Abstract
The advent of intravital microscopy in experimental rodent malaria models has allowed major advances to the knowledge of parasite-host interactions 1,2. Thus, in vivo imaging of malaria parasites during pre-erythrocytic stages have revealed the active entrance of parasites into skin lymph nodes 3, the complete development of the parasite in the skin 4, and the formation of a hepatocyte-derived merosome to assure migration and release of merozoites into the blood stream 5. Moreover, the development of individual parasites in erythrocytes has been recently documented using 4D imaging and challenged our current view on protein export in malaria 6. Thus, intravital imaging has radically changed our view on key events in Plasmodium development. Unfortunately, studies of the dynamic passage of malaria parasites through the spleen, a major lymphoid organ exquisitely adapted to clear infected red blood cells are lacking due to technical constraints.
Using the murine model of malaria Plasmodium yoelii in Balb/c mice, we have implemented intravital imaging of the spleen and reported a differential remodeling of it and adherence of parasitized red blood cells (pRBCs) to barrier cells of fibroblastic origin in the red pulp during infection with the non-lethal parasite line P.yoelii 17X as opposed to infections with the P.yoelii 17XL lethal parasite line 7. To reach these conclusions, a specific methodology using ImageJ free software was developed to enable characterization of the fast three-dimensional movement of single-pRBCs. Results obtained with this protocol allow determining velocity, directionality and residence time of parasites in the spleen, all parameters addressing adherence in vivo. In addition, we report the methodology for blood flow quantification using intravital microscopy and the use of different colouring agents to gain insight into the complex microcirculatory structure of the spleen.
Ethics statement
All the animal studies were performed at the animal facilities of University of Barcelona in accordance with guidelines and protocols approved by the Ethics Committee for Animal Experimentation of the University of Barcelona CEEA-UB (Protocol No DMAH: 5429). Female Balb/c mice of 6-8 weeks of age were obtained from Charles River Laboratories.
Keywords: Immunology, Issue 59, intravital microscopy, GFP, malaria, spleen, mobility, adhesion, Plasmodium yoelii, Balb/c mice
Protocol
This method was used in the research reported in 7.
1. Animal infection with green fluorescent protein (GFP) transgenic parasites
P. yoelii–GFP transgenic lines of 17XL and 17X were generated using the same vectors, targeting strategy and protocols described elsewhere for P. berghei8. They express the mutant 3 variant of GFP 9 under the ubiquitous promoter of P. berghei elongation factor 1 (Pbeef1a), which directs constitutive expression of GFP to parasite cytosol during the entire intra-erythrocytic developmental cycle.
Inject animals intraperitoneally with parasitized red blood cells (pRBCs) of P. yoelii-GFP transgenic lines 17XL and 17X obtained from the tail blood of donor mice at 5-10% parasitemia and diluted in PBS. Use a dose of 5x105 pRBCs/mouse to reach a peripheral parasitemia of 1% at day 3 post-infection (p.i.).
At day 3 p.i., check that parasitemia of mice infected with both parasite lines is the same by doing a blood smear with a drop of tail blood followed by Giemsa staining and observation under a light microscope with a 100x oil objective. Parasitemia is estimated by calculating the percentage of pRBCs over total RBCs in three optical fields of approximately 300 RBCs.
Control animals injected with FITC-labeled RBCs can be used to characterize the movement of these cells in normal spleens.
2. Labeling of red blood cells with FITC and injection to control animals
Collect 1 ml of total blood through cardiac puncture of a Balb/c mouse in 200 μl of PBS containing ethylene diamine tetraacetic acid (EDTA) (100 g/L, pH 7.4) and wash the RBC pellet in PBS/EDTA (0.1 g/L, pH 7.4) through centrifugation at 300 xg for 5 minutes (min) at room temperature (RT).
Resuspend 200 μl of the RBC pellet in 300 μl of PBS/EDTA (0.1 g/L, pH 8) containing FITC (10 g/L) and incubate for 2 hours at room temperature in the dark with gentle agitation. After that time, the supernatant is removed and the cells washed five times (300 xg, 5 min, RT) in PBS/EDTA (0.1 g/L, pH 7.4).
