Abstract
In many resource-limited settings, cryptococcal meningitis (CM) contributes up to 20% of all deaths with further complications due to Immune Reconstitution Inflammatory Syndrome (IRIS). We present a case report on a patient who developed CM-IRIS and then subsequent CM-relapse with a fluconazole-resistant organism and then later CM-IRIS once again, manifesting as cystic cryptococcomas, hydrocephalus, and sterile CSF. In this case we, demonstrate that CM-IRIS and persistent low level cryptococcal infection are not mutually exclusive phenomena. The management of IRIS with corticosteroids may increase the risk of culture positive CM-relapse which may further increase the risk of recurrent IRIS and resulting complications including death. We also highlight the role of imaging and fluconazole resistance testing in patients with recurrent meningitis and the importance of CSF cultures in guiding treatment decisions.
Keywords: HIV, AIDS, Cryptococcal Meningitis, antiretroviral therapy, Immune reconstitution inflammatory Syndrome
Introduction
In many resource-limited settings, HIV-infected patients continue to present late to Antiretroviral Treatment (ART) programmes with low CD4+ T cell counts and high early mortality1. Cryptococcal Meningitis (CM) contributes up to 20% of all deaths in HIV cohorts2 with further complications due to immune reconstitution inflammatory syndrome (IRIS) 1,3. We present a case highlighting the complexities of CM-IRIS diagnosis and management.
Case History
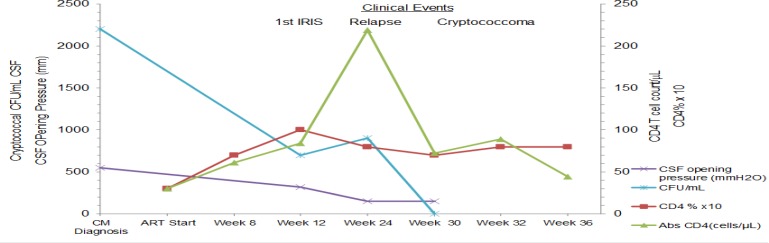
A 35 year old male presented to Mulago hospital in Kampala, Uganda in April 2008 with initial new diagnosis of HIV/AIDS and cryptococcal meningitis. Lumbar puncture (LP) opening pressure was >550 mmH2O, and cerebral spinal fluid (CSF) examination showed yeast cells on India ink, a positive cryptococcal antigen (CRAG) test at 1:1024 titre dilution, elevated protein 60 mg/dL and WBC 20 cells/µL (100% lymphocytes). CSF quantitative culture grew Cryptococcus neoformans at 2200 colony forming units (CFU)/mL of CSF.
He was treated with amphotericin B (0.7mg/kg/day) for 14 days with symptomatic improvement and no significant renal dysfunction. The patient declined any therapeutic lumbar punctures during the induction phase. This was followed by consolidation treatment with fluconazole 400mg/day for 8 weeks, and then secondary prophylaxis with 200mg daily. He received trimethoprimsulfamethoxazole daily prophylaxis and initiated ART 24 days after CM diagnosis with zidovudine, lamivudine, and efavirenz. His pre-ART CD4+ T cell count was 30cells/ µL and HIV viral load was 5.7 log10 copies/ mL (figure 1).
Figure 1.
Immune responses and CSF findings on HIV therapy
At 12 weeks on ART, he presented with headache, photophobia, neck pain, vomiting, with an unremarkable, non-focal neurological examination. Repeat lumbar puncture revealed an elevated opening pressure of 320 mmH2O with a CSF WBC count of 110 cells/µL (100% lymphocytes) and CSF protein 100 mg/dL.
The CD4 count had risen to 84 cells/µL and HIV-1 viral load decreased to 2.7 log10 copies/ml. Diagnosis of an IRIS event was made, and he received prednisolone 60mg/day for 7 days tapered by 10mg/week over 6 weeks with remarkable symptomatic improvement. However, the CSF culture eventually grew C. neoformans with a quantitative culture burden of 700 CFU/mL of CSF approximately 14 days later, but the patient was unable to be reached and maintained fluconazole at a dose of 200mg/day.
