Abstract
Human immunodeficiency virus (HIV) infection has been implicated as a trigger for various autoimmune diseases, one of which is dermatomyositis. This is a very rare autoimmune disease characterised by myopathy, typical cutaneous signs and variable systemic manifestations. To our knowledge, the association of this rare disease with HIV infection has not been previously reported in Nigeria. We therefore decided to report the case of a 40 year old HIV-1 infected Nigerian female who presented to us with muscle, skin, and systemic manifestations of dermatomyositis. Our aim is to show the effect of HIV infection, as well as HAART-induced immune reconstitution on the clinical course of dermatomyositis.
Keywords: dermatomyositis, HIV, autoimmune disease, immune reconstitution syndrome, Nigeria
Introduction
Dermatomyositis is a rare inflammatory autoimmune disease of the muscle and skin, characterised primarily by proximal myopathy and various cutaneous signs, as well as multi-systemic manifestations1,2. Although the exact aetiology of this disease remains unknown, infection with human immunodeficiency virus (HIV) has been implicated as a possible trigger3,4. To our knowledge, there has been no previous report of dermatomyositis associated with HIV infection from Nigeria. We hereby report a case of dermatomyositis in a HIV-infected Nigerian woman.
Case report
A 40 years old housewife was referred to our centre with three years history of progressive weakness of hip and shoulder muscles, resulting in difficulty rising from a chair and lifting objects. About one year after onset of muscle weakness, she developed progressive muscle pains, recurrent painful joint swellings of the hands and feet, and hyperpigmented non-pruritic rashes in sun-exposed areas of her body such as face, hands and feet. There were no sicca symptoms, dysphagia, speech abnormalities or cardiorespiratory symptoms; and there was no history of drug allergy or hypersensitivity reactions. Six months into her illness, she tested seropositive to HIV-1, confirmed by Western blot and was commenced on highly active antiretroviral therapy (HAART) consisting of daily tablet of Truvada (tenofovir 300 mg + emtricitabine 300 mg) and nevirapine 200 mg b.d, at a baseline CD4 cell-count of 168cells/ul. The referral hospital had also placed her on different analgesics for pain relief including acetaminophen and NSAIDS such as piroxicam. However, she did not receive any steroid therapy since the diagnosis of autoimmune diseases such as dermatomyositis was not considered by referring hospital. During the first year of HAART, her symptoms were characterised by periods of remission lasting two - three months. However, after one year of regular HAART, and periodic analgesics, the symptoms became worse, despite viral suppression to undetectable level (less than 400 viral copies/ul) and increase in CD4 cell count to 383cells/ul. The deterioration in her symptoms necessitated referral to our centre for expert management.
On presentation to our centre, physical examination revealed a middle aged woman who was afebrile, anicteric, and not pale. She had no leg oedema, peripheral adenopathy, malar rash, mouth ulcers, alopecia, or skin thickening. Her breast, chest, cardiovascular, abdominal, and pelvic examinations were normal.
She had hyperpigmented maculopapular rashes over the lower eye lids (heliotrope rash)-[figure1] and, over the metacarpophalangeal, proximal and distal interphalangeal joints of both hands (Guttron's papules)-[figure 2], as well as over the extensor surfaces of both knees (knee erythema)-[figure 3] and feet. The skin of both hands was rough and cracked with irregular “dirty” hyperpigmented spots on lateral, palmar surfaces of the hand and on the tips of fingers (mechanic's hands)-[figure 4]. Apart from both wrist joints that were slightly swollen and tender, other joints were normal.
Figure 1.
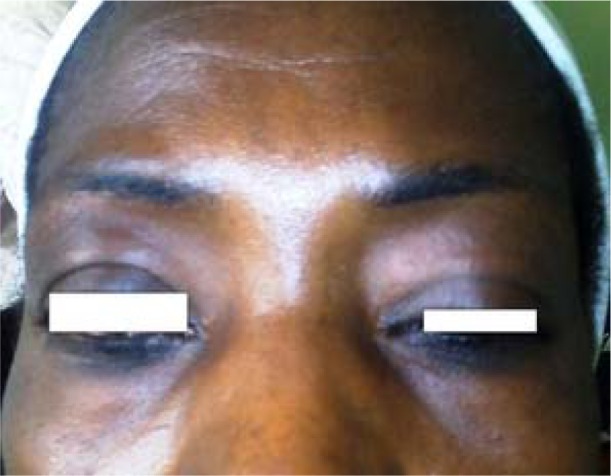
Heliotrope rash; hyperpigmentation of the lower eyelid
Figure 2.

