Abstract
We recently reported that a preponderance of small adipose cells, decreased expression of cell differentiation markers, and enhanced inflammatory activity in human subcutaneous whole adipose tissue were associated with insulin resistance. To test the hypothesis that small adipocytes exhibited these differential properties, we characterized small adipocytes from epididymal adipose tissue of Zucker Obese (ZO) and Lean (ZL) rats. Rat epididymal fat pads were removed and adipocytes isolated by collagenase digestion. Small adipocytes were separated by sequential filtration through nylon meshes. Adipocytes were fixed in osmium tetroxide for cell size distribution analysis via Beckman Coulter Multisizer. Quantitative real-time PCR for cell differentiation and inflammatory genes was performed. Small adipocytes represented a markedly greater percentage of the total adipocyte population in ZO than ZL rats (58±4% vs 12±3%, p<0.001). In ZO rats, small as compared to total adipocytes had 4-fold decreased adiponectin, and 4-fold increased visfatin and IL-6 levels. Comparison of small adipocytes in ZO versus ZL rats revealed 3-fold decreased adiponectin and PPARγ levels, and 2.5-fold increased IL-6. In conclusion, ZO rat adipose tissue harbors a large proportion of small adipocytes that manifest impaired cell differentiation and pro-inflammatory activity, two mechanisms by which small adipocytes may contribute to insulin resistance.
Keywords: insulin resistance, obesity, adipose cell size, inflammation
Introduction
There is substantial evidence that insulin resistance is the link between obesity and risk of type 2 diabetes and/or cardiovascular disease.1 Since not all obese individuals are insulin resistant,2,3 factors related to adipose cell biology likely contribute to induction of an insulin resistant state. Adipose tissue cellularity and its association with altered glucose metabolism was first described several decades ago.4–7 Obese humans with enlarged subcutaneous adipose cells were found to be more hyper-insulinemic and glucose intolerant than individuals with smaller cells.5,6,8 Rodent and human studies indicated that adipose cell enlargement was associated with decreased insulin responsiveness.4,7,9 These data prompted speculation that “hypertrophic” obesity was responsible for the metabolic derangements associated with insulin resistance and diabetes.8,10
Using an updated method to characterize fat cell size and distribution, we have recently reported several novel observations. First, we found that the mean diameter of large cells from abdominal subcutaneous adipose tissue was, in fact, not different between insulin resistant as compared to obesity-matched, insulin sensitive individuals.11 Rather, insulin resistant individuals demonstrated a preponderance of small adipose cells measuring <40 μm in size. This finding was associated with decreased expression of genetic markers related to cell differentiation in the whole adipose tissue specimens, suggesting that a defect in fat storage capacity may contribute to obesity-related insulin resistance.
Secondly, we demonstrated that inflammatory activity was modestly upregulated in subcutaneous adipose tissue of insulin resistant as compared to equally obese, insulin sensitive women.12 Furthermore, inflammatory gene expression in subcutaneous adipose tissue was associated with an increased proportion of small adipose cells in the whole adipose tissue samples, independent of sex, insulin resistance, and BMI.13 Taken together, while the above results do not provide evidence of causality, they lend support to the inter-relationships of impaired adipose cell differentiation, increased inflammation, and accumulation of small adipose cells in the development of insulin resistance. In the present study, we sought to test the hypothesis that small adipocytes may be responsible for the biological properties observed by characterizing separated small adipocytes in epididymal adipose tissue of Zucker Obese (ZO) and Lean (ZL) rats. Comparisons were made of adipose cell size distribution of whole adipose tissue and isolated adipose cell samples. Gene expression analyses of markers of cell differentiation and inflammation were performed in small adipose cells in ZO and ZL rats.
Materials and Methods
Animals
Male ZO (fa/fa) and ZL (Fa/Fa) rats 8- to 10-weeks of age (Harlan Laboratories, Livermore, CA, USA) were maintained on a normal chow diet and housed in a room with a 12h:12h light-dark cycle and ambient room temperature of 22°C. A total of 31 rats were used in the study. The study protocol was approved by the Stanford University Administrative Panel on Laboratory Animal Care and carried out in accordance with guidelines from the American Association for the Accreditation of Laboratory Animal Care.