For in vivo experiments, dilute 10 μl of the FITC-labeled RBC pellet in 200 μl of PBS and inject intravenously to a Balb/c mouse in order to reach 1% FITC-RBCs in circulation.
3. Surgical procedures
Prepare injectable anesthetic composed of 100 mg/kg of Ketamine and 5 mg/kg of Midazolam per dose according to the weight of the animal. Inject the mouse intraperitoneally with one dose of anesthetic. Readminister half of the dose every 30 min to maintain the mouse full-time anesthetized.
Keep the mouse warm and verify that the mouse is completely anesthetized (usually after 5-20 min) by pinching the foot pad before proceeding.
In order to facilitate intravenous administration of substances during the course of the experiment, cannulate the tail vein of the mouse using a 27G cannula. Check that the needle is well positioned inside the vein by injecting 20-50 μl of saline buffer and seal it with tape. If it obstructs, repeat the cannulation upstream of the vein. Be careful to not introduce air bubbles.
Expose the inferior part of the spleen through a small incision in the skin and musculature at the left dorsal side of the animal. Place the spleen where less breath movement is observed and apply PBS on the surface exposed to keep it clean of the mouse hair and hydrated.
Seal a cover-slip of 60x24mm with cyanoacrylate adhesive (Super Glue-3 Loctite) to the skin surrounding the spleen to allow visualization.
4. Imaging of living parasites in the spleen
Intravital microscopy experiments were carried out in a Leica TCS-SP5 confocal microscope (Leica Microsystems, Heidelberg, Germany) equipped with an incubation system with temperature control, an APO 63x glycerol immersion objective lens (NA 1.3), resonant scanner at 8000 lines/s and an Argon (488 nm) and HeNe (594 nm, 633 nm) lasers. Additional lasers, such as blue diode (405 nm) and diode-pump-solid state (561 nm), may be required for excitation of probes listed in Table 1.
Place the animal on the stage of the microscope with the cover-slipped spleen facing down to the objective. A general view of the microcirculatory structure of the spleen can be optionally visualized using a 20x objective. RBC reflection contrast will be helpful to select different regions of interest to image at higher magnification afterwards.
Focus the selected regions of interest with 63x glycerol immersion objective lens using tissue autofluorescence. GFP parasites are observed passing through different areas of the spleen.
Fluorescence is recorded on two different channels (excitation/emission wavelength 488/505-580 nm for FITC/GFP and 488/570-630 nm for tissue autofluorescence) with the pinhole set to 3.0 Airy units. RBC reflection (488/480-495 nm), together with fluorescent dyes to label the blood vasculature (see Table 1), are used to obtain additional information on the zone being imaged and in the blood flow experiments described below.
Capture images through five Z-stacks covering a depth of 8 μm because of the three-dimensionality of the organ, at a speed of 8 kHz to generate videos of 1.5 min.
Record videos of different zones of the spleen for quantitative analysis.
5. Intravital microscopy of the microvasculature of the spleen and image acquisition for blood flow measurement
Vital fluorescent probes dissolved in isotonic saline can be injected to the tail vein during the experiment to image the vasculature and gain insight into the structure of the spleen. A list of probes and their application is presented in Table 1 10.
To label the vascular system with fluorescent dextran, prepare 1 mg of 70 kDa dextran labeled with Texas Red in 100 μl of saline buffer.
Use the cannulated tail vein to inject the fluorescent dextran to the animal being imaged.
Set the vessels horizontally, in the direction of laser scanning, by optical field rotation (not affecting speed). Use xy and xt line scanning modes in the central lumen of the vessel. Use bidirectional scanning with a line average of 32 at a speed of 8 kHz to obtain an image of 512x512 pixels.
Acquire images of vessels on three different channels (excitation/emission wavelength 488/505-580 nm, 594/605-660 nm, 488/480-495 nm for FITC/GFP, dextran– Texas Red and erythrocyte reflection, respectively).
Take images of vessels with different diameters and over different phases of the cardiac cycle to compensate for fluctuations. In these images, the streaks resulting from moving cells will be used to quantify blood flow 11.
