Abstract
Math anxiety is a negative emotional reaction to situations involving mathematical problem solving. Math anxiety has a detrimental impact on an individual’s long-term professional success, but its neurodevelopmental origins are unknown. In a functional MRI study on 7- to 9-year-old children, we showed that math anxiety was associated with hyperactivity in right amygdala regions that are important for processing negative emotions. In addition, we found that math anxiety was associated with reduced activity in posterior parietal and dorsolateral prefrontal cortex regions involved in mathematical reasoning. Multivariate classification analysis revealed distinct multivoxel activity patterns, which were independent of overall activation levels in the right amygdala. Furthermore, effective connectivity between the amygdala and ventromedial prefrontal cortex regions that regulate negative emotions was elevated in children with math anxiety. These effects were specific to math anxiety and unrelated to general anxiety, intelligence, working memory, or reading ability. Our study identified the neural correlates of math anxiety for the first time, and our findings have significant implications for its early identification and treatment.
Keywords: cognitive neuroscience, cognitive development, educational psychology, neuroimaging, mathematical ability
Strong mathematical skills are increasingly essential for academic and professional success in today’s high-technology world (National Mathematics Advisory Panel, 2008). Research has shown that math anxiety has a negative impact on mathematical skills, which leads to adverse effects on career choice, employment, and professional success (Ma, 1999). Math anxiety is thought to influence learning and mastery of mathematics from an early age, but its precise developmental origins are not known (Rubinsten & Tannock, 2010). Although the first years of elementary schooling are an important period for acquiring basic mathematical skills, previous behavioral studies of math anxiety have mainly focused on adolescents and adults. However, across all age groups, but most notably in children, nothing is currently known about the neurobiological mechanisms underlying math anxiety. The study reported here is the first to identify the neural basis of math anxiety in young children and demonstrate its impact on brain functioning and connectivity at one of the earliest stages of formal acquisition of math skills.
Math anxiety is a negative emotional response that is characterized by avoidance as well as feelings of stress and anxiety in situations involving mathematical reasoning (Ashcraft & Ridley, 2005). It can often hinder the successful completion of tasks involving manipulation of numerical information and is a prominent cause of problem-solving difficulties across all age ranges (Ashcraft & Krause, 2007; Suinn, Taylor, & Edwards, 1988; Wigfield & Meece, 1988). Behavioral studies, mainly in adults, have shown that math anxiety has a negative effect on performance of basic numerical operations, including counting, addition, and subtraction (Ashcraft & Ridley, 2005; Maloney, Risko, Ansari, & Fugelsang, 2010). Because the detrimental impact of math anxiety on mathematical development is lifelong (Bynner & Parsons, 1997; Rubinsten & Tannock, 2010), it is important to understand its neurobiological origins, especially during the earliest stages of formal math learning in elementary school children.
Although the behavioral literature on math anxiety in adults and adolescents is extensive, there is a relative dearth of studies investigating math anxiety in young children. This is in part due to the lack of a developmentally appropriate measure of math anxiety. To address this issue, we recently extended the Mathematics Anxiety Rating Scale (MARS), a standardized method for assessing math anxiety in older children and adults (Richardson & Suinn, 1972; Suinn & Edwards, 1982), to create the Scale for Early Mathematics Anxiety (SEMA; Table S1 in the Supplemental Material available online provides details of this scale). The SEMA has been shown to be a reliable and construct-valid (Cronbach’s α = .870) measure of math anxiety in 7- to 9-year-old second and third graders (Wu, Amin, Barth, Melcarne, & Menon, 2012).
To examine the neurodevelopmental basis of math anxiety, we analyzed functional brain-imaging data from forty-six 7- to 9-year-old children, which we obtained while the children determined whether addition and subtraction problems were correct (e.g., “2 + 5 = 7”) or incorrect (e.g., “2 + 4 = 7”). In a separate session, we used the SEMA to assess math anxiety in each child. Previous studies in both children and adults have implicated multiple limbic, paralimbic and prefrontal cortex regions, including the amygdala and medial prefrontal cortex in social and generalized anxiety disorders (Etkin & Wager, 2007; Guyer et al., 2008). Normal healthy adults also show activation of these same regions while viewing negative stimuli, such as fearful faces (Sabatinelli et al., 2011). However, it is not known whether these same areas are also engaged by “neutral” numerical symbols that are perceived negatively. Specifically, it is unknown whether these limbic-prefrontal cortex circuits are differentially engaged during math problem solving in individuals with high math anxiety. We hypothesized that if children with high math anxiety view such stimuli negatively, they would show hyperactive amygdala response during math problem solving. Furthermore, amygdala connectivity with medial prefrontal cortex regions involved in emotion regulation would also be elevated when compared with such connectivity in children with low math anxiety.
We used multivariate pattern analysis (MPA; Kriegeskorte, Goebel, & Bandettini, 2006) to further investigate aberrant patterns of amygdala response in children with high math anxiety. We predicted that such children would show distinct spatial patterns of task-related activity in the amygdala when compared with children with low math anxiety and that the activation patterns of each group would be independent of overall differences in signal amplitude. Finally, we hypothesized that children with high math anxiety would show decreased engagement of the intraparietal sulcus and dorsolateral prefrontal cortex regions typically associated with mathematical cognition in children (Ansari, 2008; Rivera, Reiss, Eckert, & Menon, 2005).
