Abstract
Background: Moist wound treatment improves healing of skin graft donor site wounds. Microbial colonised wounds represent an increased risk of wound infection; while antimicrobially active, topical antiseptics may impair epithelialization. Objectives: The aim of this prospective randomised controlled clinical trial was to examine the influence of an Octenidine-dihydrochloride (OCT) hydrogel on bacterial colonisation and epithelialization of skin graft donor sites. Methods: The study was designed as a randomised, double-blinded, controlled clinical trial. Skin graft donor sites from a total of 61 patients were covered either with 0.05% OCT (n=31) or an OCT-free placebo wound hydrogel (n=30). Potential interaction with wound healing was assessed by measuring the time until 100% re-epithelialization. In addition, microbial wound colonisation was quantitatively determined in all skin graft donor sites. Results: There was no statistically significant difference in the time for complete epithelialization of skin graft donor sites in the OCT and the placebo group (7.3±0.2 vs. 6.9±0.2 days; p=0.236). Microbial wound colonisation was significantly lower in the OCT group than in the placebo group (p=0.014). Conclusions: The OCT-based hydrogel showed no delay in wound epithelialization and demonstrated a significantly lower bacterial colonisation of skin graft donor site wounds.
Keywords: Octenidine, wound gel, antimicrobial compound, skin graft donor site, skin graft, acute wound, tolerability, antiseptic efficacy
Introduction
Worldwide, more than 6 million burns are treated in health care facilities, not including the number of burns treated outside the hospitals [1]. Flame burns and scalds account for up to 75% of the reported cases, half of these occurring at home [2]. In case of burns and scald injuries, the incidence of chronic, non-healing wounds is increasing due to the aging population and the corresponding cumulative comorbidity. In 2009, it was estimated that 11 million venous ulcers and 11.3 million diabetic ulcers requiring medical treatment exist globally [1]. Chronic wounds as well as burns in general show a prolonged healing process with a high demand of medical care. The reason for a wound to become chronic is based on a multifactorial process such as repeated trauma, continued pressure, ischemia, or endocrine disorders and systemic disease [3,4].
Infection is a major complication in both chronic and burn wounds, contributing to delayed healing of wounds [2,3]. Any wound infection starts with colonisation of pathogenic bacteria, which can occur within 6 hours after a superficial epithelial lesion is present [5]. Therefore, control of microbiological colonisation, and hence, prevention of infection, is of particular importance and requires adequate wound dressings and additional antimicrobial management [6]. While topically applied antimicrobial agents, dependent of their mode of action, are able to be antibacterially effective, they may equally interfere with human cell proliferation during wound healing (i.e. immature and non-adherent keratinocytes), and ultimately result in delayed wound healing. Therefore, the general application of topical antimicrobials on chronic and burn wounds is discussed controversially, as they also may negatively affect the epithelialization of wounds [7,8]. Due to this fact, a number of compounds with high antimicrobial efficacy cannot be used on wounds because of their high cytotoxicity. Thus, a balance must be achieved between antimicrobial efficacy and cytotoxicity [9-11,22].
In recent years, preclinical studies and clinical observational trials have confirmed the applicability and suitability of antimicrobial preparations containing Octenidine-dihydrochloride (OCT). Due to its low cytotoxicity and high antimicrobial efficacy, OCT is a potent candidate for preventing wound infection while not affecting wound healing [12,23]. In order to verify the available preclinical results, the aim of this study was to investigate the clinical effect of an OCT-based hydrogel on epithelialization and bacterial colonisation in a prospective, double-blinded, randomised clinical trial. As there is no standardised model for chronic, non-healing wounds, and finding perfectly matched patients with chronic wounds is futile, this study was conducted using skin graft donor site wounds in burn patients, which allows controlled standardisation. Skin graft donor sites in particular are suitable as a standardised model of human wounds and allow comparative assessment of the epithelialization process and tissue compatibility, as they are readily available, reproducible in other settings and most importantly, do not cause additional stress to the patient [13].
In this context, the aim of this present randomised clinical trial was to determine the tissue compatibility and the antimicrobial effectiveness of OCT in comparison to an OCT-free hydrogel preparation (placebo) in order to verify its safety and effectiveness for use in wounds.