6. Image processing and quantitative analysis of parasite mobility using ImageJ software
Create a real-time video from the image sequence generated using ImageJ software (version 1.39o, Wayne Rasband, NIH, www.macbiophotonics.ca).
Open the ".lif" file in ImageJ keeping "xyzct" sequence and separated channels.
Register some useful information from the metadata file: dblvoxelX-voxel-width, dblvoxelY-voxel-height, dblvoxelZ-voxel-depth and frame interval between consecutive Z-frames and between stacks. This information will be used for calibration.
Substract autofluorescence (channel 2) to the GFP-parasite images (channel 1). Filter the images with Gaussian Blur=1. Please recall that using gaussian blur filter in images needs to be declared for publications. Save file "animal1_m1_substract.seq".
A Z-coded color video is created as supportive material for quantitative analysis of parasite mobility, as it will facilitate single-particle identification in fast moving particles and Z-movement characterization.
Convert the stack to Image 5D, with third dimension being the Z and fourth dimension being the time. Give a different color to each Z and overlay.
Project all the Z using maximum intensity over all the time frames to create a Z-coded color video. Save as "animal1_m1_Z_color.avi".
Classify and label all the particles that appear in the first 10 time frames of the video according to the number of frames of residence (from 1 to 10). In each video, 20 particles will be tracked following the proportions obtained. In total, 120 parasites will be quantified from 3 animals, using six videos/animal representing different zones of the spleen.
Report the frames of residence on each Z and over the entire movie for all the particles to be quantified.
Perform 4D (x,y,z,t) manual tracking of particles using the MTrackJ plugin (written by E. Meijering). Open the file "animal1_m1_substract.seq" as Image 5D and set image properties using the information registered before from pixel width, height, depth (in μm) and stack interval (in sec). Configure the track settings as follows: "move to next time" and "apply local cursor-bright centroid/25x25pixel". Configure displaying: "show origin", "show image", "show active track", "show only tracks present in current channels", "show only track point at current time".
Add a track for each particle. Consider movement in Z-axis only if displacement is higher than 6 μm (average diameter for a pRBC). Follow the particle over a maximum of 100 frames.
Save the x, y, z and t coordinates measured from the track as "animal1_m1_p1".xls.
Measures for displacement (D=SQRT((xfinal-xinital)2+(yfinal-yinital)2+(zfinal-zinital)2); path length (P=Σn=0→final SQRT((xn+1-xn)2+(yn+1-yn)2+(zn+1-zn)2) with n indicating each position tracked; mean velocity and residence time can be calculated using the values from x,y,z,t coordinates tracked calibrated according to the data registered. Directionality of the particles is defined as the quotient of displacement vs. path length, with values of close to 1 indicating directed movement and values of close to 0 indicating restrained movement 12. A template for calculations is facilitated enclosed.
7. Calculation of volumetric blood flow
Volumetric blood flow is estimated as Q=V*π*Dv2/4, with V, erythrocyte velocity over the cross section and Dv, lumen vessel diameter 11.
To calculate V, measure the angles (θ) of five particle streaks showing bright reflection (RBC) and four particle streaks showing green fluorescence (pRBC-GFP) in each xt image using ImageJ software. Measure lumen vessel diameter on the xy image.
The velocity is then expressed as V=1/tan(θ)*De/Dv to normalize for erythrocyte (De=6 μm) and lumen vessel diameters.
Quantify a minimum of three vessels with different diameters and five xt images for each vessel.
8. Statistical analysis
For statistical analysis, plot directionality, mean velocity and residence time as density distributions and use the equality-of-medians test in STATA (IC10) to assess differences between the two parasite lines.
9. Representative Results
Intravital imaging of GFP parasites in the spleen revealed differences in mobility between the two strains of parasites. Quantitative analysis of mobility parameters of single parasites indicated reduced velocity, lack of directionality and augmented residence time of parasites of mice infected with 17X strain. Moreover, volumetric blood flow in vessels was not altered between strains 7. The technical procedure is presented in Figure 1A. Figure 1B shows a general view of a normal spleen of a mouse injected with FITC-labeled RBCs, with a zoom in into the red pulp and another to a vessel (Figure 1B, zoom in 1 and 2, respectively). Vasculature was evidenced by injecting 70 kDa Dextran-Texas Red together with erythrocyte reflection contrast. Other fluorescent dyes summarized in Table 1 can be used to gain information on the organ being imaged, such as Hoechst (Figure 1C, 1D).