Method
Participants
A total of 54 children from the San Francisco area were originally recruited for this study. Eight participants were excluded because of poor functional MRI (fMRI) data quality. The remaining sample (n = 46) consisted of 28 boys and 18 girls in the second and third grades (age range = 7.17–9.33 years, M = 8.42 years, SD = 0.61 years). All but 4 children were right-handed. None of the participants had a history of psychiatric illness, neurological disorders, or learning disabilities. Participants were recruited via flyers sent to elementary schools as well as advertisements posted in libraries, in magazines, on Web sites, and with learning-disability groups. All protocols were approved by the institutional review board at Stanford University School of Medicine, and participants were treated in accordance with the American Psychological Association Code of Conduct.
Neuropsychological assessments
Before the fMRI scan, we conducted a neuropsychological assessment on each child. This assessment consisted of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to measure IQ, the Wechsler Individual Achievement Test (Wechsler, 2001) to assess performance, and the Working Memory Test Battery for Children (Pickering & Gathercole, 2001) to determine working memory capacity. Parents of study participants also completed the Child Behavior Checklist for Ages 6–18 (CBCL/6–18; Achenbach & Rescorla, 2001), and trait anxiety was determined using the DSM-Oriented Anxiety Problems subscale of the CBCL. The SEMA (Wu et al., 2012) was used to assess math anxiety. SEMA scores were used both as a continuous measure and to divide participants into high-math-anxiety (HMA) and low-math-anxiety (LMA) groups. All measures have been shown to have accurate validity and reliability and have been normed for use in children.
Functional brain imaging
Each child completed two runs in the fMRI scanner: the addition run and the subtraction run. Each run had four task conditions: (a) complex arithmetic problems, (b) simple arithmetic problems, (c) number identification, and (d) passive fixation. Only the two arithmetic-task conditions were examined in this study. In both arithmetic conditions, full equations were given, and the child indicated via a button box whether the answer shown was correct or incorrect (see fMRI Experimental Design in the Supplemental Material).
In the addition run, complex problems consisted of equations with one addend ranging from 2 to 9 and the other addend ranging from 2 to 5 (e.g., “5 + 2 = 7”). There were no problems in which both addends were the same (e.g., “5 + 5 = 10”). The format of simple addition problems was identical to that of complex problems, except that one of the addends was 1 (e.g., “5 + 1 = 7”). The design of the subtraction run was the same as its addition counterpart, such that complex subtraction problems were the inverse of complex addition problems (e.g., “7 – 5 = 2”), and simple subtraction problems contained a subtrahend of 1 (e.g., “7 – 1 = 5”). In each case, incorrect answers deviated by ±1 or ±2 from the correct answer. Critically, for both addition and subtraction, the complex and simple problems had equivalent numerical and symbolic formats as well as the same response-selection requirements.
fMRI data acquisition and analysis
Acquisition and preprocessing
Functional brain images were acquired on a 3-T GE Signa scanner (see fMRI Data Acquisition in the Supplemental Material). Data were analyzed using a general linear model implemented in the Statistical Parametric Mapping program (SPM8; Wellcome Trust Centre for Neuroimaging, London, United Kingdom). Images were realigned to correct for movement, denoised, spatially normalized to Montreal Neurological Institute (MNI) space, and smoothed with an effective Gaussian kernel of 6 mm (see fMRI Preprocessing in the Supplement Material). A repeated measures analysis of variance of movement parameters using group (HMA, LMA) as a between-subjects factor and operation (addition, subtraction) and direction (x, y, z) as within-subjects factors revealed that there were no group differences in movement, F(1, 44) = 0.661, p = .420. A linear regression analysis confirmed that SEMA scores were not correlated with movement (p > .380).
Statistical analysis
Data from the two runs were combined in a single analysis. Brain activity related to each task condition was modeled by convolving boxcar functions with a canonical hemodynamic response function and a temporal derivative to account for voxel-wise latency differences in hemodynamic response. Voxel-wise t statistics were generated for each participant by contrasting complex addition and subtraction problems with simple addition and subtraction problems. Brain responses in the HMA and LMA groups were then compared using a t test on contrast images from each participant. Significant clusters of activation were determined at a voxel-wise height threshold of p < .01, with family-wise error (FWE) correction for multiple spatial comparisons (p < .01, k = 133 voxels). The FWE correction was determined using Monte Carlo simulations (Ward, 2000).
In addition to conducting the dichotomous group analysis, we used SEMA scores as a continuous variable to identify brain regions that showed increases and decreases in brain activation related to math anxiety. Cytoarchitectonic maps (Eickhoff et al., 2005) were used to determine the percentage of fMRI activation clusters that fell within individual subdivisions of the amygdala and anterior hippocampus as well as to conduct (anatomically) unbiased MPA. MPA was used to examine whether multivoxel spatial activation patterns, above and beyond overall differences in signal level, were different between the two groups (see fMRI Multivariate Pattern Analysis in the Supplemental Material). Finally, psychophysiological interaction analysis was used to examine group differences in effective connectivity of the amygdala independent of overall task-related activation (see fMRI Effective Connectivity Analysis in the Supplemental Material).