Materials and methods
Study design and implementation
The study was designed as a prospective, double-blinded randomised controlled clinical trial. It was expected that in-vivo no difference was observable in the epithelialization of skin graft donor site wounds treated with an OCT-based hydrogel compared to an identical non-antimicrobial hydrogel (placebo without OCT) and that the epithelialization occurred at a comparable time frame.
Data were collected starting October 2007 until May 2009 at three German study centres: Department of Plastic Surgery, University Medical Centre Schleswig-Holstein in Lübeck, Department of Trauma Surgery, University Medical Centre of Greifswald, and Centre for Serious Burns with Plastic Surgery, Trauma Centre Berlin. The design and microbial analysis was performed at the Institute for Hygiene and Environmental Medicine, University of Greifswald. The study was supervised by an independent clinical monitor, Mediconomics GmbH Hannover.
The study was approved by the German Ethical Committees of the Medical Faculty of the University of Greifswald (Reg.-Nr. MPG 03/07), the Clinical Centre at the Charité Universitätsmedizin Berlin (EA 1/090/07), and the Medical Faculty of the University of Lübeck (AZ 07-093).
Volunteers
Inclusion criteria were patients of both gender between 18 and 65 years of age, suffering from burns or chronic wounds in need of skin grafts, skin graft areas >25 cm2, and the possibility of harvesting skin grafts from the thighs. Exclusion criteria were atopic dermatitis, diabetes mellitus, hypersensitivity to any of the applied ingredients, skin disease requiring therapy, any malignancy, HIV infection, immune system disorder, pregnant women or breastfeeding mothers, patients taking cortisone, anticoagulants, cytostatic drugs, prostaglandins, anabolic steroids, and patients with any drug addiction. Patients were excluded from the study in case of withdrawal of consent, the occurrence of non-tolerable adverse events, non-compliance, and breaches of protocol.
Study procedure
After signing a consent form, participants (n=61) were double blinded, randomly divided into two groups (Figure 1): one group (n=31) was treated with the OCT-based hydrogel (Octenilin® wound gel, Schuelke & Mayr GmbH, Norderstedt/Germany); the second group (n=30) received treatment with an OCT-free identical hydrogel (placebo). In all patients, wound treatment was performed in the same manner over a period of 3 days as described below.
Figure 1.
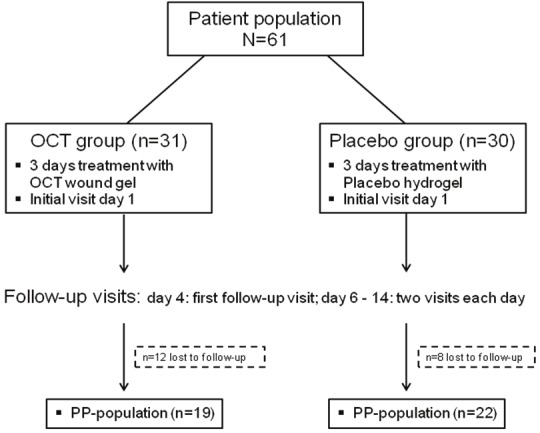
Study overview of patients included, therapy used, and follow-up characteristics (PP = per protocol; OCT = Octenidine-dihydrochloride).
Demographic data of all patients were recorded and physical examination of all patients was done before the treatment started.
Harvesting of skin grafts was performed in both groups following a standardised procedure: after ethanol (80 v/v%)-based skin antisepsis with AHD 2000® (Lysoform GmbH Berlin, Germany) and complete drying on air following a minimum contact time of 60 sec, harvesting was done by excision of a 0.2 mm deep skin graft from the donor site using a power cell supplied dermatome (Aesculap, B. Braun Melsungen AG). After excision, wound edges were topically defatted with wound benzene. Subsequently, wounds were treated double-blind either with 1 mL OCT or placebo hydrogel per 5 cm2 wound area. This ensured that the wound area was completely covered with an adequate amount of hydrogel.