At week 26 on ART, he presented with another episode of headache, general malaise, anorexia, and weight loss that had developed over the preceding month. He was generally lethargic without features of meningismus or focal neurologic deficit. The CSF opening pressure was 150 mmH2O with CSF protein of 400mg/dL and WBC count 30 cells/µL (100% lymphocytes). The absolute CD4+ T cell count had increased to 219 cells/µL, although the CD4% was almost unchanged at 7%. He had virologic suppression (<400 copies/mL). CSF culture had increased growth of 900 CFU/ mL of C. neoformans. The plan was to admit the patient and use Amphotericin re-induction therapy; however, the patient declined admission and hence was treated as a CM relapse with high dose fluconazole 800 mg/day for 14 days followed by 400 mg daily for 8 weeks with minimal clinical improvement.
At 31 weeks on ART, he presented with worsening headache, dizziness, malaise, vomiting, photophobia, anorexia, and behavioural changes characterized by loquaciousness, uncoordinated speech, visual hallucinations, inattention, and poor concentration. His was disorientated in time, had a staggering gait and urinary incontinence. The CSF opening pressure was normal (150 mmH2O). CSF analysis revealed a raised protein of 120mg/dL, CSF WBCs of 45cells/µL, negative culture for C. Neoformans.
Plasma HIV-1 viral load remained suppressed and CD4+ T cell count was 89 cells/µL. Computerized Tomography (CT) of the brain showed a cystic mass in the right parietal area with evidence of hydrocephalus. The imaging differential diagnosis included neurocysticercosis and echinococcosis. High dose albendazole 400mg twice daily for 7 days was empirically added, without improvement. The patient's medication adherence on both fluconazole and ART was reported to be 100% as assessed through self-report and pill counts. He remained HIV virologically suppressed.
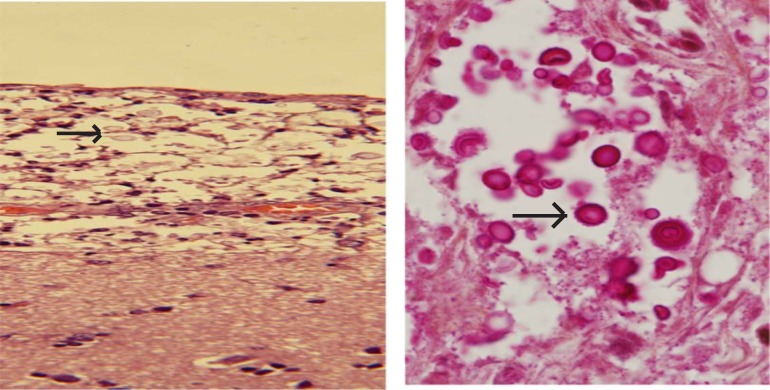
At 36 weeks on ART, the patient was admitted with progressively worsening symptoms. A repeat head CT showed worsening hydrocephalus without change in the cystic lesions. He was scheduled for neurosurgical placement of a ventriculoperitoneal shunt, but the patient died prior to the procedure. Autopsy revealed extensively disseminated fungal organisms consistent with C.neoformans on hematoxylin and eosin stain and mucicarmine stain in the subarachnoid space and cystic areas of the parietal lobe (figure 2).
Figure 2.
Post mortem histopathology of the cystic mass in the parietal region of the cerebral cortex
Laboratory evaluation retrospectively performed on the stored Cryptococcal isolates revealed the relapse strain to be the same by multilocus sequence typing (MLST) as the isolate at the initial CM diagnosis. However, there was interval development of fluconazole resistance, by E® test. The initial minimum inhibitory concentration (MIC) was 12µg/mL at baseline rising to >96µg/mL at the 26-week relapse episode.
Discussion
This case illustrates the complexities of diagnosis and management of cryptococcal-IRIS. Though this case occurred in a resource-limited setting, we believe the insight provided by the case is broadly generalizable. We demonstrate that CM-IRIS, persistent low-level CM infection, and CM -relapse are not mutually exclusive phenomena. The management of CM-IRIS with corticosteroids may increase the risk of persistent C. neoformans infection and complications which may further increase the risk of recurrent IRIS.