Adipose tissue harvesting and cell size analysis
After lethal anesthetization of the rats with isoflurane, whole blood was obtained for analysis of plasma insulin (rat ELISA kit, ALPCO Diagnostics, Salem, NH, USA). Intra-abdominal epididymal fat pads were removed from rats and 500 mg to 1 gm of adipose tissue immediately flash frozen in liquid nitrogen. These samples were stored at −80°C for subsequent analysis. Two samples of 30 mg of epididymal fat tissue were immediately fixed in osmium tetroxide and incubated in a water bath at 37°C for 48 hours in accordance with previously described methodology.14 Adipose cell sizes from these samples were then determined by a Beckman Coulter Multisizer III with a 400 μm aperture, as previously reported. 11,15 The range of cell sizes that can effectively be measured using this aperture is 20–240 μm. The instrument was set to count 6,000 particles, and the fixed-cell suspension was diluted so that coincident counting was <10%. After collection of pulse sizes, the data were expressed as particle diameters and displayed as histograms of counts against diameter using linear bins and a linear scale for the x-axis.
Analysis of adipose cell size distribution from Multisizer graphs entailed identification of the nadir, defined as the low point between the two cell populations.11 The number of adipose cells above and below this point was calculated by the Multisizer software and expressed as the % above’ and % below’ the nadir, as well as the ratio of small to large cells. In addition, the peak diameter’ of the large adipose cells was defined as the mean diameter at which the frequency of the large cell population reached a maximum. This method was found to be concordant with a mathematical modeling technique described in a prior study.11 We therefore chose to analyze results in this study using the former method. Finally, the Multisizer software calculated the mean, median, and mode of the adipose cell sizes in aggregate.
Adipocyte isolation and separation
The remainder adipose tissue was used for isolation of adipocytes. Two to three grams of ZO and ZL rat adipose tissue were transferred into 6 ml Krebs Ringer Bicarbonate HEPES Buffer containing 5% BSA. Because ZL rats had less ‘total’ fat mass, epididymal adipose tissue from two ZL rats was pooled for digestion to comprise one sample. After tissue was mechanically minced, 6 mg of collagenase was added and fat allowed to digest for 90 minutes at 37°C in a shaking water bath. The digested cells were then filtered through a 250 μm mesh and buoyant adipocytes and supernatant removed after washing with buffer three times. The remaining stromal cell pellet was flash frozen on liquid nitrogen. These adipocytes are subsequently referred to as total adipocytes, i.e. cells prior to separation. An aliquot of digested adipocytes was taken and fixed in osmium tetroxide for cell size distribution analysis via Beckman Coulter Multisizer.
Separation of small adipocytes in ZO rats was initiated by gentle filtration of digested adipocytes through an 84 μm and then 41 μm mesh. Adipocytes that filtered through the 41 μm mesh were labeled small adipocytes. For ZL rats, filtration through an additional 25 μm mesh was required to separate small from larger cells. An aliquot of small adipose cells was removed and fixed with osmium tetroxide to confirm successful separation of small adipocytes by Multisizer curve analysis, as well as visual inspection of cells by light microscopy. Of note, cells that stayed on top of the 84 μm mesh during filtration were also removed in an attempt to collect large adipocytes. In ZL rats, large cell separation was successful. However, since Multisizer curves for separated large adipocytes in ZL rats were essentially identical in appearance to curves for total adipocytes, total adipocytes were used in further analyses. When large cell separation in ZO rats was attempted, a significant proportion of small adipocytes were mixed with large adipocytes on top of the mesh. Therefore, further gene expression comparisons were made between the small and ‘total’ (mixed large and small) adipose cell populations.
Gene expression with rtPCR
Total RNA was extracted from flash frozen adipose tissue, total adipocytes, and small adipocytes using Qiazol and the Adipose Tissue RNAeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturers’ instructions. cDNA was synthesized from total RNA using the SuperScript II kit (Invitrogen, Carlsbad, CA, USA). Taqman primer/probe sets for mRNA transcripts of the following were purchased from Applied Biosystems (Foster City, CA, USA): adiponectin, leptin, GLUT4, peroxisome proliferator-activated receptorγ 1/2)(PPARγ1/2), sterol receptor element binding protein 1c (SREBP1c), CCAAT/enhancer binding protein alpha (C/EBPα), monocyte chemotactic protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), TNF-α, visfatin, and IL-6. 18S ribosomal RNA was used as a reference to normalize expression levels between samples. Amplification was carried out in triplicate on an ABI Prism 7700 sequence detection system at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15s and 60°C for 1 min. A threshold cycle (Ct value) was obtained from each amplification curve and a ΔCt value was first calculated by subtracting the Ct value for 18S ribosomal RNA from the Ct value for each sample. A ΔΔCt value was calculated by subtracting the ΔCt value of a ZO or ZL rat, chosen arbitrarily to serve as control. Fold-changes compared with the control were then determined by calculating 2−ΔΔ Ct. Because gene expression data were similar between whole adipose tissue and isolated adipocyte samples in ZO and ZL rats, we have chosen to present data for isolated adipocytes only.