Gottron's sign; hyperpigmented macules over the metacarpophalangeal and interphalangeal joints of both hands
Figure 3.
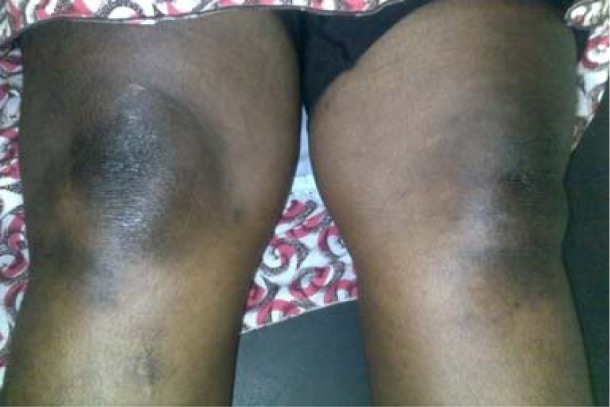
Knee erythema; hyperpigmented macules over the both knees
Figure 4.
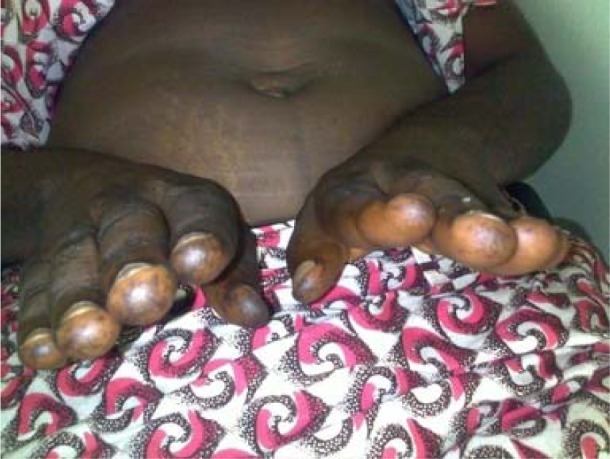
Mechanic's hands; hyperpigmented ‘dirty’ macular spots on the tips and lateral surfaces of the fingers of both hands
The deltoids and quadriceps muscles were tender to deep pressure and wasted bilaterally with power of 3 and 4 respectively (BMRC grading). The biceps and triceps were hypertonic and stiff, although reflexes were variable, with diminished biceps jerk and normal knee jerk. There were no sensory abnormalities.
Anciliary investigations: electrocardiogram, Xrays of the wrist joints, chest X-ray, abdominopelvic ultrasound, total while cell count (5 × 109/I), packed cell volume (33%), and renal function tests were negative, but erythrocytes sedimentation rate (ESR) (120mm/hr) and aspartate transaminase (142mmol/l) were both elevated. Creatine kinase, anti-Jo-1 (histadyl tRNA synthetase) antibody, and electromyography were not done due to resource constraints, and the patient refused muscle biopsy.
She was maintained on her HAART regimen and physiotherapy was commenced along with tablets of diclofenac potassium 50mg b.d and prednisolone 60mg daily. After four weeks of therapy, her symptoms gradually resolved, power in the proximal muscles increased to grade 4/5 and tghe hyperpigmented rashes diminished. She was maintained on tablets of prednisolone 10mg daily for four weeks and then 5mg for a further two weeks. She continued to improve and was discharged to be followed up on an outpatient basis.