Cryptococcal IRIS of the central nervous system (CNS) has been described as aseptic meningitis associated with high intracranial pressure and localized inflammatory lesions which may be found in the brain4. The diagnosis of cryptococcal IRIS is a diagnosis of exclusion. The factor supporting this diagnosis include: temporal association between starting ART and clinical presentation, evidence of immune restoration, exclusion of alternative explanations (e.g. noncompliance or resistance to fluconazole, a second possible diagnosis), cytology (i.e. CSF white cell count) or histopathology consistent with an increased cell-mediated immune response and negative cryptococcal cultures5. The consensus International Network for the Study of HIV-associated IRIS (INSHI) defines two forms of CM-IRIS: i) unmasking CM-IRIS which is the unmasking of subclinical disease, which is detectable pre-ART by cryptococcal antigen screening6,7; and ii) paradoxical CM-IRIS which is the symptomatic recrudescence of a previously treated infection which is an immune mediated reaction, most typically in the presence of sterile CSF cultures4.
Our patient presented with an event at 12-weeks of ART that fulfilled the INSHI paradoxical CM-IRIS case definition criteria. He was treated with anti-inflammatory medications with remarkable improvement. He then presented with another clinical event at week 26 on ART with persistently positive CSF cultures for C. neoformans. Although he was virologically suppressed and his CD4+ T cell count had risen, the CD4% remained stable. Due to the persistence as well as the increased quantitative culture growth, these findings suggest persistent CM disease with treatment failure and/or relapse. The Infectious Disease Society of America (IDSA) guidelines define relapse of cryptococcal disease as the recovery of viable cryptococci from a previously sterile body site and recrudescence of signs and symptoms at the previous site of disease supporting presence of disease8. The patient was treated with oral fluconazole (800mg) for 2 weeks and the subsequent lumbar punctures were culture negative for C. neoformans.
Our patient later developed a cystic cryptococcoma and hydrocephalus but with a suppressed viral load and maintained CD4%. Cryptococcoma(s) are characterized by localized, solid, tumour-like masses in which the fungus has invaded the parenchyma, producing a chronic granulomatous reaction composed of macrophages, lymphocytes, and foreign body-type giant cells. The incidence of these lesions appears to be the highest in immunocompetent patients infected with C. neoformans var. gattii. Granuloma formation, however, is uncommon in immunosuppressed patients because of the inability to initiate a robust inflammatory response (in the absence of ART) 9,10. The findings in this case would suggest that the cryptococcomas could be still be part of a CNS IRIS event similar to a report by Briton G et al, in which cultures were negative following stereotactic brain biopsy despite histologic evidence of cryptococcal organisms11. We were limited in our case by the inability to do fungal cultures on the brain at autopsy.
Fluconazole resistance tests showed an initial susceptible organism but with a relatively high MIC, which in the presence of fungistatic dosing of fluconazole at ≤400mg/day likely contributed to the interval development of high level fluconazole resistance and recurrence of the CM disease. Fluconazole resistance has been noted to be associated with initial treatment with fluconazole as opposed to Amphotericin B and this resistance is associated with high six month mortality. Our patient had Amphotericin B for induction therapy12,13.
Therapy for cryptococcal IRIS remains a clinical challenge as the relative benefit remains unknown for 1) anti-inflammatory therapies; 2) intracranial pressure control; 3) intensification of anti-fungal therapy; 4) merits and disadvantages of ART continuation during severe CNS IRIS events. This patient had several suspected IRIS events. Cattelan et al have reported two patients who developed brain cryptococcomas after initiating ART(14). These patients had virologic and immunologic responses on ART coupled with falls in CSF and serum CRAG titres and negative CSF cultures. They reported improvement when amphotericin was used14.
The IDSA guidelines define paradoxical IRIS as consisting of clinical manifestations compatible with exuberant tissue inflammation in patients experiencing rapid improvement in cellular immunity, and generally, results of cultures for the site of infection are negative. IDSA guidelines recommend observation for most of these patients except for patients with major complications who may require steroids8. The use of steroids in our patient at 12-weeks of ART was in respect to the inflammatory pleocytosis and the timing of the event in an addition to the evidence of improved immune response. The culture growth was reported 2-weeks later. In view of the IDSA guidelines, this would probably not be advisable without intensifying antifungal therapy in view of the positive fungal cultures.