The hydrogels were applied and spread on hydrofiber dressing Aqua cell (ConvaTec GmbH, Munich, Germany). The size of the dressing depended on the size of the skin graft. Then, the coated dressing was applied to the wound and covered with the hydrocolloid dressing (Varihesive®, ConvaTec GmbH, Munich, Germany), and remained on the wound for 3 days, unless an additional bandage change was required, e.g. due to high exudation. After 3 days, wounds were cleaned with Ringer’s solution and were covered with the hydrocolloid dressing without any hydrogel. From the sixth day onward, wounds were covered with the transparent hydrogel film HydroSorb Plus (Hartmann AG, Heidenheim, Germany).
The total area of the skin graft donor site was observed until epithelialization. During this period, follow-up (FU) visits were conducted immediately after the removal of the skin grafts, and again after removal of the first dressing on the fourth day (FU1). After the second dressing change on the sixth day and after the hydrogel film was applied, follow-up visits (FU2) were conducted twice daily. During these follow-up visits, the re-epithelialization process was documented using a digital camera (Digital IXUS 70, Canon) together with a standardised measuring scale without removal of the hydrogel film dressing (Figure 2). The time needed to achieve complete (100%) epithelialization was documented by two independent observers.
Figure 2.
Visual documentation of skin donor site reepithelialization progress after treatment with OCT-Hydrogel.
Microbiological examinations
At FU1 (day 4) and FU2 (day 6) contact cultures (RODAC contact plate; Heipha Dr. Müller GmbH, Eppelheim, Germany) were obtained and microbes on the skin graft donor sites were quantitatively assessed. All RODAC contact plates were examined and counted twice, once after incubation at 36°C for 48h, second after 3 days of incubation at 22°C. Bacterial counts were analysed semi-quantitatively and denoted as colony forming units (cfu), stratified into five categories: ≤300 cfu, >300-1,000 cfu, >1,000-1,500 cfu, >1,500-2,300 cfu, and >2,300 cfu.
Adverse events
In order to evaluate the safety of the OCT-based hydrogel application, all adverse events (AEs) were documented on separate report form. The following concomitant information was documented: severity, duration, relation, action taken, and clinical consequence of the AE. The AEs were retrospectively assigned to the treatment (OCT-based hydrogel) or placebo group.
Statistical analysis and sample size considerations
The main outcome was the time for complete (100%) epithelialization of treated skin graft donor site. The secondary outcome was the cfu of bacteria on wounds.
For analysis of the per protocol (PP) patient group, only patients with complete (100%) epithelialization were included. In case of missing values, the Lost Observation Carried Forward (LOCF)-method was used for primary analysis.
Data were analysed using descriptive statistical methods. Uniform distributed continuous data were expressed as mean together with standard deviation (SD). Categorical variables were expressed as absolute numbers together with percentages (%). For statistical comparison, twosided p-values were calculated. Continuous data were further analysed using the t-test. Categorical variables were compared using the Chi2-test or Fisher’s exact test, where appropriate. P-values of ≤0.05 were considered to indicate statistical significant differences. For all calculations, SPSS software, version 11.5.1 (SPSS GmbH, Munich, Germany), was used.
It was assumed that a large effect size (≥ 0.8) for the differences between the two groups would be clinically relevant. Using a two-sided parametric test (t-test) for the analysis of the difference between groups with respect to time for complete epithelialization, a significance level of α=0.05 and a power (1-β) of 0.80, 16 and 25 cases per group would be needed to detect a difference with an effect size of 1.0 and 0.8, respectively. To account for possible drop-out, a sample size of 30 cases per group was planned.
Results
No gender differences were observed between the treatment (female/male: 5/14) and the placebo group (female/male: 4/18; p=0.530, Chi2-test; Table 1). Also, age did not differ between the treatment group (42.2±13.5) vs. the placebo group (41.4±14.3 years; p=0.856, Chi2-test). Patients in both groups were comparable in terms of their mean body size (p=0.376, t-test) or body weight (p=0.934, t-test; Table 1). The most frequent general risk factors were tobacco smoking (51.2%), allergies (46.3%), and obesity (22.0%) with equal distribution in both groups.
Table 1.