Real-time imaging of parasites of the 17XL and 17X strain is presented in Movies 1 and 2, with some 17X-pRBC (Movie 2) showing a rolling-circle behavior. Quantitative analysis of mobility parameters was achieved through tracking of individual parasites with the help of Z-coded color images. Figure 2A shows a Z-projection of a Z-coded color stack, where the encircled particle appears moving in different planes. Figure 2B and 2C represent the tracks for different parasites in 17X and 17XL infection, respectively. Results from directionality and residence time of all the particles quantified are presented as a density distribution map of parasite population in Figure 2D and 2E, respectively. To monitor blood flow in the spleen using intravital microscopy, the streaks obtained in xt images of the central lumen of vessels resulting from the erythrocyte movement were measured to calculate velocity 11. The images show a xy scan of a vessel (Figure 2F) with the corresponding xt line scan (Figure 2G).
| Fluorescent Probe | Localization | 1 photon Excitation (nm) | 2 photon Excitation (nm) | Detected emission (nm) | Quantity/ mouse weight |
| Hoechst 33342 | Membrane-permeant DNA-binding probe. It labels nuclei of all cells (live and dead) after Intravenous injection. | 405 | 800 | 410-480 | 12.5 g/Kg |
| Propidium iodide | Membrane-impermeant DNA-binding probe. It labels nuclei of cells with compromised membrane (apoptotic and necrotic cells). | 561 | 800 | 570-650 | 250 mg/Kg |
| 70,000 mol wt Dextran- Fluorescent (FITC, Texas Red) | Fluid-phase marker that enhances contrast of plasma. | FITC 488 Texas Red 594 | 800 | 500-540 600-650 | 50 mg/Kg |
| Sodium Fluorescein | Bulk fluid-phase albumin marker that enhances contrast of plasma. | 488 | 800 | 500-540 | 2 mmol/Kg |
| Evans Blue | Bulk fluid-phase albumin marker that enhances contrast of plasma. | 633 | nd | 645-700 | 20 mg/Kg |
| Rhodamine R6 | Vital probe that accumulates in active mitochondria. It labels endothelia and circulating white cells after intravenous injection. | 561 | 800 | 570-650 | 25 mg/Kg |
| Fluospheres- 1micron diameter | Beads that are uptaken by cells with phagocytic activity. | 488 | 800 | 500-540 | nd |
| Alexa488-labeled fibrin IIβ chain-specific antibody | Probe that labels fibrin IIβ chain | 488 | 800 | 500-540 | 0.3 mg/Kg |
Table 1. Fluorescent probes for intravital microscopy. Vital fluorescent dyes with different localizations that may be used to label the spleen in vivo. Excitation/emission (Exc/em) ranges to be used with one-photon (or two-photon microscopy) are provided. The dose indicated is dissolved in 0.1-0.2 ml of saline buffer and injected to the tail vein of the mouse. [nd: not determined in this study.
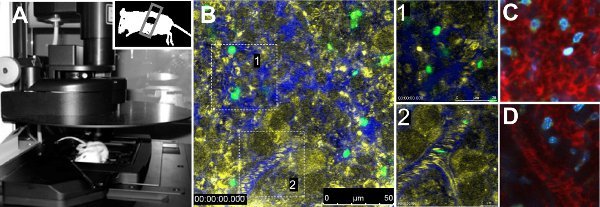 Figure 1. Intravital microscopy of the spleen. A. Leica TCS-SP5 confocal microscope with one mouse placed on the stage of the microscope. The mouse has the inferior part of the spleen exposed and sealed with a cover-slip. B. Image of a representative area of the spleen of a non-infected animal injected with FITC-labeled RBCs and 70 kDa Dextran- Texas Red to visualize the vasculature. Reflection (yellow), Dextran (blue) and FITC-RBCs (green) are shown. Blow-ups in white boxes represent open-circulation (1) and close-circulation (2) areas. Open-circulation (C) and close-circulation(D) stained with 70 kDa dextran (red) and Hoechst 33342 (blue).