Statistical analysis
Student’s unpaired t-tests were used to compare adipose cell size variables in ZO versus ZL rats, and gene expression analyses between groups. Student’s paired t-tests were used to compare adipose cell size variables between total and small adipocytes among ZO or ZL rats. Where data were not normally distributed, values for gene expression were logarithmically transformed prior to analysis. Outliers were also removed if they were greater than or equal to two standard deviations (SDs) above or below the mean. Data were expressed as mean ± standard error of the mean (SEM). P< 0.05 was taken to indicate statistical significance.
Results
Animal characteristics and adipose cell size distribution
ZO rats were heavier than their lean counterparts (349 ± 6.5 vs 256 ± 12 g, p< 0.001) and had higher fasting insulin levels (365 ± 113 vs 62.7 ± 26 pmol/L, p< 0.001).
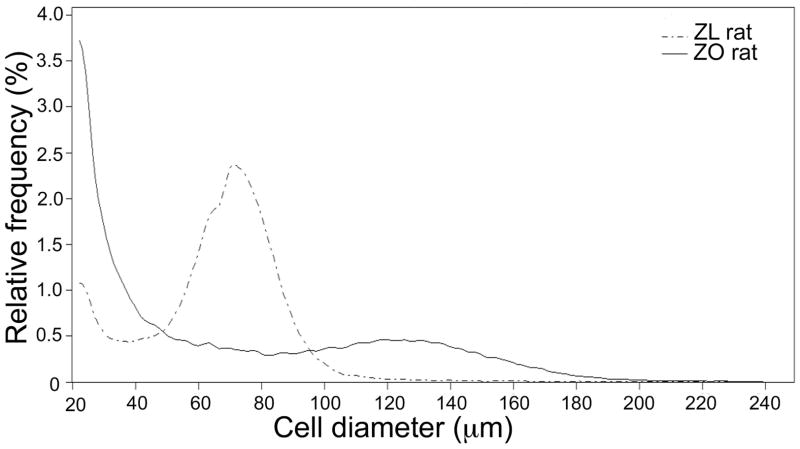
Figure 1 represents the averaged Multisizer curves of the total adipocyte population isolated from ZO and ZL rats, respectively. It is apparent that the two curves are quite different. Small adipocytes, measuring <40 μm comprised a large proportion of total adipocytes in ZO rat fat pads. In contrast, the majority of adipocytes from ZL rats measured between 60 to 90 μm. Quantitative comparisons of the cell size distribution of the two total adipocyte populations are presented in Table 1. These data show that small cells represented a markedly greater percentage of total cells in ZO than ZL rats (58 ± 4% vs 12 ± 3%, p< 0.001), also reflected in the ratio of small to large cells (1.6 ± 0.2 vs 0.1 ± 0.04, p< 0.001). In addition, the peak diameter of large cells was nearly twice as large in ZO than ZL rats (123 ± 2.6 μm vs 72 ± 1.0 μm, p< 0.001). Multisizer curves and data for whole adipose tissue were similar to that of the isolated adipocytes and are therefore not shown. Photographic evidence of small adipose cells using scanning electron microscopy of paraformaldehyde-fixed tissue as illustrated in our previous study in human subjects11 support that small cells identified by Multisizer are, indeed, adipose cells.
Figure 1.
Comparison of averaged Multisizer adipose cell profile curves from total adipocytes of Zucker Obese (ZO) and Zucker Lean (ZL) rats.
Table 1.