Demographic data (mean±SD) from patients with one skin graft donor site treated either OCT-Hydrogel (OCT) or placebo
| Treatment group | Significance | ||
|---|---|---|---|
|
|
|||
| OCT | Placebo | p-value | |
| Gender (f/m) | 5/14 | 4/18 | 0.530 |
| Age (years) | 42.2±13.5 | 41.4±14.3 | 0.856 |
| Body size (cm) | 175.8±10.5 | 178.7±10.1 | 0.376 |
| Weight (kg) | 82.2±13.2 | 85.1±13.9 | 0.934 |
Nineteen patients in the treatment group and 22 patients in the placebo group completed the per protocol (PP) treatment.
Duration of epithelialization
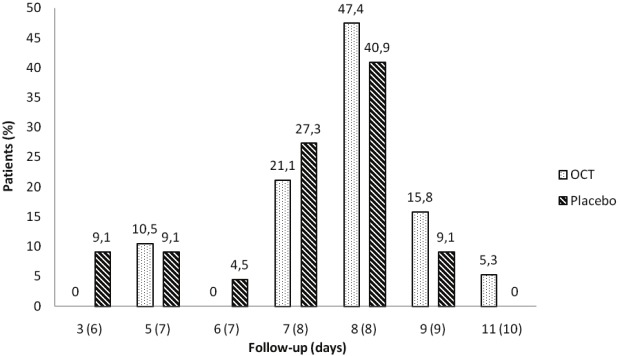
The maximum number of follow-up visits until epithelialization and the corresponding number of days until complete epithelialization did not differ between patients in the treatment and the placebo group (7.3 ± 0.2 vs. 6.9 ± 0.2 days; p=0.236, t-test; Figure 3). None of the following patients’ variables: age, gender, size of wound, and risk factors serving as covariates had a significant influence on the duration of the epithelialization.
Figure 3.
Maximum number of follow-up visits (days) until 100% reepithelialization in patients with skin donor sites treated either with OCT-Hydrogel (OCT) or placebo.
Bacterial colonisation
After FU1, significantly more skin graft donor site wounds in the treatment group showed bacterial colonisation with numbers lower than 300 cfu (p=0.014, Chi2-test; 2nd count; Figure 4A). After FU2, three days after using non-antimicrobial wound dressings, bacterial colony counts were comparable in both groups yielding >2.300 cfu in all wounds (p=0.363, Chi2-test; 2nd count; Figure 4B).
Figure 4.
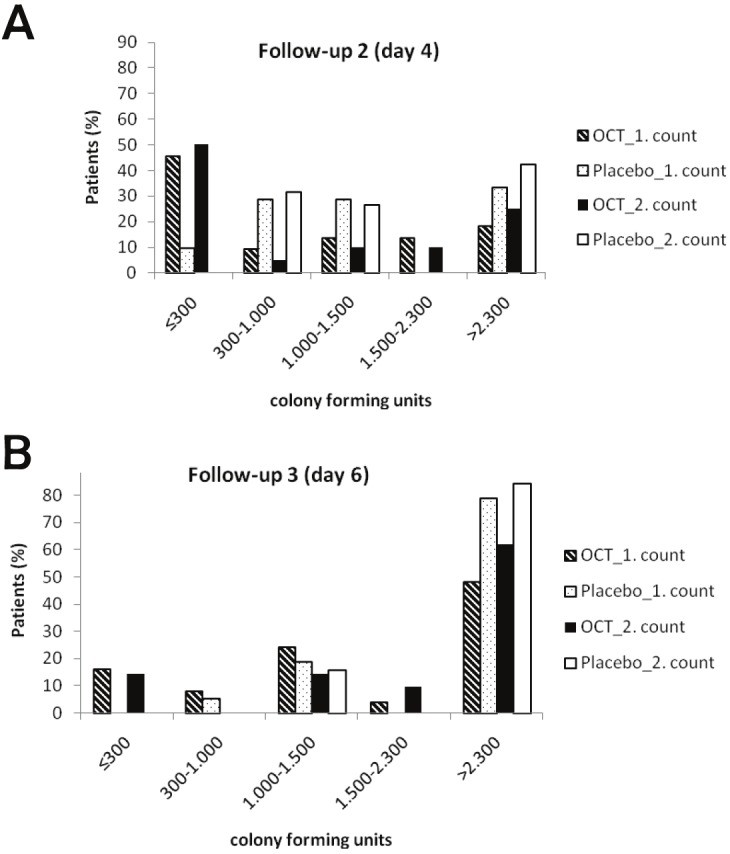
Number of colony forming units from skin donor sites treated either with OCT-Hydrogel (OCT) or placebo at two different colony countings in (A) follow-up on day 4 and (B) follow-up on day 6.