ART interruption is generally unnecessary and has been recommended only for patients with severe, life-threatening symptoms until their condition is stabilised. IRIS can recur during re-initiation of ART, so this has to be monitored carefully. However, stopping ART in the setting of incompletely suppressed HIV replication may be associated with an increased risk of virologic resistance. In most cases ART has been continued without any significant events13
The current practice at our institution for patients with suspected IRIS is to give analgesics and increase fluconazole dose after the LP while awaiting fungal cultures. When there are positive CSF cultures, amphotericin re-induction is used for at least 5–7 days with later 8-weeks of consolidation and then maintenance phases with fluconazole. In suspected IRIS cases which are indeed culture negative, we give analgesics and control ICP. High ICP is treated with repeated lumbar punctures and steroids may be employed if the CSF is inflammatory and the patient condition is not improving with all the above measures or under life threatening conditions. Further clinical trials are needed to define what is optimal CM-IRIS management.
Conclusion
This case demonstrates the possible pathogenic interaction and co-existence of cryptococcal-IRIS, persistent C. neoformans infection, and CM-relapse which poses challenges for management especially where diagnostic capacity is limited. In addition, the case highlights the challenges of making an upfront clinical decision regarding patient management which is ultimately influenced by CSF culture that may take 7–14 days to grow in the presence of low antigen burden. The patient may have suppressed but not eradicated Cryptococcus possibly due to undiagnosed intermediate fluconazole resistance with negative CSF cultures for cryptococcal disease and hence recurrent IRIS events. This patient may have required both anti-inflammatory and intensified antifungal treatment to have altered his outcome.
We also highlight the role of imaging and fluconazole resistance testing in patients with recurrent meningitis and the importance of CSF cultures in guiding treatment decisions. We would have considered induction therapy with amphotericin B had we discovered the fluconazole resistance earlier. This case was managed in a tertiary institution with research facilities that made it possible to have CSF quantitative cultures and MICs. However we would advise other clinicians in resource limited settings faced with this similar presentation without some of those resources to consider induction with either amphotericin or high dose fluconazole and symptom control initially with therapeutic lumbar punctures. Failure to control symptoms with these measures would perhaps warrant use of steroids for a limited period of time.
Acknowledgements
DRB was supported by the NIH National Institute of Allergy and Infectious Diseases (L30AI066779; K23AI073192). AM was supported by U01AI089244
References
- 1.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 3.Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7(12):e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddow LJ, Colebunders R, Meintjes G, Lawn SD, Elliott JH, Manabe YC, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21(16):2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 6.Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51(4):448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48(7):856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drouet A, Amah Y, Pavic M, Gerome P, Meyer X P D. Subacute meningoradiculomyeloencephalitis due to cryptococcosis infection. Rev Med Interne. 2005;26:403–408. doi: 10.1016/j.revmed.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Gultasli NZ, Ercan K, Orhun S, Albayrak S. MRI findings of intramedullary spinal cryptococcoma. Diagnostic and interventional radiology. 2007;13(2):64–67. [PubMed] [Google Scholar]
- 11.Breton G, Seilhean D, Cherin P, Herson S, Benveniste O. Paradoxical intracranial cryptococcoma in a human immunodeficiency virus-infected man being treated with combination antiretroviral therapy. Am J Med. 2002;113(2):155–157. doi: 10.1016/s0002-9343(02)01130-0. [DOI] [PubMed] [Google Scholar]
- 12.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43(8):1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis JN, Meintjes G, Williams Z, Rebe K, Harrison TS. Symptomatic relapse of HIV-associated cryptococcal meningitis in South Africa: the role of inadequate secondary prophylaxis. S Afr Med J. 2010;100(6):378–382. doi: 10.7196/samj.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cattelan AM, Trevenzoli M, Sasset L, Lanzafame M, Marchioro U, Meneghetti F. Multiple cerebral cryptococcomas associated with immune reconstitution in HIV-1 infection. AIDS. 2004;18(2):349–351. doi: 10.1097/00002030-200401230-00034. [DOI] [PubMed] [Google Scholar]