Comparison of adipose cell size variables in ZO (n=11) and ZL (n=10) rats
| Cell size variables | ZO ratsa | ZL ratsa | p value |
|---|---|---|---|
| Mean (μm) | 75 ± 3.0 | 66 ± 1.3 | 0.028 |
| Median (μm) | 63 ± 6.4 | 69 ± 1.4 | 0.36 |
| Mode (μm) | 23 ± 0.1 | 59 ± 8.0 | <0.01 |
| Nadir (μm) | 78 ± 3.3 | 39 ± 2.2 | <0.001 |
| % of cells below nadir | 58 ± 3.7 | 12 ± 2.7 | <0.001 |
| Ratio (small:large) | 1.6 ± 0.2 | 0.1 ± 0.04 | <0.001 |
| Peak diameter of large cells (μm) | 123 ± 2.6 | 72 ± 1.0 | <0.001 |
| Mean diameter of small cells (μm) | 35 ± 1.8 | 32 ± 1.4 | 0.07 |
Data are expressed as mean ± standard error of the mean (SEM)
Gene expression of total adipocytes
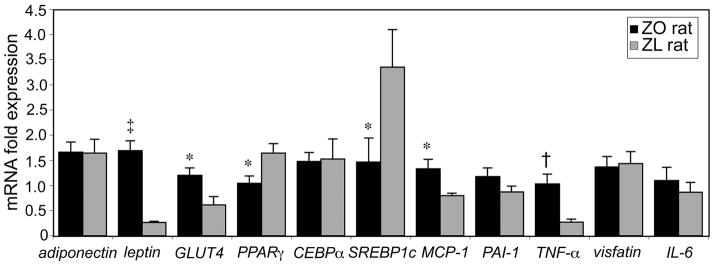
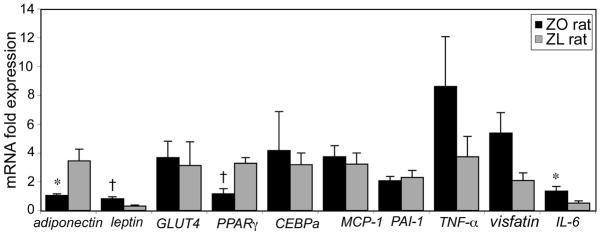
Figure 2 compares the expression of genes related to differentiation and inflammation in the total adipocyte populations from ZO and ZL rats. Of the genes related to differentiation, adipocytes isolated from ZO rats had a 6-fold increased expression of leptin (p< 0.001), 2-fold increased expression of GLUT4 (p< 0.05), and 1.5- and 2.3- fold decreased expression of PPARγ and SREBP1c, respectively (p< 0.05). In addition, inflammatory markers, MCP-1 and TNF-α were upregulated in ZO rat adipocytes (p< 0.05).
Figure 2.
Mean relative gene expression levels in total adipocytes from ZO (n=11) and ZL rats (n=10). Genes related to adipose cell differentiation and inflammation are presented. *p< 0.05; †p< 0.01; ‡p< 0.001.
Small adipocytes: Multisizer curves and gene expression
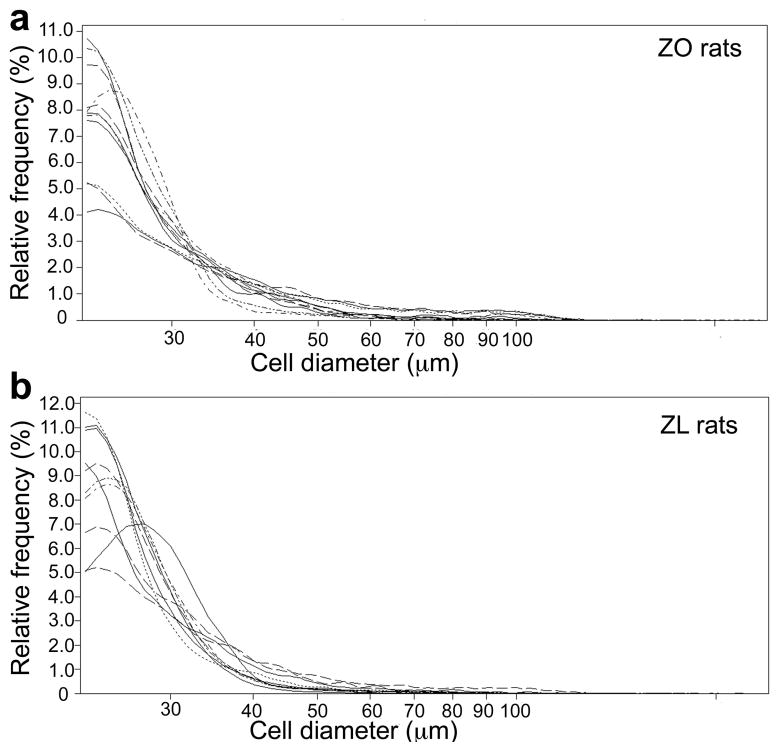
Figure 3 illustrates the separated small adipocytes in ZO (Figure 3a) and ZL (Figure 3b) rats. Each curve represents an individual ZO and ZL rat sample. These curves demonstrate that small cells were separated successfully and therefore validated for subsequent gene expression analyses.