Results
Math anxiety and behavior
SEMA scores were neither uniformly distributed, χ2(4, N = 46) = 19.00, p = .001, nor normally distributed (Shapiro-Wilk test W = 0.91, p = .002). To increase sensitivity and robustness, and to facilitate interpretability and multivariate classification analysis of fMRI data, we used a median split on the total-anxiety score to divide children into two groups of equal size (HMA group score: M = 38.391, SD = 7.590; LMA group score: M = 25.348, SD = 2.673). The two groups differed on math anxiety but not on IQ, working memory, or overall reading and math proficiency (Table 1). Math-anxiety scores did not differ between boys and girls, F(1, 44) = 1.751, p = .193, or between second and third graders, F(1, 44) = 0.018, p = .894, so data from both genders and grades were pooled for subsequent analysis. Critically, although the two groups differed in math anxiety, F(1, 44) = 60.425, p < .001, they did not differ in trait anxiety, F(1, 39) = 0.508, p = .480. Additional analyses using math anxiety and trait anxiety as continuous variables showed that these two measures were not significantly correlated (r = −.132, p > .400).
Table 1.
Mean Neuropsychological Scores and Analysis of Variance Results for the High-Math-Anxiety (HMA) and Low-Math-Anxiety (LMA) Groups
| Measure | HMA group | LMA group | F test | p |
|---|---|---|---|---|
| Scale for Early Mathematics Anxiety | 38.39 (1.58) | 25.35 (0.56) | F(1, 44) = 60.43 | < .001 |
| Child Behavior Checklist for Ages 6–18 | ||||
| DSM-Oriented Anxiety Problems subscale | 1.25 (0.32) | 1.67 (0.48) | F(1, 39) = 0.51 | .48 |
| Wechsler Abbreviated Scale of Intelligence | ||||
| Verbal IQ subscale | 108.52 (1.99) | 110.57 (3.00) | F(1, 44) = 0.32 | .57 |
| Performance IQ subscale | 112.09 (3.30) | 106.74 (2.34) | F(1, 44) = 1.74 | .19 |
| Full-Scale IQ subscale | 111.35 (2.34) | 109.57 (2.24) | F(1, 44) = 0.30 | .58 |
| Working Memory Test Battery for Children | ||||
| Digit Recall subscale | 104.04 (3.57) | 100.41 (3.69) | F(1, 43) = 0.50 | .48 |
| Block Recall subscale | 93.82 (3.03) | 96.82 (2.47) | F(1, 43) = 0.59 | .45 |
| Count Recall subscale | 90.45 (3.73) | 87.65 (3.36) | F(1, 43) = 0.31 | .58 |
| Backwards Digit Recall subscale | 92.96 (2.42) | 93.48 (3.20) | F(1, 44) = 0.02 | .90 |
| Wechsler Individual Achievement Test | ||||
| Word Reasoning subscale | 105.96 (2.22) | 110.22 (2.82) | F(1, 44) = 1.40 | .24 |
| Number Operations subscale | 105.35 (3.45) | 105.39 (3.31) | F(1, 44) = 0.00 | .99 |
| Reading Composition subscale | 105.39 (2.15) | 107.39 (2.84) | F(1, 44) = 0.32 | .58 |
| Math Reasoning subscale | 111.83 (3.06) | 112.52 (3.12) | F(1, 44) = 0.03 | .88 |
Note: Standard errors of the mean are given in parentheses. The degrees of freedom varied because of missing data and because standardized scores could not be computed for every subject. The following measures were used in this study: Scale for Early Mathematics Anxiety (Wu, Amin, Barth, Melcarne, & Menon, 2012), Child Behavior Checklist for Ages 6–18 (Achenbach & Rescorla, 2001), Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), Working Memory Test Battery for Children (Pickering & Gathercole, 2001), and Wechsler Individual Achievement Test (Wechsler, 2001).
Neural correlates of math anxiety
Behavioral analysis of fMRI task
We first examined behavioral performance during the fMRI session (results are shown in Table S2 in the Supplemental Material). Group differences in accuracy and response time (RT) were analyzed using a three-way repeated measures analysis of variance with operation (addition, subtraction) and problem type (complex, simple) as within-participants factors and group (HMA, LMA) as a between-participants factor. The main effect of group was marginally significant, F(1, 44) = 3.81, p = .057, with a trend toward reduced accuracy in the HMA group. The main effect of operation was significant, F(1, 44) = 21.03, p < .001; participants were more accurate at solving addition problems than at solving subtraction problems. Finally, there was a significant main effect of problem type; accuracy was higher on simple problems than on complex problems, F(1, 44) = 30.28, p < .001. No other interactions were significant.
For RT, there was a significant Group × Problem Type interaction, F(1, 44) = 4.60, p = .038; the HMA group showed smaller RT differences between complex and simple problems than did the LMA group. In addition, the main effect of operation was significant, F(1, 44) = 7.75, p < .008; participants solved addition problems more quickly than they solved subtraction problems. Finally, there was a significant main effect of problem type; participants solved simple problems more quickly than complex problems, F(1, 44) = 47.56, p < .001. No other main effects or interactions were significant. These results indicate that high math anxiety is associated with marginally lower accuracy and lesser differentiation between RTs across problem types during math problem solving.
Brain-activation differences between the HMA and LMA groups
Next, to identify the neural correlates of math anxiety, we compared fMRI responses during math problem solving in the HMA and LMA groups. Because the Group × Operation interaction was not significant for both accuracy and RT, brain-imaging data from the two operations were combined to increase sensitivity to detect group differences. Brain responses during math problem solving in the HMA and LMA groups were then contrasted using a between-groups t test. Significant clusters of activation were identified at the whole-brain level using a height threshold of p < .01, with FWE corrections at p < .01 for multiple spatial comparisons.
