Abstract
Objectives
To determine the relative frequency of prostate cancer among surgical specimens, and among prostate specimens received at the pathology department ,University Hospital Calabar.
Methods
Histology records were reviewed for the following: total number of histology specimens received; total number of prostate specimens; total number of prostate cancer; and the total number of cancers in males during the study period. Histology sections 4–5microns thick were cut from paraffin blocks and stained by Haematoxylin and Eosin (H&E). Histopathologic specimens were classified using the grading system of tumour differentiation described by Gleason and associates.
Results
One hundred and twenty three cancers of the prostate were received, constituting 2% of the total surgical specimens and 31% of prostate specimens. Thirty three cases (27%) could not be analyzed; therefore the study is based on 90 prostate cancer specimens. Eighty nine (99%) cases were epithelial tumours (adenocarcinoma.) There was a single mesenchymal tumour (rhabdomyosarcoma) (1%). The commonest grade in this study was the high grade (Gleason grade IV).
Conclusions
We observed that prostate cancer is a common among males (all sites) diagnosed at the University Hospital Calabar, with a peak incidence between the ages of 61 – 70 years (seventh decade).
Keywords: prostate, cancer, analysis, histologic, pattern, role, grade
Introduction
Prostate cancer is predominantly a disease of the elderly and is rarely found before the age of 50 years1. There are many reported series showing an increasing incidence of latent cancers in elderly patients.2 Prostate cancer is second only to lung cancer in males worldwide and is the most common cause of cancer death in the western world.3
According to the World Health Organization (WHO), a total of 508 534 new prostate cancers were diagnosed in 2000; with an estimated mortality of 212 558 deaths.1
There are remarkable national and racial differences in the incidence of this disease4 The highest incidence worldwide is said to occur in American blacks and the lowest incidence is among oriental Asians and in the Honduras5. In the African continent prostate cancer ranks among the top five cancers in men.1 Studies in the West African sub-region, showed it is a common cancer in the population, and is the fourth most common cancer among males.6
Prostate cancer is one of the most common causes of surgical admission of elderly men, second only to benign prostatic hyperplasia.7 In one report, it was stated to be the most common cancer in Nigerian men contributing 11% of male cancers.8 Another report stated it as the most common cause of cancer death in men older than 50 years.9 A review showed a relatively early peak at 55– 64 years in Africans compared to 65 – 74 years for whites.10 One Nigerian report put the median age at 67.5 years, and mean age at 71.4 years.8 Other Nigerian studies similarly reported the mean age to be the seventh decade.7
Gleason grading of prostatic specimens remains as one of the most powerful factors predicting prognosis in the patient with prostate cancer. For most carcinomas, the finding of poor differentiation (high grade) offers a reliable and powerful index of aggressive biologic behavior, including high risk of metastasis11–13. The most aggressive tumour found in a prostate biopsy, even if it is the smallest component may determine prognosis of patient's tumour.11–13 The natural history of the three groups (low risk, intermediate risk and high risk) is quite distinct. Therefore, the clinician must be cognizant of the gleason grade of the prostate cancer when making treatment. There are various studies that demonstrate that the amount of gleason grade 4 and 5 relate to the overall prognosis of the patient.
This study aimed at: determining the relative frequency of prostate cancer in men as documented in our cancer registry, its frequency in prostate specimens, the age distribution of the disease, the commonest histological types and the various grades
Methods
This retrospective study involves an audit of histologically verified prostate cancer specimens from surgical pathology records of the Department of Pathology, University of Calabar Teaching Hospital (UCTH) Calabar, Nigeria from May 1994 to April 2004(10 years). The UCTH is one of the centres with histopathological services in the South-South Zone of Nigeria. The hospital is a reference centre to many government, missionary and private hospitals and clinics within the zone.
Histology records of the department were reviewed for the following: total number of histology specimens received; total number of prostate specimens; total number of prostate cancer; and the total number of cancers in males during the study period. Histopathologic specimens of the prostate biopsies were also graded using the grading system of tumour differentiation described by Gleason and associates. Records were also analyzed for age distribution. Histology sections 4–5microns thick were cut from paraffin blocks and stained by Haematoxylin and Eosin (H&E). Serial sections cut were examined at 40x magnification.The lesions were histologically typed according to the 2004 WHO histological classification of prostate tumours.