Adverse events
The total number of adverse events (AEs) was low in all patients in the present study. In general, all observed AEs were of mild intensity and were assessed as not related to the test compounds by the treating physicians. Eight patients (12.3% of all patients) presented with 11 AEs, whereas in two patients both wound pain and infection was reported. Pertaining to patients treated per protocol (PP), 4 AEs were observed in 3 patients (4.6% of all study patients). In one patient allocated to the placebo group, the blood circulation in the transplanted skin graft had to be stimulated. This patient also showed nausea after anaesthesia. Another patient in the placebo group developed night cramps in the calves. In the treatment group, 1 patient exhibited pruritus at the site of the applied dressing.
Discussion
The application of antiseptics to burns and chronic wounds is of importance because of any wound’s high susceptibility for bacterial colonisation with subsequent risk of infection. The antiseptics’ beneficial prophylactic use, however, needs to be weighed against the risk of potential cell cytotoxicity, and hence, a possible delay in wound healing. The general strategy for treatment of burns and chronic wounds comprises removal of necrotic or infected tissue (debridement), establishment of adequate blood circulation, maintenance of moist wound environment, and prevention of wound infection. Beside these direct interventions, underlying conditions supporting the chronic state of wounds such as malnutrition, blood glucose control in diabetic patients, and urine and stool incontinence in patients with e.g. pressure ulcers at risk for contamination is obligatory [14,15]. Depending on the degree of severity of burn injuries, skin graft transplantations may be necessary. Generally, correct wound care management should be ensured by moist wound dressings and the supportive use of antiseptic compounds.
Hydrogels containing the antimicrobial compound Octenidine dihydrochloride [16] were developed to keep wounds moist and to create a bacterial free environment during continuous exposure of the wound under occlusive conditions [12]. While beneficial from a microbiological aspect, antiseptic agents may not only affect microorganisms, but depending on their mode of action, may also interfere with human fibroblasts and keratinocytes, resulting in tissue cytotoxicity and delayed wound healing [5,7,8]. Pertaining to the potential cytotoxicity of OCT-based antiseptics, previously published data are limited to in vitro/ex vivo and preclinical experiments, e.g. in porcine skin [17,18]. Many established standardised examination protocols, however, are limited due to the fact that the examined cells form a monolayer, or the methods applied to assess viability of biological cells. The initially reported unfavourable cytotoxicity of OCT found in an in vitro peritoneal rat cell study [17] later was rescinded by the same team of investigators, as they could demonstrate in further studies that OCT forms a dense layer over test cells [19]. This layer inhibits the uptake of dyes such as neutral red, and hence, mimics a cell-death in still viable cells. Additionally, it was shown that cells incubated with OCT for 1 hour followed by a meticulous removal of OCT showed no cytotoxicity, but still were able to reduce the number of Escherichia coli or Staphylococcus aureus added to the cell culture medium. This was interpreted as strong binding of OCT to cells resulting in an antiseptic depot effect on the surface of the treated cells [19].
One of the main disputed issues is the transferability of laboratory based in vitro data for OCT to clinical observations concerning the effect of OCT application in patients, and consequently to the tolerability of OCT in vivo. For that reason, the present study was finally conducted in a human randomised, double-blind, controlled clinical trial, and the time needed for complete epithelialization of skin graft donor site wounds was used as primary endpoint [20].
In accordance with the results of another in vivo experiment, in which wound epithelialization after treatment with OCT combined with phenoxyethanol in comparison to povidoneiodine under occlusive conditions was examined in porcine skin [12], no differences were found in the epithelialization of skin donor site wounds from patients treated with OCT compared to placebo in the present study. Concerning the comparison of wound healing rates under occlusive and non-occlusive conditions, considerable differences were observed in porcine skin demonstrating that occlusive cover accelerates wound re-epithelialization [12]. This clearly demonstrates the fact that a moist wound environment favours wound healing.