In comparison with the LMA group, math problem solving in the HMA group was associated with significantly greater activation in the right amygdala extending posteriorly into the anterior hippocampus (Fig. 1a, Table 2). According to previously published cytoarchitectonic maps of the amygdala (Eickhoff et al., 2005), 40.8% of the activation cluster was in the basolateral nucleus of the amygdala, 11.9% in the subiculum region of the hippocampus, 10.6% in the entorhinal cortex, and 3.9% in the cornu ammonis section of the hippocampus. No other brain regions showed greater responses in the HMA group than in the LMA group. Additional laterality analyses provided quantitative evidence for the specificity of right, compared with left, amygdala hyperactivity in math anxiety (see Results in the Supplemental Material).
Fig. 1.
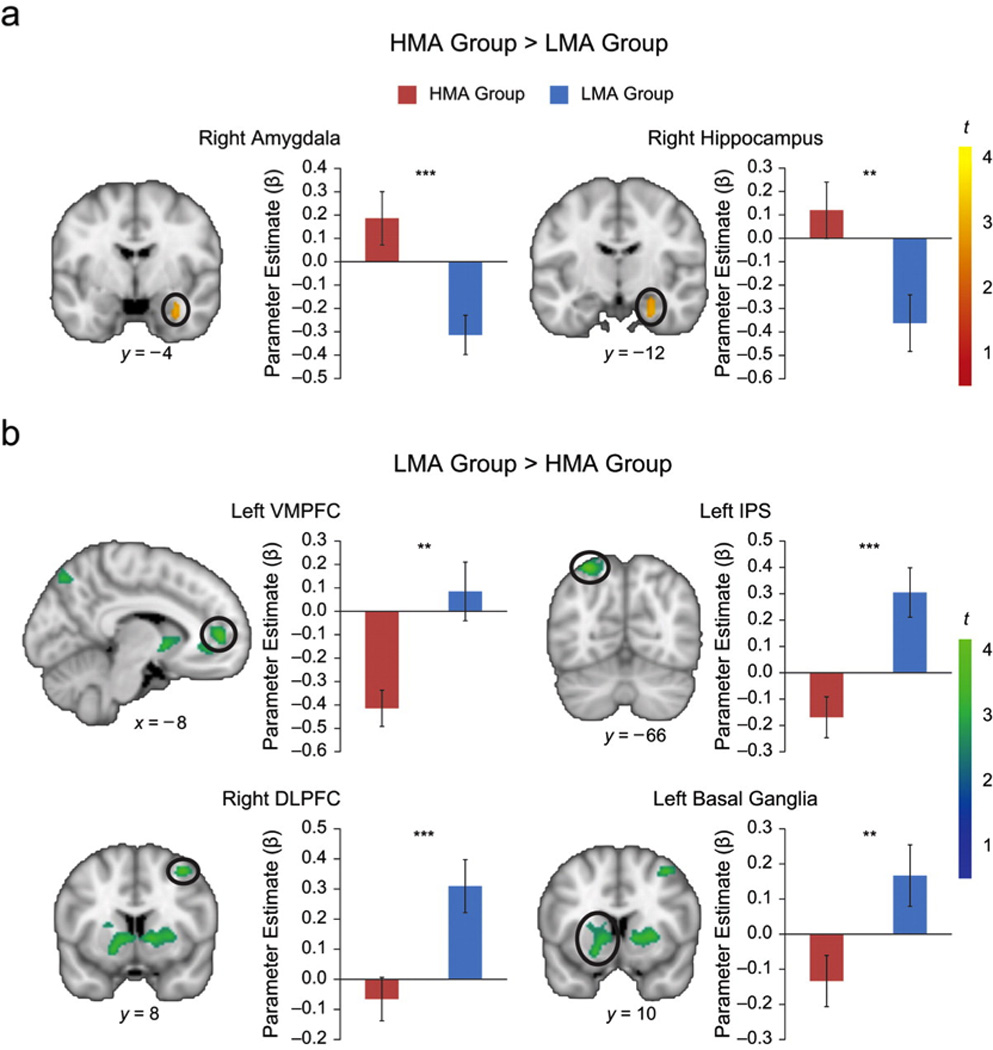
Brain-activation differences between the high-math-anxiety (HMA) group and the low-math-anxiety (LMA) group. The figure shows (a) brain areas in which activation was higher for the HMA group than for the LMA group and (b) brain areas in which activation was higher for the LMA group than for the HMA group. Coronal slices (with Montreal Neurological Institute y-axis coordinates) and a sagittal slice (with a Montreal Neurological Institute x-axis coordinate) are shown. Color coding in the brain images indicates the results of t tests comparing brain activation between the two groups. The graphs present mean parameter estimates for the circled areas of activation as a function of group. Asterisks indicate significant differences between groups (**p < .01, ***p < .001). Error bars show standard errors of the mean. DLPFC = dorsolateral prefrontal cortex; IPS = intraparietal sulcus; VMPFC = ventromedial prefrontal cortex.
Table 2.