Discussion
In this patient, a clinical diagnosis of dermatomyositis was made based on typical features of heliotrope rash, Gottron's sign, knee erythema, proximal myopathy, elevated ESR, muscle pain and tenderness, and nondestructive arthritis. This is in agreement with established definite diagnostic criteria for dermatomyositis by Tanimoto et al 5 While the exact aetiology of dermatomyositis remains unknown, malignancies (breast, lung, ovary, and stomach), drugs (hydroxyurea, penicillamine, and statins, among others), and viruses (coxsackievirus, echovirus, human T-cell lymphotrophic virus type 1, HIV) have been implicated as possible triggers.1,2 Furthermore, in the overlap syndromes, dermatomyositis may rarely be associated with other autoimmune collagen vascular diseases such as scleroderma and SLE.1,2 Our patient had no history of ingestion of any of the drugs implicated as triggers for dermatomyositis. There was no apparent evidence of malignancies and other autoimmune diseases such as SLE or scleroderma were excluded by absence of diagnostic signs and symptoms, as well as by negative anti-nuclear antibodies. While it was not possible to confirm other viral infectious triggers due to resource constraints, our patient was HIV-1, infected confirmed by western blot. Consequently, it may be suggested that the trigger for dermatomyositis in our patient is HIV-1 infection. This assertion is supported by previous reports of dermatomyositis associated with HIV-infection in the literature4, 6–8.
The endothelium of muscle capillaries and small arterioles is the primary antigenic target for the complement-mediated autoimmune attack in dermatomyositis.1,2,4 HIV may trigger this autoimmune attack by inducing polyclonal activation of B cells with formation of autoantibodies, by increasing expression of autoantigens due to direct infection of the endothelium, and by molecular mimicry between HIV and the endothelium.3,4
There is an intriguing relationship between the stage of HIV disease and the development of autoimmune diseases. In early HIV infection, autoimmune diseases may occur due to polyclonal activation of B cells with formation of auto-antibodies.3 In the later stages of HIV-infection, when immune responses are significantly suppressed, autoimmunity is also suppressed and autoimmune diseases occur rarely.3 However, after HAART-induced immune reconstitution, immune responses may become abnormally exaggerated and uncoordinated, leading to a resurgence of autoimmune diseases.3 Perhaps the above explains the clinical course of our patient which was characterised by progression of symptoms before HIV diagnosis, periods of remissions of symptoms at HIV diagnosis and during first year of HAART (when patient's CD4 cell count was 168/µl) and worsening of symptoms after one year of HAART-induced immune reconstitution. Our patient's illness improved with prednisolone which is the mainstay of therapy of dermatomyositis. 1,2 However, such potentially immunosuppressive therapy must be implemented cautiously in HIV-infected patients, who are inherently immunosuppressed.
Conclusion
We have reported this case to remind clinicians of the associations between HIV-1 infection, HAART-induced immune reconstitution and the clinical course of dermatomyositis.
References
- 1.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 2.Gondim FA, Brannagan TH. Dermatomyositis/Polymyositis. Updated: Mar 8, 2010. Available at emedicine.medscape.com.
- 3.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmunity Reviews. 2002;1:329–337. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 4.Carroll MB, Holmes R. Dermatomyositis and HIV infection: case report and review of the literature. Rheumatol Int. 2009 Oct; doi: 10.1007/s00296-009-1231-x. [DOI] [PubMed] [Google Scholar]
- 5.Tanimoto K, Nakano K, Kano S, Mori S, Ueki H, Nishitani H, et al. Classification criteria for polymyositis and dermatomyositis. J Rheumatol. 1995 Apr;22(4):668–674. [PubMed] [Google Scholar]
- 6.Baguley E, Wolfe C, Hughes GRV. Dermatomyositis in HIV infection [letters to the editor] Br J Rheum. 1988;27:493–500. doi: 10.1093/rheumatology/27.6.493-a. [DOI] [PubMed] [Google Scholar]
- 7.Gresh JP, Aguilar JL, Espinoza LR. Human immunodeficiency virus (HIV) infection-associated dermatomyositis. J Rheum. 1989;16(10):1397–1398. [PubMed] [Google Scholar]
- 8.Marfatia YS, Ghiya RA, Chaudhary D. Dermatomyositis in a human immunodeficiency virus-infected person. Indian J Dermatol Venereol Leprol. 2008;74(3):241–243. doi: 10.4103/0378-6323.41370. [DOI] [PubMed] [Google Scholar]