Results
During the ten-year period, May 1994 and April 2004 a total of 6 121 surgical specimens (male and female) from various anatomic sites were received in the department of pathology of the U.C.T.H Calabar, of which 374 (6.1%) were from the prostate and 123(32.9%) were histologically proven to be cancer of the prostate. Prostate cancer comprised 123(2%) of the 6 121 surgical specimens (male and female) and 32.9% of the 374 prostate specimens. Three hundred and ninety-four (6.4%) of the 6 121 specimens were the total number of malignant neoplasms (all sites) in males and prostate cancer comprised 123 (31.2%) of all male cancers (all sites). Thirty-three of the one hundred and twenty-three prostate cancer specimens could not be analyzed for age and Gleason's grading/ccoring due to missing slides, blocks and inadequate clinical information. Ninety (73.1%) prostate cancer specimens were then analyzed for histologic type, grade and score .The records were also analyzed for age of patients.
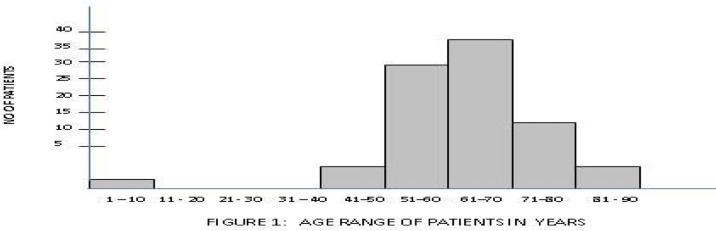
The peak age of the disease was in the 61–70 years age group (seventh decade as shown in figure 1). The mean age of the patients was 64.5 with a range between 45 and 90 years. Five (5.5%) patients were below 50 years and five were above 80 years of age; majority (89%) were between 51 and 80 years. Epithelial tumours predominated and adenocarcinomas were found in 89 of the 90 patients (99%). One four year old patient (1%) had embroyonal rhabdomyosacoma.
Figure 1.
Age distribution of prostate cancer patients
About 47 of the 90 Ca Prostate specimens (53%) were of primary Gleason grade 4, 22 of 90 (25%) of grade 3, 11 (12%) of grade 5 and 9 (10%) of grades 1 and 2. - Figure 2. Intraepithelial neoplasia (PIN) was observed in two cases (2.2%).
Figure 2.
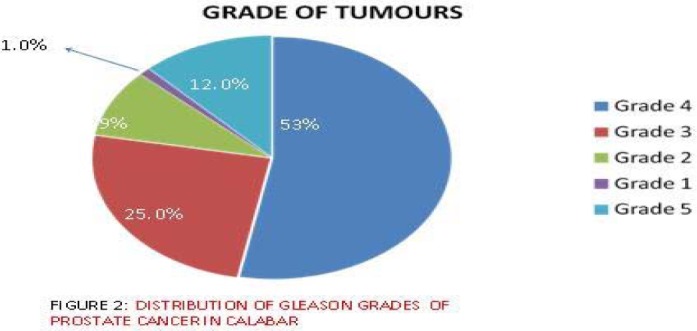
Gleason grade distribution of prostate cancers
Discussion
This study was carried out to determine the relative frequency, histological types, Gleason's grade and score, as well as the age distribution of patients with prostate cancer in surgical specimens received at the pathology department of the University of Calabar Teaching Hospital (UCTH), Calabar, Nigeria from May 1994 April 2004,10 years in total.
During the period, a total of 123 prostate cancer specimens were received accounting for 2% of the total 6 121 surgical specimen received. The total number of male malignancies (all sites) were 394 and prostate cancer accounted for 31.2% of male cancer, during the period. This contrasted with the observation of Ogunbiyi et al8 in Ibadan, Nigeria who had 11% in his study. However our finding is similar to those of Mosli et al14 in Saudi Arabia and Akang15 in Benin; both had 2% of histologically proven prostate cancer in surgical specimens. It is possible that the previously observed low incidence of prostate cancer in surgical specimen in Calabar16 may be attributed to the paucity of trained urologists in the centre at that time.
Most of the cancers (41.1%) were in patients between the ages of 61–70 years (seventh decade). This agrees with most studies across the globe2–5,17, but differs from the studies in Enugu18 and Kenya19 where the age ranges were 50–70 and 70–79 years respectively. The mean age at diagnosis was 64.5 years which is similar to the experience of other researchers in Nigeria7, 16 except Udeh18 and Ogunbiyi et al8 who had values of 59.8 years and 71.4 years respectively.