Brain-Activation Differences Between the High-Math-Anxiety (HMA) and Low-Math-Anxiety (LMA) Groups
| Contrast and brain region | Size of cluster (voxels) |
Peak t score |
Peak MNI coordinates (mm) |
||
|---|---|---|---|---|---|
| x | y | z | |||
| HMA group > LMA group | |||||
| Right basolateral amygdala | 175 | 3.72 | 38 | 4 | −26 |
| Right anterior hippocampus | — | 2.89 | 26 | −12 | −26 |
| LMA group > HMA group | |||||
| Left intraparietal sulcus/superior parietal lobule | 443 | 3.80 | −28 | −66 | 60 |
| Right primary somatosensory cortex | 433 | 3.57 | 68 | −8 | 30 |
| Left ventromedial prefrontal cortex | 170 | 3.34 | −8 | 48 | 6 |
| Right dorsolateral prefrontal cortex | 143 | 3.30 | 38 | 8 | 54 |
| Right and left basal ganglia (caudate, putamen) | 1,074 | 3.23 | 6 | 6 | 0 |
Note: MNI = Montreal Neurological Institute.
The HMA group also showed less activation than did the LMA group in multiple cortical and subcortical areas, including the intraparietal sulcus and superior parietal lobule, the right dorsolateral prefrontal cortex and adjoining premotor cortex, and the bilateral caudate and putamen nuclei of the basal ganglia (Fig. 1b, Table 2). In addition, the HMA group displayed greater deactivation of the ventromedial prefrontal cortex regions implicated in emotion regulation than did the LMA group. These results demonstrate that math anxiety in 7- to 9-year-old children is associated with significant differences in activation of brain areas that mediate affective and cognitive information processing. Critically, the HMA and LMA groups did not differ on measures of trait anxiety, which suggests that the observed behavioral and brain differences arose from math anxiety rather than from general anxiety.
Relation between brain activation and the continuous SEMA measure: confirmatory analysis
Our primary analysis focused on comparing dichotomized groups because (a) math-anxiety scores in our sample were not normally or uniformly distributed; (b) we wanted to examine whether multivoxel amygdala response patterns were abnormal, controlling for differences in activation levels; and (c) we were able to detect stronger amygdala effects (p < .01, FWE-corrected for multiple comparisons at the whole brain level) without having to resort to any small-volume corrections with the dichotomized group analysis.
To examine the generalizability of our findings, we conducted three additional sets of analyses. First, we confirmed that brain areas that showed activation differences between the HMA and LMA groups also demonstrated significant linear increases and decreases in signal level with math anxiety (see Fig. S1 in the Supplemental Material). Second, group differences were examined using multiple analysis of covariance models with several potential confounding variables, including trait anxiety, working memory, overall accuracy, and overall RT on arithmetic problems. In each case, all the significant effects noted in the main analysis were confirmed (p < .05, small-volume-corrected in the right amygdala; p < .01, FWE-corrected for all other brain regions). Third, math anxiety was used as a continuous variable in multiple analysis of covariance models with trait anxiety, working memory, overall accuracy, and overall RT as potential confounding variables. In each case, all the significant effects noted in the main analysis were confirmed (p < .05, small-volume-corrected in the right amygdala; p < .01, FWE-corrected for all other brain regions).
MPA of amygdala distinguishes HMA and LMA groups
We used MPA to examine whether the spatial pattern of amygdala responses could be discriminated between the HMA and LMA groups. We used a searchlight method with a support-vector-machine algorithm (Abrams et al., 2011) to conduct two separate analyses involving functional and anatomical specification of amygdala regions of interest. First, we examined multivoxel activation patterns in the right amygdala cluster that showed group differences in the univariate analysis. MPA of regional t scores revealed a leave-one-out classification accuracy of 76.09% (p = .001). The second analysis compared multivoxel activity patterns in the cytoarchitectonically defined basolateral nucleus of the amygdala (Amunts et al., 2005). This analysis revealed that activation patterns in the basolateral nucleus could be distinguished with a classification accuracy of 71.74% (p = .002). These results show that children with high math anxiety and children with low math anxiety have distinct fine-scale activation patterns within the basolateral nucleus of the right amygdala. Furthermore, because fMRI responses were variance-normalized within each child’s data, differences in multivoxel patterns were independent of group differences in task-related signal strength within the amygdala.
Amygdalar connectivity differences between the HMA and LMA groups
To gain additional insights into functional circuits mediating math anxiety, we next compared the effective connectivity of the amygdala in the HMA and LMA groups. We focused on the right amygdala cluster that showed hyperactive responses in children with high math anxiety. Psychophysiological interaction analysis was used to examine math-task-related influences of the right amygdala on other brain regions (Fig. 2c). The peak seed voxel for this analysis was located in the right amygdala (x = 32 mm, y = −4 mm, z = −22 mm). Effective connectivity was estimated after discounting the influence of overall task-related activation and the effects of common driving inputs. This analysis revealed that, compared with the LMA group, the HMA group showed greater effective connectivity between the right amygdala and multiple brain areas associated with social and general anxiety, specifically the left amygdala and the ventromedial prefrontal cortex, as well as the anterior thalamic nucleus (Fig. 2a, Table 3). In sharp contrast, the right amygdala showed less effective connectivity with the posterior parietal cortex, including the intraparietal sulcus, superior parietal lobule, and angular gyrus, in the HMA group than in the LMA group (Fig. 2b, Table 3).
Fig. 2.