Figure 3.
Multisizer curves demonstrating separation of small adipocytes in (a), ZO rats (n=11) and (b), ZL rats (n=10). Each curve represents an individual ZO and ZL rat sample.
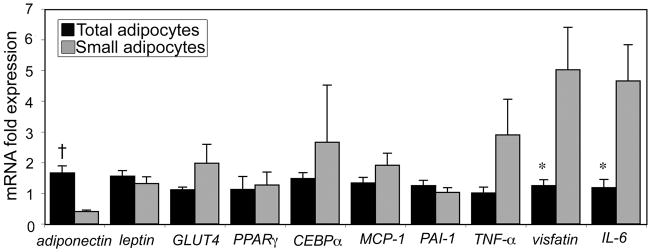
Figure 4 compares the gene expression profile in small versus total adipocytes isolated from ZO rats. Small adipocytes from ZO rats had significantly decreased adiponectin levels (4-fold, p< 0.01), and 4-fold increased visfatin and IL-6 levels (p< 0.05). Because Ct values were undetectable for SREBP1c in almost all samples of small adipocytes from ZO rats, this gene was not included in analysis.
Figure 4.
Mean relative gene expression levels in small and total adipocytes from ZO rats (n=11). *p< 0.05; †p< 0.01.
On the other hand, expression of adipogenic gene markers was not different when small adipocytes from ZL rats were compared with total adipocytes (data not shown). Although small adipocytes from ZL rats did have a 4.5- fold increase in TNF-α levels (p< 0.05) as compared to total adipocytes, there was no significant increase in gene expression of the other inflammatory markers.
Potential differences in gene expression in small adipocytes from ZO and ZL rats are further explored in Figure 5. When directly compared, small adipocytes from ZO rats demonstrated 3-fold decreased expression of adiponectin and PPARγ (p< 0.05), as well as 2.5- fold increases in expression of leptin and IL-6 levels (p< 0.05) as compared with ZL rats.
Figure 5.
Small adipocytes in ZO (n=11) and ZL rats (n=10) are compared for differences in relative gene expression levels. *p< 0.05; †p< 0.01.
Discussion
The results presented here have documented striking differences in cell size distribution between adipocytes from obese, insulin resistant ZO rats and non-obese, insulin sensitive ZL rats, as well as delineate the adipogenic and inflammatory characteristics of small adipocytes that help to substantiate the observations we have made on insulin resistance, accumulation of small adipose cells, and adipose tissue gene expression in our human studies.11–13
The most notable difference between adipocytes from the ZO and ZL rats was in the cell size distribution. The ratio of small to large cells was increased 16-fold in adipocytes from ZO rats. Put in another way, small cells occupied more than half of the total number of cells in the ZO rat epididymal fat depot. By contrast, small cells comprised only 12% of the total adipose cell population in ZL rats. Moreover, the mean peak diameter of the large cells was significantly greater in ZO rats. Our ability to demonstrate this striking difference in cell size distribution was made possible by the use of updated Multisizer cell sizing methodology to characterize adipose tissue cellularity. Earlier Coulter Counter techniques were set to count cells above a certain plateau level that unintentionally overlooked smaller cells.5,6,16 Other methods such as microscopic or photographic visualization use a representative sample of cells that may not sufficiently account for adipose cell size spread. Histologic method to evaluate adipose cell diameter is dependent on the plane through which the adipocyte is sliced, and thereby subjected to the artifact of off-center sectioning. Through Multisizer technology we have been able to document the presence of an expanded population of small adipose cells in the epididymal adipose tissue of ZO rats, associated with a significant increase in the size of the large cells.