No evidence of significant cytotoxicity of OCT, which continually is discussed controversially in the literature when compared to other antiseptics [18,21], was found in the present study. Contrary, OCT showed good tissue tolerability together with significant antimicrobial efficacy. These findings are in accordance with in vitro findings for OCT [22], categorising OCT as an highly effective antiseptic with favourable cytotoxicity compared to other antiseptics, such as benzalkonium chloride, chlorhexidine digluconate, triclosan, silver proteins, and povidoneiodine.
Conclusion
The observed antimicrobial hydrogel containing 0.05% OCT showed no undesirable interaction with wound epithelialization and demonstrated a significant antimicrobial efficacy on skin graft donor sites. It is concluded that OCT may be used safely in burns and chronic wounds to provide a moist environment and an added antimicrobial effect on the wound surface.
Acknowledgments
We thank Mrs. Andrea Rathmann-Schmitz, Ph.D. and Mrs. Nicole Scholtz, Ph.D. (Bonn, Germany) for their assistance in preparing the manuscript for publication. This work is dedicated to our friend and college Professor Werner Eisenbeiß, who sadly passed away after a long and brave battle with pancreatic cancer on May 17, 2011. Prof. Eisenbeiß always used his research for mentoring and for introducing medical students to the highest standards of patient care. He was generous in sharing credit with those who worked with him. The successful intellectual and professional careers of many medical doctors today owe a great deal to the support Werner Eisenbeiß gave them as graduate students. He will not be forgotten.
Sources of funding and conflict of interest statement
The study was funded by Schuelke & Mayr, Norderstedt. The authors have no financial or other conflict of interest to declare in relation to this manuscript and declare no financial or other relationships leading to a conflict of interest. Prof. Assadian O and Prof. Kramer A have received speaker’s honoraria for public medical education and invited congress presentations related to clinical use of antiseptics, including Octenidine dihydrochloride in the past from Schuelke & Mayr, Norderstedt.
Bulleted statements
Microbially colonised wounds have an increased risk of infection; previous studies have shown that topical antiseptics may negatively affect reepithelialization. Skin graft donor sites treated with an Octenidine-based hydrogel healed as fast as wounds treated with placebo wound hydrogel. Octenidine significantly reduced the bacterial colonisation of skin graft donor sites. No impaired effect of Octenidine on wound healing was observed on skin graft donor sites.
References
- 1.MedMarket Diligence, LLC. Worldwide Wound Management, 2008-2017. Established and Emerging Products, Technologies and Markets in the U.S., Europe, Japan & Rest of World. URL http://mediligence.com/rpt/rpt-s247.htm.
- 2.Evans J. Massive Tissue Loss: Burns. In: Bryant RA, Nix DP, editors. Acute & chronic wounds. Current management concepts. 3rd ed. Mosby, Elsevier; 2007. pp. 361–362. [Google Scholar]
- 3.Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, Moroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30:145–151. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Moreo K. Understanding and overcoming the challenges of effective management for patients with chronic wounds. Case Manager. 2005;16:62–63, 67. doi: 10.1016/j.casemgr.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis. 2004;32:88–94. doi: 10.1016/j.bcmd.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Kramer A, Hübner NO, Weltmann D, Lademann J, Ekkernkamp A, Hinz P, Assadian O. Polypragmasia in the therapy of infected wounds - conclusions drawn from the perspectives of low temperature plasma technology for plasma wound therapy. GMS Krankenhaushyg Interdiszip. 2008;3:1–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Innes ME, Umraw N, Fish JS, Gomez M, Cartotto RC. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns. 2001;27:621–627. doi: 10.1016/s0305-4179(01)00015-8. [DOI] [PubMed] [Google Scholar]
