DE, Wing R, Buonocore C, et al. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1287–1293. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 170.Cohen RV, Schiavon CA, Pinheiro JS, et al. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg Obes Relat Dis. 2007;3:195–197. doi: 10.1016/j.soard.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 171.Cohen RV, Rubino F, Schiavon C, et al. Diabetes remission without weight loss after duodenal bypass surgery. Surg Obes Relat Dis. 2011 doi: 10.1016/j.soard.2011.07.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Klein S, Fabbrini E, Patterson BW, et al. Moderate effect of duodenal-jejunal bypass surgery on glucose homeostasis in patients with type 2 diabetes. Obesity (Silver Spring) 2012;20:1266–1272. doi: 10.1038/oby.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jiang FZ, Zhu HL, Zheng XF, et al. Duodenojejunal bypass in treatment for 7 cases with non-severe obese type 2 diabetes mellitus. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:36–38. [PubMed] [Google Scholar]
- 174.Ferzli GS, Dominique E, Ciaglia M, et al. Clinical improvement after duodenojejunal bypass for nonobese type 2 diabetes despite minimal improvement in glycemic homeostasis. World J Surg. 2009;33:972–979. doi: 10.1007/s00268-009-9968-7. [DOI] [PubMed] [Google Scholar]
- 175.Geloneze B, Geloneze SR, Fiori C, et al. Surgery for nonobese type 2 diabetic patients: an interventional study with duodenal-jejunal exclusion. Obes Surg. 2009;19:1077–1083. doi: 10.1007/s11695-009-9844-4. [DOI] [PubMed] [Google Scholar]
- 176.Ramos AC, Galvao Neto MP, de Souza YM, et al. Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI<30 kg/m2 (LBMI) Obes Surg. 2009;19:307–312. doi: 10.1007/s11695-008-9759-5. [DOI] [PubMed] [Google Scholar]
- 177.Sarson DL, Scopinaro N, Bloom SR. Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes. 1981;5:471–480. [PubMed] [Google Scholar]
- 178.Naslund E, Gryback P, Hellstrom PM, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord. 1997;21:387–392. doi: 10.1038/sj.ijo.0800418. [DOI] [PubMed] [Google Scholar]
- 179.Naslund E, Backman L, Holst JJ, et al. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg. 1998;8:253–260. doi: 10.1381/096089298765554449. [DOI] [PubMed] [Google Scholar]
- 180.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 181.Korner J, Bessler M, Inabnet W, et al. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22:1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 183.Whitson BA, Leslie DB, Kellogg TA, et al. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res. 2007;141:31–39. doi: 10.1016/j.jss.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 184.Falken Y, Hellstrom PM, Holst JJ, et al. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 185.Bose M, Teixeira J, Olivan B, et al. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes. 2010;2:47–55. doi: 10.1111/j.1753-0407.2009.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meirelles K, Ahmed T, Culnan DM, et al. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shin AC, Zheng H, Townsend RL, et al. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tichansky DS, Boughter JD, Jr, Harper J, et al. Gastric bypass surgery in rats produces weight loss modeling after human gastric bypass. Obes Surg. 2008;18:1246–1250. doi: 10.1007/s11695-008-9556-1. [DOI] [PubMed] [Google Scholar]
- 190.Chambers AP, Stefater MA, Wilson-Perez HE, et al. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav. 2011;105:120–123. doi: 10.1016/j.physbeh.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chelikani PK, Shah IH, Taqi E, et al. Comparison of the effects of Roux-en-Y gastric bypass and ileal transposition surgeries on food intake, body weight, and circulating peptide YY concentrations in rats. Obes Surg. 2010;20:1281–1288. doi: 10.1007/s11695-010-0139-6. [DOI] [PubMed] [Google Scholar]
- 192.Suzuki S, Ramos EJ, Goncalves CG, et al. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–290. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 193.Aprahamian CJ, Tekant G, Chen M, et al. A rat model of childhood diet-induced obesity: Roux-en-Y gastric bypass induced changes in metabolic parameters and gastric peptide ghrelin. Pediatr Surg Int. 2007;23:653–657. doi: 10.1007/s00383-007-1944-4. [DOI] [PubMed] [Google Scholar]
- 194.Zhang H, Wang Y, Zhang J, et al. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31:2063–2069. doi: 10.1161/ATVBAHA.111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yin DP, Gao Q, Ma LL, et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Ann Surg. 2011;254:73–82. doi: 10.1097/SLA.0b013e3182197035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg. 2012;255:294–301. doi: 10.1097/SLA.0b013e31823e71b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bueter M, Lowenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 198.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17:1839–1847. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nestoridi E, Kvas S, Kucharczyk J, et al. Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology. 2012;153:2234–2244. doi: 10.1210/en.2011-2041. [DOI] [PubMed] [Google Scholar]
- 200.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pacheco D, de Luis DA, Romero A, et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am J Surg. 2007;194:221–224. doi: 10.1016/j.amjsurg.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 202.de Luis D, Domingo M, Romero A, et al. Effects of duodenal-jejunal exclusion on beta cell function and hormonal regulation in Goto-Kakizaki rats. Am J Surg. 2012 doi: 10.1016/j.amjsurg.2011.07.020. in press. [DOI] [PubMed] [Google Scholar]
- 203.Wang Y, Zhang ZZ, Wang L, et al. Effect of diabetes control after small intestine exclusion surgery in Goto-Kakizaki rat with non-obese type 2 diabetes mellitus. Zhonghua Wai Ke Za Zhi. 2009;47:1736–1740. [PubMed] [Google Scholar]
- 204.Kindel TL, Yoder SM, Seeley RJ, et al. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13:1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 205.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Speck M, Cho YM, Asadi A, et al. Duodenal-jejunal bypass protects GK rats from β-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300:E923–E932. doi: 10.1152/ajpendo.00422.2010. [DOI] [PubMed] [Google Scholar]
- 207.Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012 doi: 10.1038/nm.2745. in press. [DOI] [PubMed] [Google Scholar]
- 208.Rubino F, Zizzari P, Tomasetto C, et al. The role of the small bowel in the regulation of circulating ghrelin levels and food intake in the obese Zucker rat. Endocrinology. 2005;146:1745–1751. doi: 10.1210/en.2004-1181. [DOI] [PubMed] [Google Scholar]
- 209.Kindel TL, Martins PJ, Yoder SM, et al. Bypassing the duodenum does not improve insulin resistance associated with diet-induced obesity in rodents. Obesity (Silver Spring) 2011;19:380–387. doi: 10.1038/oby.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 211.Pories WJ, Dohm LG, Mansfield CJ. Beyond the BMI: the search for better guidelines for bariatric surgery. Obesity (Silver Spring) 2010;18:865–871. doi: 10.1038/oby.2010.8. [DOI] [PubMed] [Google Scholar]
- 212.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]