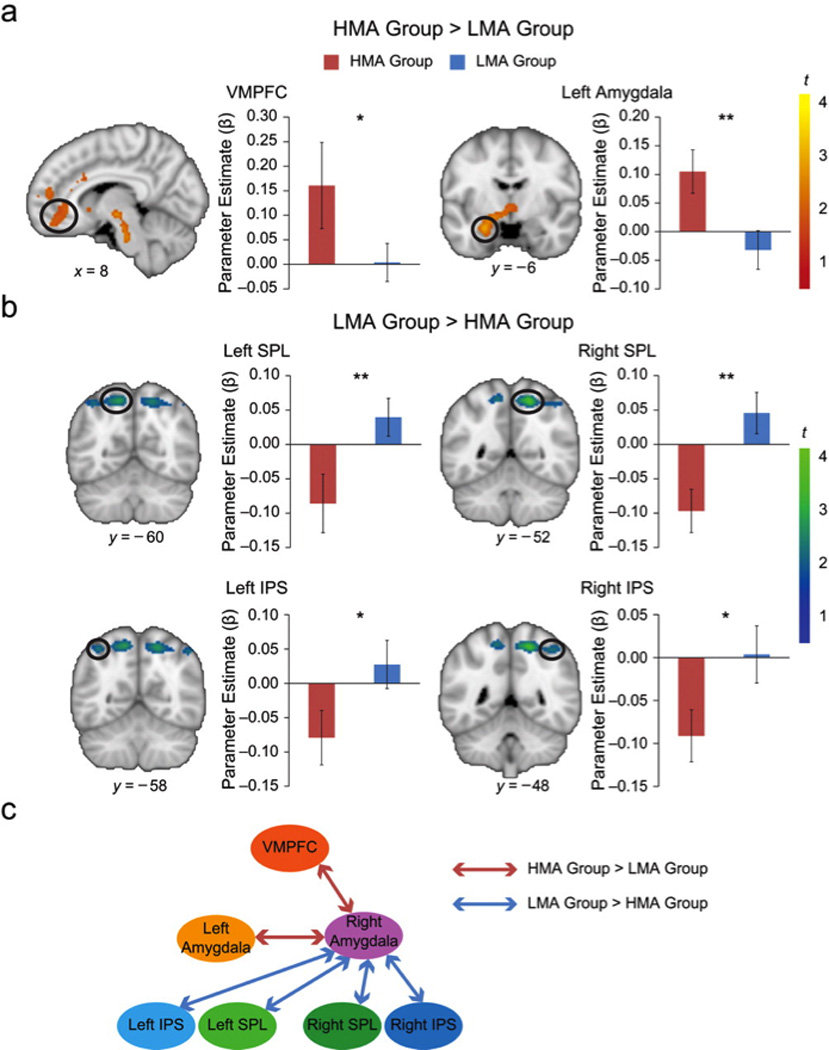
Amygdalar connectivity differences between the high-math-anxiety (HMA) group and the low-math-anxiety (LMA) group. The figure shows (a) brain areas in which activation was higher for the HMA group than for the LMA group and (b) brain areas in which activation was higher for the LMA group than for the HMA group. Amygdala effective connectivity was examined using psychophysiological interaction analysis with seed voxels located in the right amygdala (x = 32, y = −4, z = −22 mm). Coronal slices (with Montreal Neurological Institute y-axis coordinates) and a sagittal slice (with a Montreal Neurological Institute x-axis coordinate) are shown. Color coding in the brain images indicates the results of t tests comparing brain activation between the two groups. The graphs present mean parameter estimates for the circled areas of activation as a function of group. Error bars show standard errors of the mean. Asterisks indicate significant differences between groups (*p < .05, **p < .01). The network diagram (c) summarizes these results. IPS = intraparietal sulcus; SPL = superior parietal lobule; VMPFC = ventromedial prefrontal cortex.
Table 3.
Brain Areas Showing Differences in Amygdalar Connectivity Between the High-Math-Anxiety (HMA) and Low-Math-Anxiety (LMA) Groups
| Contrast and brain region | Size of cluster (voxels) |
Peak t score |
Peak MNI coordinates (mm) |
||
|---|---|---|---|---|---|
| x | y | z | |||
| HMA group > LMA group | |||||
| Left and right thalamus | 3235 | 3.46 | 0 | −12 | −6 |
| Left basolateral amgydala | — | 2.79 | −24 | −6 | −20 |
| Left and right ventromedial prefrontal cortex | — | 2.74 | 14 | 26 | −4 |
| LMA group > HMA group | |||||
| Right superior parietal lobule/intraparietal sulcus | 1118 | 3.38 | 18 | −50 | 56 |
| Left superior parietal lobule/intraparietal sulcus | 681 | 2.70 | −14 | −60 | 56 |
Note: Results are from an effective connectivity (psychophysiological interaction) analysis. MNI = Montreal Neurological Institute.
Discussion
In the experiment reported here, we examined the neurodevelopmental basis of math anxiety by investigating brain response and connectivity during arithmetic problem solving in 7- to 9-year-old children. We showed, for the first time, that in children as young as 7 to 9 years of age, math anxiety is associated with hyperactivity and abnormal effective connectivity of the amygdala, a brain region associated with processing negative emotions and fearful stimuli (Phelps & LeDoux, 2005). Furthermore, children with high math anxiety also showed distinct multivoxel patterns of neural activity within the amygdala, above and beyond overall differences in signal level.