The age range in this study was 45–90 years (between fifth to ninth decade), which agrees with the work of Abbiyesiku et al20. However a few researchers have recorded a younger age range; Ekwere et al had 35–88 years16 range, and Udeh had 32–85 years18. In Udeh's study, 11% of the patients were above 70 years and 15% were below 50 years. In this work 22.5% of the patients were above 70 years of age while 5.6% were below the age of 50 and about 88.8% are between the 50 and 70 year age bracket. This finding contrasts with the review from Saudi Arabia14, which showed 56% of patients were over 70 years and 44% were under 70 years. It is possible that certain environmental/social factors may be responsible for these differences. Furthermore, Mosli's14 review in Saudi Arabia showed a predominance of Grade III lesion. This agrees with the work of Gaeta and associates21, but differs with our finding, which showed a predominance of grade IV cancers. These differences could imply disparities in screening awareness. There are also established disparities in biology of prostate cancer among different races and ethnic groups.22, 23
Most cancers (98.9%) were adenocarcinoma which is in line with the work of Akang et al 15. High risk cancers (Gleason score 8–10) made up the largest percentage of cases which is similar to the work done in Zaria24 that showed predominantly poorly differentiated cancers. This work contrasts sharply with the work done in Benin 15, which showed that 64% of adenocarcinomas were well differentiated. The reason for the difference in grade and degree of differentiation compared to some other parts of Nigeria is difficult to explain. It is possible that environmental factors may be responsible.
We must appreciate the important limitations of this study. This is a hospital based study and cannot be said to be a reflection of the wider community. It is also based on surgical specimens submitted for histopathological examination. This implies several selection biases, because it excludes conditions in which a surgical (or biopsy) specimen is not usually obtained, it excludes patients who may not have had access to this diagnostic modality and it excludes patients who may have been treated without a histological diagnosis, or who died without a surgical specimen having been obtained. The study also provides no information on the clinical stage, serum PSA, treatment, or duration of survival in the patients. Other deficiencies such as incomplete records are also appreciated
Conclusion
We observed that prostate cancer comprises a large proportion (31.3%) of all male cancers (all sites) histologically diagnosed in the University of Calabar Teaching Hospital, with a peak incidence between the ages of 61 – 70 years. Adenocarcinoma was the commonest histological pattern, while the Gleason grade IV was the predominant grade seen. The majority of the case were high risk (Gleason score 8–10) to intermediate risk (Gleason score 7) cancers. Our findings are comparable to similar studies within Nigeria and other countries. A prospective study is necessary, during which the role of environmental factors in the aetiopathogenesis of prostate cancer especially, in the African setting, may be defined. .
Table 1.
Gleason score distribution of prostate cancers
| Gleason score | Risk | No of patients |
| 2–6 | Low risk | 33 (37%) |
| 7 | Intermediate risk | 22 (25%) |
| 8–10 | High risk | 34 (38%) |
| Total | 89 |
One case of embroyonal rhabdomyosacoma was excluded
References
- 1.Ferlay J, Parkin DM, Pisani P. Cancer Incidence and Mortality Worldwide. GLOBOCAN 1 IARC. WHO; 1998. [Google Scholar]
- 2.Meike AW, Smith JA. Epidemiology of Prostate Cancer. Urol Clin North Am. 1990;17:709–718. [PubMed] [Google Scholar]
- 3.Haas GP, Sakr WA. Epidemiology of Prostate Cancer. Cancer J Clin. 1997;47:273–287. doi: 10.3322/canjclin.47.5.273. [DOI] [PubMed] [Google Scholar]