Figure 1. Intravital microscopy of the spleen. A. Leica TCS-SP5 confocal microscope with one mouse placed on the stage of the microscope. The mouse has the inferior part of the spleen exposed and sealed with a cover-slip. B. Image of a representative area of the spleen of a non-infected animal injected with FITC-labeled RBCs and 70 kDa Dextran- Texas Red to visualize the vasculature. Reflection (yellow), Dextran (blue) and FITC-RBCs (green) are shown. Blow-ups in white boxes represent open-circulation (1) and close-circulation (2) areas. Open-circulation (C) and close-circulation(D) stained with 70 kDa dextran (red) and Hoechst 33342 (blue).
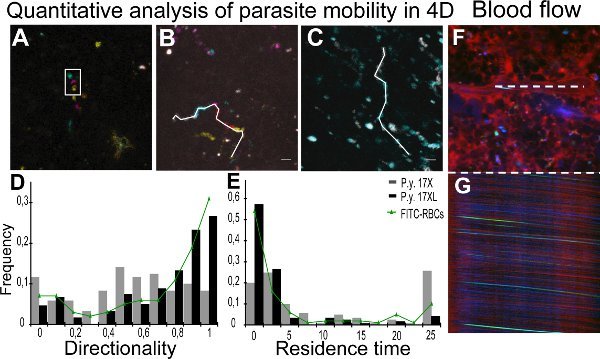 Figure 2. Quantification of parasite mobility and blood flow. A-C. Quantitative analysis of particle movement in the four dimensions (4D) is facilitated by using color-coded image processing. A. Tracking was performed with the depth information from Z-coded color images, represented using maximum intensity projection of five different depths. White rectangle represents the same particle at different Z in one time point. Different positions are due to time lapses between the acquisition of different Z images. Depth code: yellow (0 μm), orange (2 μm), pink (4 μm), blue (6 μm), green (8 μm). B, C. Time projections of particle movement with each time interval coloured as: gray (0-2.4 sec), cyan (2.4-4.8 sec), magenta (4.8-7.0 sec), red (7.0-9.4 sec) and yellow (9.4-11.8 sec). White line represents 4D manual tracking of particles of 17X (11.8 s) (B) and 17XL (4.8 sec) (C) GFP parasites using MTrackJ. D,E. Distribution of the density of GFP particles by values of directionality (D) and residence time (E). Data correspond to 120 particles of each line of parasites and 100 FITC-labeled RBCs from three independent experiments analysed with the equality-of-medians test. The 17X/17XL/FITC-RBCs medians are 0.53/0.75/0.85 (D) and 4.61/0.67/0.9 sec (E). Differences between the two lines in (D) and (E) are statistically significant (P < 0.001). Differences between FITC-labeled RBCs and 17XL parasites are not statistically significant (P > 0.05). F,G. Spleen blood flow measurements. Representation of xy image (F) and xt image (G) from a line-scan of the central lumen of the same vessel (white line). Spleen vessel showing plasma with 70 kDa dextran (red), pRBC (green) and erythrocyte reflection (blue).
Figure 2. Quantification of parasite mobility and blood flow. A-C. Quantitative analysis of particle movement in the four dimensions (4D) is facilitated by using color-coded image processing. A. Tracking was performed with the depth information from Z-coded color images, represented using maximum intensity projection of five different depths. White rectangle represents the same particle at different Z in one time point. Different positions are due to time lapses between the acquisition of different Z images. Depth code: yellow (0 μm), orange (2 μm), pink (4 μm), blue (6 μm), green (8 μm). B, C. Time projections of particle movement with each time interval coloured as: gray (0-2.4 sec), cyan (2.4-4.8 sec), magenta (4.8-7.0 sec), red (7.0-9.4 sec) and yellow (9.4-11.8 sec). White line represents 4D manual tracking of particles of 17X (11.8 s) (B) and 17XL (4.8 sec) (C) GFP parasites using MTrackJ. D,E. Distribution of the density of GFP particles by values of directionality (D) and residence time (E). Data correspond to 120 particles of each line of parasites and 100 FITC-labeled RBCs from three independent experiments analysed with the equality-of-medians test. The 17X/17XL/FITC-RBCs medians are 0.53/0.75/0.85 (D) and 4.61/0.67/0.9 sec (E). Differences between the two lines in (D) and (E) are statistically significant (P < 0.001). Differences between FITC-labeled RBCs and 17XL parasites are not statistically significant (P > 0.05). F,G. Spleen blood flow measurements. Representation of xy image (F) and xt image (G) from a line-scan of the central lumen of the same vessel (white line). Spleen vessel showing plasma with 70 kDa dextran (red), pRBC (green) and erythrocyte reflection (blue).