These results provide converging evidence for aberrant processing within local functional circuits in the amygdala of children with high math anxiety. Children with high math anxiety also showed reduced responses in cortical and subcortical areas that have been consistently associated with mathematical and numerical reasoning in children and adults (Menon, Rivera, White, Glover, & Reiss, 2000). These differences were related to arithmetic complexity and were independent of sensory, motor, decision-making, or response-selection processes. Additional analysis using SEMA scores as a continuous variable confirmed the observed pattern of increased right basolateral amygdala responses and decreased fronto-parietal activation with math anxiety. Furthermore, these effects occurred independently of individual differences in trait anxiety, working memory, and performance.
Our findings suggest that math anxiety is associated with aberrant activity in the right amygdala. The recent availability of cytoarchitectonic maps based on the spatial distribution of cortical and subcortical neurons provided more detailed information about amygdala subregions involved in math anxiety (Amunts et al., 2005). Using these maps, we identified the basolateral nucleus as the most prominent site of hyperactive amygdala response in our study. The basolateral nucleus of the amygdala plays an important role in learned fear, as demonstrated by classical conditioning studies in healthy adults (Buchel, Dolan, Armony, & Friston, 1999; Phelps, Delgado, Nearing, & LeDoux, 2004). Our study extends these findings to problem-solving situations outside the traditional experimental contexts involving viewing fearful or angry faces (McClure et al., 2007), and our results further suggest that these amygdala regions are specifically involved in anxiety experienced during math problem solving.
Network-level analysis (Bressler & Menon, 2010; Rowe, 2010) provided novel insights into impaired functional circuits underlying math anxiety in children, and two findings are noteworthy here. First, the right amygdala showed greater effective connectivity with the ventromedial prefrontal cortex in the HMA group than in the LMA group. Previous studies in adults have suggested that the ventromedial prefrontal cortex regulates negative emotions by modulating amygdala activity (Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010; Etkin & Wager, 2007). Enhanced effective connectivity between these regions may facilitate compensatory mechanisms that allowed children with high math anxiety to perform well, albeit at a lower level than children with low math anxiety. Second, the posterior parietal cortex regions known to be involved in numerical and math problem solving (Wu et al., 2009) also showed reduced effective connectivity with amygdala regions that were hyperactive in children with high math anxiety. In conjunction with this, children with high math anxiety showed weaker activation in the posterior parietal cortex than did children with low math anxiety.
Thus, in children in the LMA group, the amygdala was coupled with brain areas that facilitate efficient task processing, whereas in children in the HMA group, the amygdala showed greater coupling with cortical regions involved in processing and regulating negative emotions. An emerging body of research suggests that the amygdala is involved in complex cognitive-emotional behaviors arising from its dynamic interactions with multiple brain areas (Pessoa, 2008). Our findings are consistent with this view and suggest that hyperactive amygdala function contributes to aberrant functional interactions during mathematical problem solving.
Our findings support the notion that math anxiety is stimulus- and situation-specific. Amygdala hyperactivity in the HMA group was observed in conjunction with lower problem-solving accuracy, despite the fact that the HMA group was matched with the LMA group on multiple domain-general measures, including IQ, working memory, reading, and trait anxiety. One mechanism by which anxiety is thought to influence performance is through reduced capacity for working memory, attention, and cognitive-control processes engaged during math problem solving (Beilock & Decaro, 2007). Two key results support this interpretation. First, compared with the LMA group, children in the HMA group showed reduced responses in regions involved in working memory and attention, including the dorsolateral prefrontal cortex, presupplementary motor area, and basal ganglia (Chang, Crottaz-Herbette, & Menon, 2007). Second, compared with children in the LMA group, children in the HMA group also showed reduced responses in posterior parietal cortex regions known to play a critical role in numerical and mathematical cognition (Rivera et al., 2005; Wu et al., 2009).
In both children and adults, functional neuroimaging studies have consistently implicated the intraparietal sulcus, within the posterior parietal cortex, as a region specifically involved in the representation and manipulation of numerical quantity (Ansari, 2008; Dehaene, Piazza, Pinel, & Cohen, 2003). Performance on mathematical information processing also critically involves activation and deactivation in a more distributed network of regions, such as the superior parietal lobule and the angular gyrus (Delazer et al., 2003; Menon et al., 2000; Wu et al., 2009). These observations support our hypothe- sis that math anxiety is associated with reduced cognitive information-processing resources during arithmetic task performance in the developing brain.
It is remarkable that cognitive information-processing deficits arising from math anxiety can be traced to brain regions and circuits that have been consistently implicated in specific phobias and generalized anxiety disorders in adults. In this context, it is also noteworthy that children as young as 7 to 9 years of age can consciously report on their own anxiety in situations involving mathematical problem solving and that the effects of this subjective measure can be traced to individual differences in amygdala response and connectivity. Our findings not only emphasize parallels between math anxiety and other anxiety disorders but also validate math anxiety as a genuine type of stimulus and situation-specific anxiety.
Our study provides new insights into the neurobiological mechanisms and developmental basis of math anxiety in children and highlights the importance of assessing math anxiety at a young age. Brain-imaging data can be particularly useful in the identification of domain-specific and domain-general brain systems related to math anxiety; these identifications can, in turn, be used to design remediation strategies based on treatments that work on other phobias. In addition, studies such as ours can also provide crucial information on how problem solving and reasoning are affected by math and performance anxiety. Further elucidation of the relationship between math anxiety and general academic anxiety as well as the neurodevelopmental mechanisms underlying math anxiety can spur new ways of thinking about early treatment of a disability that has significant implications for an individual’s long-term academic and professional success.