- 4.Scardino PT, Wearer R, Hudson MA. Early detection of prostate cancer. Human Pathol. 1992;23:211. doi: 10.1016/0046-8177(92)90102-9. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. IARC Scientific Publications; 2003. p. 155. [Google Scholar]
- 6.Jackson MA, Ahluwalia BS, Heshmat MY. Characterization of Prostate Carcinoma among blacks,a continuation report. Cancer Treat Rep. 1977;61:167–172. [PubMed] [Google Scholar]
- 7.Osegbe DN. Prostate Cancer in Nigerians. Facts and Nonfacts. J Urol. 1997;157:1340–1343. [PubMed] [Google Scholar]
- 8.Ogunbiyi JO, Shittu OB. Increased Incidence of prostate Cancer in Nigerians. JNatl Med Assoc. 1999;91:159–164. [PMC free article] [PubMed] [Google Scholar]
- 9.Osegbe D N, Ogunlewe J O. Androgen Concentration in Blacks with Benign and Malignant Prostatic Disease. J Urol. 1988;140:160–164. doi: 10.1016/s0022-5347(17)41518-7. [DOI] [PubMed] [Google Scholar]
- 10.Boring CC, Squires TS, Heath CW., Jr Cancer Statistics for African Americans. C A Cancer J Clin. 1992;42:7–17. doi: 10.3322/canjclin.42.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Stamey TA, McNeal JE, Yemoto CM, et al. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 12.McNeal JE. Cancer volume and site of origin of adenocarcinoma in the prostate: relationship to local and distant spread. Hum Path. 1992;23:258–266. doi: 10.1016/0046-8177(92)90106-d. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi M, Stamey TA, McNeal TA, et al. Preoperative prostate specific antigen does not reflect biochemical failure rates after radical prostatectomy in men with large volume cancer. J Urol. 2000;164:1596–1600. [PubMed] [Google Scholar]
- 14.Mosli HA. Prostate cancer in Saudi Arabia: A review of the literature. Ann Saudi Med. 1997;17:510–514. doi: 10.5144/0256-4947.1997.510. [DOI] [PubMed] [Google Scholar]
- 15.Akang EE, Aligbe JU, Olisa EG. Prostatic cancer in Benin city Nigeria. West Afri J Med. 1996;15:56–60. [PubMed] [Google Scholar]
- 16.Ekwere PD, Egbe SN. The Changing pattern of prostate cancer in Nigerians: current status in the South Eastern State. J Natl Med Assoc. 2002;94:619–627. [PMC free article] [PubMed] [Google Scholar]
- 17.Talukder SI, Roy MK, Azam MS, Huq MH, Haque MA, Saleh AF. Histopathological Patterns of Prostate Specimens in Mymensingh. Dinajpur Med Col J. 2008 Jul;1(2):29–32. [Google Scholar]
- 18.Udeh FN. Prostate carcinoma in Nigeria. A ten year retrospective study. Int Urol Neph. 1981;13:159–165. doi: 10.1007/BF02082060. [DOI] [PubMed] [Google Scholar]
- 19.Mahoga GA. Epidemiological and Clinical Aspects of Incidental cancer of Prostate in Africans. East Afr Med J. 1995;72:283–287. [PubMed] [Google Scholar]
- 20.Abbiyesiku FM, Shittu OB, Oduwole OO, Osotimehin BO. Prostate specific antigen in the Nigerian Africans. Afr J Med Sci. 2000;29:97–100. [PubMed] [Google Scholar]
- 21.Gaeta JF, Asirwatham JE, Miller G, Murphy G. Histologic Grading of Primary Prostate Cancer: A new approach to an old problem. J Urol. 1980;123:689–693. doi: 10.1016/s0022-5347(17)56093-0. [DOI] [PubMed] [Google Scholar]
- 22.Sneyd MJ. Ethnic differences in prostate cancer survival in New Zealand: a national study. Cancer Causes Control. 2008 Nov;19(9):993–999. doi: 10.1007/s10552-008-9166-1. Epub 2008 May 14. [DOI] [PubMed] [Google Scholar]
- 23.Caruso RP, Levinson B, Melamed J, Wieczorek R, Taneja S, Polsky D, Chang C, Zeleniuch-Jacquotte A, Salnikow K, Yee H, Costa M, Osman I. Altered N-myc Downstream-Regulated Gene 1 Protein Expression in African-American Compared with Caucasian Prostate Cancer Patients. Clin Cancer Res. 2004;10:222. doi: 10.1158/1078-0432.ccr-0604-3. [DOI] [PubMed] [Google Scholar]
- 24.Dawam D, Rafindadi AH, Kalayi GD. Benign Prostate Hyperplasia and Prostate Cancer in Native Africans. BJU Int. 2000;85:1074–1077. doi: 10.1046/j.1464-410x.2000.00677.x. [DOI] [PubMed] [Google Scholar]