Movies 1 and 2. Time-lapse intravital microscopy images of the murine spleen infected with 17XL (1) or 17X (2) GFP-transgenic parasites at 10% parasitemia (Z-maximum projection). Parasite and tissue autofluorescence are shown in green and red respectively. Scale bars represent 10 μm and the time interval is in sec. Click here to watch Movie 1.Click here to watch Movie 2.
Discussion
The implementation of intravital microscopy of the spleen in this rodent malaria model opened the possibility of investigating the dynamic passage of parasites through this organ which until now has been considered a "black-box" due to technical considerations. In here, a major effort was put to adapt a quantitative method that allows comparative analysis of different parasite lines at the single and population levels. In contrast to other tissues and cells that had been imaged before in malaria 3,5, imaging the passage of pRBCs through the spleen needs to take into consideration the three-dimensionality and compartmentalization of the organ, the presence of different circulations with fast and slow flux 13, as well as the rapid erythrocyte velocities. With this aim, a specific methodology using online available ImageJ software was developed to enable single parasite tracking, mobility analysis and comparison between lines at the population level. However, the application of automatic software that solves identification and tracking of single parasites in this context is still needed. Of note, the parameters used to describe parasite mobility have been previously described in other studies to report lymphocyte recruitment and adhesion in vivo12,14,15. Thus, this methodology and parameters should be considered a new tool to the in vivo studies of adherence in malaria. In the future, we will use this technology to gain insight into the immunobiology and parasite-spleen cell interactions by imaging infection in transgenic mice expressing fluorescent reporter genes in different cells. Moreover, the generation of transgenic parasites expressing fluorescent markers other than GFP may be used in combination to image dual infections in this model.
In vivo imaging is a powerful tool to study the dynamic interplay of parasites within their hosts. However, there exist several factors affecting cell mobility that must be taken into consideration. Changes in the tissue architecture in response to infection with different Plasmodium strains can modify cell passage and interaction with the tissue 7,16 and the rheological properties of red blood cells, as well as changes in the hematocrit or other hematologic parameters, can affect blood flow and hence the interaction of cells with the tissue. For this reason, we recommend to analyze vessel blood flow with the procedure provided. To avoid any confounding effects, we imaged the spleen of infected mice at a time point when hematocrit, reticulocytemia and parasite tropism is comparable in both infections 7.
The resolution of this technique allows for the observation of single fluorescent cells passing through the spleen at earlier time points (<1% parasitemia). However, quantitative analysis of the dynamic behavior of parasites was performed at 1% parasitemia, when sufficient numbers of pRBCs were observed passing through the spleen at time lapses that allow tracking of single cell movement. In movies 1 and 2, which correspond to animals at 10% parasitemia, we showed the general pattern of parasite passage through the spleen in each infection; however, quantitative analysis of moving cells was performed in movies from animals at 1% parasitemia, where single cells are easily followed. Due to the rapid three-dimensional movement of infected red blood cells, we couldn’t differentiate between developmental stages of the parasite, as different fluorescence intensities could be attributed to different depths or penetrability of the laser.
With this procedure, fluorescent cells can be visualized in the subcapsular zone of the spleen, composed mainly of red pulp 17. By using plasma dyes, we could discern between the fast/closed and slow/open circulations of the red pulp. Other studies have reported imaging of fluorescent T-cells in the white pulp using confocal 18 or two-photon microscopy 19, with the last offering greater tissue penetration. In those, off-line analysis and ex vivo characterization of the zone being imaged is an important factor to accurately interpret the data. Thus, efforts to enlighten the microcirculatory structure of the spleen, as well as the development of probes that label specific cells and structures, are of importance to facilitate the study of parasite-host interactions.