These findings are consistent with the hypothesis that adipose cells enlarge to a maximal state, followed by proliferation of small cells to accommodate further fat storage.17 Indeed, we have extended these observations further by assessing, in vivo, adipose tissue development in the ZO rat.18 By performing sequential micro-biopsies over a 21-week period, we observed that adipose tissue expansion exhibited a temporal periodicity of roughly 55 days during which cells increase in size and new, smaller adipose cells are recruited. This mechanism is triggered when the flux of lipid needing storage exceeds that of capacity for lipid uptake. In the present study, the preponderance of small adipose cells in the ZO rat fat pad indicates that the ability to recruit new cells is retained, although they appear to be dysfunctional in nature, as evidenced by their adipogenic and inflammatory characteristics.
The second main findings of our study are in the differential properties of small adipocytes. As compared to total cells, ZO rat small adipocytes exhibited significantly lower expression of adiponectin, an adipocytokine made exclusively by mature adipocytes, and whose plasma and adipose tissue levels are decreased in human insulin resistance, independent of obesity.11,19 That differential expression of adiponectin was robust despite comparison of small cells with the total mixed cell population intimates that the differences between small and large cells may, in fact, be greater. As compared to small adipocytes from ZL rats, small adipocytes from ZO rats also had decreased levels of adiponectin and PPARγ, highlighting not only that the small adipocytes from ZO rats have an impaired capacity for differentiation, but that they may be functionally disparate from the ZL rat small adipose cells. These findings help to substantiate our findings in human subcutaneous adipose tissue, in which accumulation of small adipose cells was associated with decreased adipose tissue expression of genes related to adipocyte differentiation in insulin resistance, independent of obesity.11 Taken together, it is reasonable to postulate that small adipose cells may contribute to systemic insulin resistance via their impaired capacity for fat storage.
The other defining properties of small adipocytes are their modest pro-inflammatory nature. Of the five inflammatory genes tested, visfatin and IL-6 were upregulated in small as compared with total adipocytes from ZO rats, suggesting that inflammation may serve as an additional mechanism by which small adipocytes may contribute to insulin resistance. Small adipocytes from ZO rats also expressed increased levels of IL-6 when compared with small cells from ZL rats. These findings are supportive of our previous data in humans, in which we showed that inflammatory activity was independently associated with increased proportion of small adipose cells in subcutaneous adipose tissue, as well as measures of insulin resistance.12,13 Thus, it seems plausible that the inflammatory properties of small adipose cells may contribute to development of insulin resistance at a systemic level, although causality remains to be elucidated.
It is valuable to consider results of the small adipocytes within the larger context of the adipose tissue and total adipocyte population as a whole. Isolated adipocytes from ZO as compared with ZL rats demonstrated evidence of impaired differentiation and greater inflammatory activity. Taken together with data from the small adipocytes, one may infer that these characteristics of the ZO rat adipose tissue depot as a whole are accounted for by the expanded presence of small adipocytes. While upregulated GLUT4 levels in ZO rat adipocytes as compared to their lean counterparts may appear to be an exception, these data are consistent with well-established evidence that regulation of adipose cell GLUT4 occurs in an age-dependent pattern in ZO rats;20,21 adipocytes of young ZO rats exhibit hyper-responsiveness to insulin-stimulated glucose transport (despite systemic hyper-insulinemia/insulin resistance), whereas adipocytes from older ZO rats exhibit impaired glucose uptake. Likewise, increased leptin in ZO as compared to ZL rat adipocytes is ascribed to the ZO rat genetic makeup.
Overall, these findings are consistent with previous studies from our group and others supporting the association of obesity-related insulin resistance with impaired adipogenesis and inflammatory states.11,12,15,22–25 How impaired differentiation, increased inflammation, and accumulation of small adipocytes may interrelate to promote insulin resistance remains more speculative. One possibility is that inflammatory cytokines secreted by small cells impair terminal adipocyte differentiation, leading to ineffective triacylglycerol storage and accumulation of further small cells. An alternate explanation is that non-adipose cell inflammatory mediators, i.e. macrophages or other myeloid-derived cells, residing in adipose tissue exert negative effects on adipocyte function.26–28 In this context, it is important to re-emphasize that the small cells we have detected in our studies are adipose cells, and not macrophages.11,13 This is an important distinction, for while it is known that macrophages can promote inflammatory activity in adipose tissue,29 our findings address the direct role small adipose cells may have in inflammation. Finally, as another hypothesis, it is also plausible that the primary defect lies in adipose cell differentiation, with development of an inflammatory phenotype in the small adipocytes as a secondary process.