- 8.Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: An area of controversy. Wounds. 2003;15:149–166. [Google Scholar]
- 9.Steen M. Review of the use of povidone-iodine (PVP-I) in the treatment of burns. Postgrad Med J. 1993;69:S84–92. [PubMed] [Google Scholar]
- 10.Al-Tannir MA, Goodman HS. A review of chlorhexidine and its use in special populations. Spec Care Dentist. 1994;14:116–122. doi: 10.1111/j.1754-4505.1994.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 11.Hidalgo E, Dominguez C. Mechanism’s underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro. 2001;15:271–276. doi: 10.1016/s0887-2333(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 12.Stahl J, Braun M, Siebert J, Kietzmann M. The effect of a combination of 0.1% octenidine dihydrochloride and 2% 2-pheoxyethanol (octenisept®) on wound healing in pigs in vivo and its in vitro percutaneous permeation through intact and barrier disrupted porcine skin. Int Wound J. 2010;7:62–68. doi: 10.1111/j.1742-481X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weingart D, Stoll P. The epithelisation of split skin graft donor sites - a test model for the efficacy of topical wound therapeutic agents. Eur J Plast Surg. 1993;16:22–25. [Google Scholar]
- 14.Food and Drug Administration. Guidance for Industry. Chronic Cutaneous Ulcer and Burn Wounds - Developing Products for Treatment. doi: 10.1046/j.1524-475x.2001.00258.x. URL http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071324.pdf. [DOI] [PubMed] [Google Scholar]
- 15.AWMF Leitlinien. Thermische und chemische Verletzungen. URL http://www.awmf.org/uploads/tx_szleitlinien/044-001_S1_Thermische_und_chemische_Verletzu ngen_01-2010_01-015.pdf http://www.uniduesseldorf.de/awmf/ll. [Google Scholar]
- 16.Hübner NO, Siebert J, Kramer A. Octenidine Dihydrochloride, a Modern Antiseptic for Skin, Mucous Membranes and Wounds. Skin Pharmacol Physiol. 2010;23:244–258. doi: 10.1159/000314699. [DOI] [PubMed] [Google Scholar]
- 17.Kramer A, Adrian V, Adam C. Comparison of the Toxicity of Lavasept and Selected Antiseptic Agents. Hyg Med. 1993;18:9–16. [Google Scholar]
- 18.Kramer A, Roth B, Müller G, Rudolph P, Klöcker N. Influence of the antiseptic agents polyhexanide and octenidine on FL cells and on healing of experimental superficial aseptic wounds in piglets. A double-blind, randomised, stratified, controlled, parallel-group study. Skin Pharmacol Physiol. 2004;17:141–146. doi: 10.1159/000077241. [DOI] [PubMed] [Google Scholar]
- 19.Müller G, Kramer A. Microbicidal efficacy, further biological activities, tolerance and biodegradation of octenidine-dihydrochlorid. GMS Krankenhaushyg Interdiszip. 2007;2:Doc46. (20071228) [Google Scholar]
- 20.Cihantimur B, Kahveci R, Ozcan M. Comparing Kaltostat with Jelonat in the treatment of splitthickness skin graft donor sites. Eur J Plast Surg. 1997;20:260–263. [Google Scholar]
- 21.Kramer A, Adrian V, Rudolph P, Wurster S, Lippert H. Explantationstest mit Haut und Peritoneum der neonatalen Ratte als Voraussagetest zur Verträglichkeit lokaler Antiinfektiva für Wunden und Körperhöhlen. Chirurg 1998, 69:840–845. doi: 10.1007/s001040050498. [DOI] [PubMed] [Google Scholar]
- 22.Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281–1287. doi: 10.1093/jac/dkn125. [DOI] [PubMed] [Google Scholar]
- 23.Dissemond J, Assadian O, Gerber V, Kingsley A, Kramer A, Leaper DJ, Mosti G, Piatkowski de Grzymala A, Riepe G, Risse A, Romanelli M, Strohal R, Traber J, Vasel-Biergans A, Wild T, Eberlein T. Classification of wounds at risk and their antimicrobial treatment with polihexanide: a practice-oriented expert recommendation. Skin Pharmacol Physiol. 2011;24:245–255. doi: 10.1159/000327210. [DOI] [PubMed] [Google Scholar]
- 24.Koburger T, Hübner NO, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. Antimicrob Chemother. 2010;65:1712–1719. doi: 10.1093/jac/dkq212. [DOI] [PubMed] [Google Scholar]