Supplementary Material
Acknowledgments
V. M. designed the study, S. S. W. collected the data, and C. B .Y. and V. M. analyzed the data and wrote the manuscript.
Funding
This research was supported by the National Institutes of Health (HD047520, HD059205, HD057610) and the National Science Foundation (DRL-0750340).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Abrams DA, Bhatara A, Ryali S, Balaban E, Levitin DJ, Menon V. Decoding temporal structure in music and speech relies on shared brain resources but elicits different fine-scale spatial patterns. Cerebral Cortex. 2011;21:1507–1518. doi: 10.1093/cercor/bhq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington: University of Vermont, Research Center for Children, Youth & Families; 2001. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nature Reviews Neuroscience. 2008;9:278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- Ashcraft MH, Krause J. Working memory, math performance, and math anxiety. Psychonomic Bulletin & Review. 2007;14:243–248. doi: 10.3758/bf03194059. [DOI] [PubMed] [Google Scholar]
- Ashcraft MH, Ridley KS. Math anxiety and its cognitive consequences. In: Campbell JID, editor. Handbook of mathematical cognition. New York, NY: Psychology Press; 2005. pp. 315–327. [Google Scholar]
- Beilock SL, Decaro MS. From poor performance to success under stress: Working memory, strategy selection, and mathematical problem solving under pressure. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:983–998. doi: 10.1037/0278-7393.33.6.983. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. The Journal of Neuroscience. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynner J, Parsons S. Does numeracy matter? Evidence from the national child development study on the impact of poor numeracy on adult life. London, England: The Basic Skills Agency; 1997. [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. NeuroImage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, Benke T. Learning complex arithmetic: An fMRI study. Cognitive Brain Research. 2003;18:76–88. doi: 10.1016/j.cogbrainres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Nelson EE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proceedings of the National Academy of Sciences, USA. 2006;103:3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. Meta-analysis of the relationship between anxiety toward mathematics and achievement in mathematics. Journal for Research in Mathematics Education. 1999;30:520–540. [Google Scholar]
- Maloney EA, Risko EF, Ansari D, Fugelsang J. Mathematics anxiety affects counting but not subitizing during visual enumeration. Cognition. 2010;114:293–297. doi: 10.1016/j.cognition.2009.09.013. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL. Dissociating prefrontal and parietal cortex activation during arithmetic processing. NeuroImage. 2000;12:357–365. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- National Mathematics Advisory Panel. Washington, DC: U. S. Department of Education; 2008. Foundations for Success: The Final Report of the National Mathematics Advisory Panel. [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pickering S, Gathercole S. Working memory test battery for children. London, England: The Psychological Corp.; 2001. [Google Scholar]
- Richardson FC, Suinn RM. The Mathematics Anxiety Rating Scale: Psychometric data. Journal of Counseling Psychology. 1972;19:551–554. [Google Scholar]
- Rivera SM, Reiss AL, Eckert MA, Menon V. Developmental changes in mental arithmetic: Evidence for increased functional specialization in the left inferior parietal cortex. Cerebral Cortex. 2005;15:1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Rowe JB. Connectivity analysis is essential to understand neurological disorders. Frontiers in Systems Neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00144. Article 144. Retrieved from http://www.frontiersin.org/systems_neuroscience/10.3389/fnsys.2010.00144/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsten O, Tannock R. Mathematics anxiety in children with developmental dyscalculia. Behavioral and Brain Functions. 2010;6 doi: 10.1186/1744-9081-6-46. Article 46. Retrieved from http://www.behavioralandbrainfunctions.com/content/6/1/46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Jeffries J. Emotional perception: Meta-analyses of face and natural scene processing. NeuroImage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Suinn RM, Edwards R. The measurement of mathematics anxiety: The mathematics anxiety rating scale for adolescents—MARS-A. Journal of Clinical Psychology. 1982;38:576–580. doi: 10.1002/1097-4679(198207)38:3<576::aid-jclp2270380317>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Suinn RM, Taylor S, Edwards RW. Suinn mathematics anxiety rating scale for elementary school students (MARS-E): Psychometric and normative data. Educational and Psychological Measurement. 1988;48:979–986. [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 Retrieved from http://homepage.usask.ca/~ges125/fMRI/AFNIdoc/AlphaSim.pdf. [Google Scholar]
- Wechsler D. The Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corp.; 1999. [Google Scholar]
- Wechsler D. The Wechsler Individual Achievement Test Second Edition (WIAT-II) San Antonio, TX: The Psychological Corp.; 2001. [Google Scholar]
- Wigfield A, Meece JL. Math anxiety in elementary and secondary school students. Journal of Educational Psychology. 1988;80:210–216. [Google Scholar]
- Wu S, Amin H, Barth M, Melcarne V, Menon V. Mathematics Anxiety in 2nd and 3rd Grades and its relation to math achievement. 2012 doi: 10.3389/fpsyg.2012.00162. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Chang TT, Majid A, Caspers S, Eickhoff SB, Menon V. Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cerebral Cortex. 2009;19:2930–2945. doi: 10.1093/cercor/bhp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