This study is one of several studies published on the genetic or metabolic characteristics of small adipocytes, of which mixed results have been reported. One study showed that small and large adipose cells in mice fed a high-fat diet did not differ in various measures of insulin resistance.30 Immune-related genes were reported to be upregulated in large versus small human adipocytes.31
Results from another study were contrary to ours, namely, that pro-inflammatory factors correlated with increasing adipocyte size.32 Among studies that did not specifically separate or characterize small and/or large cells, several described positive relationships between adipose cell enlargement and insulin resistance.33, 34 On the other hand, Pasarica et al35 reported that patients with type 2 diabetes had an increased proportion of small adipose cells in the subcutaneous adipose tissue depot as compared with BMI-matched, non-diabetic individuals. It is also important to discuss briefly our recent findings that treatment with pioglitazone led to recruitment of small adipocytes in human abdominal subcutaneous adipose tissue, as well as redistribution of fat from visceral to subcutaneous depots.36 While these findings may appear to conflict with that of the present study, we have postulated that small adipocytes that accumulate in response to the adipogenic effect of thiazolidinedione therapy36 may differ from those found in insulin resistant, untreated individuals,11 or as in the present case, the hyper-insulinemic, ZO rat. Functional studies could help to elicit whether these two small adipose cell populations differ in their capacity for lipid storage.
The strengths of our approach lie in the use of updated cell size techniques to accurately assess adipose tissue cellularity, and separation of small adipocytes for characterization and comparison. Adipocyte isolation eliminated the potential confounding effects of macrophages on inflammatory changes in the fat depot. A limitation of our study is its cross-sectional design, which precludes assumptions on causality. In addition, comparison of total adipose cells with small cells in the ZO rats may have underestimated the true effect size, given that small cells contribute substantially to the total adipose cell population. Nonetheless, that we were able to elicit the reported gene expression differences suggest that these findings are real. It is also possible that gene and protein expression levels in the adipose cells may vary. Finally, while ZO rats are useful animal models of insulin resistance, these findings may be strain-specific. We recently reported that recruitment of small adipose cells was greater in an obesity-prone mouse as compared with an obesity-resistant mouse strain, an effect enhanced under high fat feeding conditions.37 That we have been able to document an expanded proportion of small adipose cells using two different rodent models of obesity helps to validate these findings. It would be important to perform future investigations in human subjects, as well as conduct functional studies in the small adipose cells to support the genetic characteristics found in the present study.
We had postulated based on our studies in humans that small, rather than large adipose cells, were closely associated with insulin resistance. Our results in ZO rats have shown that small adipocytes manifest evidence of impaired adipogenesis and increased inflammatory activity, two mechanisms by which small adipocytes may contribute to whole-body insulin resistance in the ZO rat. These small adipocytes in the ZO rat appear to be distinct from those of the ZL rat, underscoring the relevance of metabolic phenotype in evaluating small adipocyte functionality. These findings provide novel insight into the role small adipocytes may have in the development of insulin resistance.
Acknowledgments
Funding for this study was provided by study grants NIH/NIDDK 1 R01 DK071309-01, 5 R01 DK071333-04, 5 F32 DK079578-02, and by the NIDDK Intramural Research Program.