Disclosures
No conflicts of interest declared.
Acknowledgments
We are particularly grateful to S. Graewe and V. Heussler for the initial training and continuous input in intravital microscopy of malaria parasites, to J. Burns for donating GFP transgenic parasites, to A. Bosch (Confocal Unit, CCiT-UB, IDIBAPS) for assistance in image analysis and quantification and to P. Astola for technical assistance. We thank R. Tous and I. Caralt for video production. MF is a recipient of a graduate fellowship from the Generality of Catalonia. HAP is an ICREA research professor. Work in the laboratory of HAP is funded by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement N° 242095, by the Private Foundation CELLEX (Catalonia, Spain), and by the Spanish Ministry of Science and Innovation (SAF2009-07760).
References
- Amino R, Menard R, Frischknecht F. In vivo imaging of malaria parasites--recent advances and future directions. Curr. Opin. Microbiol. 2005;8:407–414. doi: 10.1016/j.mib.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Heussler V, Doerig C. In vivo imaging enters parasitology. Trends. Parasitol. 2006;22:192–195. doi: 10.1016/j.pt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Amino R, Thiberge S, Blazquez S, Baldacci P, Renaud O, Shorte S. Imaging malaria sporozoites. in the dermis of the mammalian. 2007;2:1705–1712. doi: 10.1038/nprot.2007.120. [DOI] [PubMed] [Google Scholar]
- Gueirard P, Tavares J, Thiberge S, Bernex F, Ishino T, Milon G. Development of the malaria parasite in the skin of the mammalian host. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18640–18645. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- Gruring C, Heiber A, Kruse F, Ungefehr J, Gilberger TW, Spielmann T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat. Commun. 2011;2:165–165. doi: 10.1038/ncomms1169. [DOI] [PubMed] [Google Scholar]
- Martin-Jaular L, Ferrer M, Calvo M, Rosanas-Urgell A, Kalko S, Graewe S. Strain-specific spleen remodelling in Plasmodium yoelii infections in Balb/c mice facilitates adherence and spleen macrophage-clearance escape. Cell. Microbiol. 2011;13:109–122. doi: 10.1111/j.1462-5822.2010.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden Mvander, R A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am. J. Physiol. Cell. Physiol. 2002;283:C905–C916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Petrig BL, Qi X, Burns SA. In vivo measurement of erythrocyte velocity and retinal blood flow using adaptive optics scanning laser ophthalmoscopy. Opt. Express. 2008;16:12746–12756. doi: 10.1364/oe.16.012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Bowdler AJ. The complete spleen. Humana Press: Totowa; 2002. [Google Scholar]
- Grayson MH, Hotchkiss RS, Karl IE, Holtzman MJ, Chaplin DD. Intravital microscopy comparing T lymphocyte trafficking to the spleen and the mesenteric lymph node. Am. J. Physiol. Heart. Circ. Physiol. 2003;284:H2213–H2226. doi: 10.1152/ajpheart.00999.2002. [DOI] [PubMed] [Google Scholar]
- Khandoga AG, Khandoga A, Reichel CA, Bihari P, Rehberg M, Krombach F. In vivo imaging and quantitative analysis of leukocyte directional migration and polarization in inflamed tissue. PLoS. One. 2009;4:4693–4693. doi: 10.1371/journal.pone.0004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Geduldig U, Weidanz W. Mechanisms of splenic control of murine malaria: reticular cell activation and the development of a blood-spleen barrier. Am. J. Anat. 1986;176:251–285. doi: 10.1002/aja.1001760303. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson MH, Chaplin DD, Karl IE, Hotchkiss RS. Confocal fluorescent intravital microscopy of the murine spleen. J. Immunol. Methods. 2001;256:55–63. doi: 10.1016/s0022-1759(01)00437-9. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J. Immunol. 2008;181:3947–3954. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]