References
- 1.Reaven GM. Insulin resistance: The link between obesity and cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37:581–601. vii–viii. doi: 10.1016/j.ecl.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: Effect of differences in insulin sensitivity. Arch Intern Med. 2007;167:642–648. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 4.Salans LB, Bray GA, Cushman SW, Danforth E, Jr, Glennon JA, Horton ES, Sims EA. Glucose metabolism and the response to insulin by human adipose tissue in spontaneous and experimental obesity. Effects of dietary composition and adipose cell size. J Clin Invest. 1974;53:848–856. doi: 10.1172/JCI107625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968;47:153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JS, Batchelor BR, Hollander N, Cohn CK, Hirsch J. Adipose-cell size and immunoreactive insulin levels in obese and normal-weight adults. Lancet. 1972;2:948–951. doi: 10.1016/s0140-6736(72)92474-9. [DOI] [PubMed] [Google Scholar]
- 7.Salans LB, Dougherty JW. The effect of insulin upon glucose metabolism by adipose cells of different size. Influence of cell lipid and protein content, age, and nutritional state. J Clin Invest. 1971;50:1399–1410. doi: 10.1172/JCI106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olefsky JM. Mechanisms of decreased insulin responsiveness of large adipocytes. Endocrinology. 1977;100:1169–1177. doi: 10.1210/endo-100-4-1169. [DOI] [PubMed] [Google Scholar]
- 10.Stern MP, Olefsky J, Farquhar JW, Reaven GM. Relationship between fasting plasma lipid levels and adipose tissue morphology. Metabolism. 1973;22:1311–1317. doi: 10.1016/0026-0495(73)90275-8. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50:1707–1715. doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin T, Deng A, Gonzales O, Aillaud M, Yee G, Lamendola C, Abbasi F, Connolly AJ, Sherman A, Cushman SW, Reaven G, Tsao PS. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. 2008;51:2303–2308. doi: 10.1007/s00125-008-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin T, Deng A, Yee G, Lamendola C, Reaven G, Tsao PS, Cushman SW, Sherman A. Inflammation in subcutaneous adipose tissue: Relationship to adipose cell size. Diabetologia. 2009;53:369–377. doi: 10.1007/s00125-009-1496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch J, Knittle JL. Cellularity of obese and nonobese human adipose tissue. Fed Proc. 1970;29:1516–1521. [PubMed] [Google Scholar]
- 15.Liu A, McLaughlin T, Liu T, Sherman A, Yee G, Abbasi F, Lamendola C, Morton J, Cushman SW, Reaven GM, Tsao PS. Differential intra-abdominal adipose tissue profiling in obese, insulin-resistant women. Obes Surg. 2009;19:1564–1573. doi: 10.1007/s11695-009-9949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PR, Zucker LM, Cruce JA, Hirsch J. Cellularity of adipose depots in the genetically obese zucker rat. J Lipid Res. 1971;12:706–714. [PubMed] [Google Scholar]
- 17.Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: A new model of obesity. Am J Physiol. 1978;235:E279–286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 18.Mackellar J, Cushman SW, Periwal V. Waves of adipose tissue growth in the genetically obese zucker fatty rat. PLoS One. 5:e8197. doi: 10.1371/journal.pone.0008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, Reaven PD. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–590. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 20.Cushman SW, Zarnowski MJ, Franzusoff AJ, Salans LB. Alterations in glucose metabolism and its stimulation by insulin in isolated adipose cells during the development of genetic obesity in the zucker fatty rat. Metabolism. 1978;27:1930–1940. doi: 10.1016/s0026-0495(78)80010-9. [DOI] [PubMed] [Google Scholar]
- 21.Hainault I, Guerre-Millo M, Guichard C, Lavau M. Differential regulation of adipose tissue glucose transporters in genetic obesity (fatty rat). Selective increase in the adipose cell/muscle glucose transporter (glut 4) expression. J Clin Invest. 1991;87:1127–1131. doi: 10.1172/JCI115077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 23.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS, Cam MC, Cushman SW, Smith U. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun. 2004;317:1045–1051. doi: 10.1016/j.bbrc.2004.03.152. [DOI] [PubMed] [Google Scholar]
- 26.Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81–92. doi: 10.1016/s0303-7207(01)00394-x. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson B, Smith U. Cytokines promote wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3t3-l1 preadipocytes. J Biol Chem. 2006;281:9507–9516. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 28.Hammarstedt A, Isakson P, Gustafson B, Smith U. Wnt-signaling is maintained and adipogenesis inhibited by tnfalpha but not mcp-1 and resistin. Biochem Biophys Res Commun. 2007;357:700–706. doi: 10.1016/j.bbrc.2007.03.202. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wueest S, Rapold RA, Rytka JM, Schoenle EJ, Konrad D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia. 2009;52:541–546. doi: 10.1007/s00125-008-1223-5. [DOI] [PubMed] [Google Scholar]
- 31.Jernas M, Palming J, Sjoholm K, Jennische E, Svensson PA, Gabrielsson BG, Levin M, Sjogren A, Rudemo M, Lystig TC, Carlsson B, Carlsson LM, Lonn M. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 32.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 33.Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–633. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 34.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type ii diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 35.Pasarica M, Xie H, Hymel D, Bray G, Greenway F, Ravussin E, Smith SR. Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes Care. 2009;32:900–902. doi: 10.2337/dc08-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin TM, Liu T, Yee G, Abbasi F, Lamendola C, Reaven GM, Tsao P, Cushman SW, Sherman A. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring) 2010;18:926–931. doi: 10.1038/oby.2009.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]