Abstract
Background
The immunopathogenesis of type 1 diabetes mellitus (T1DM) is associated with T-lymphocyte autoimmunity. To be fully active, immune T-lymphocytes require a co-stimulatory signal in addition to the main antigen driven signal. Abatacept modulates co-stimulation andprevents full T-lymphocyte activation. We evaluated the effect of abatacept in recent-onset T1DM.
Methods
In this multicentre, double-masked, randomised controlled trial, 112 subjects (ages 6–36) recently diagnosed with T1DM; 77 received abatacept (10 mg/kg, maximum 1000 mg/dose) and 35 received placebo infusions intravenously on days 1, 14, 28, and monthly for a total of 27 infusions over two years. Primary outcome was baseline-adjusted geometric mean 2-hour area under the curve (AUC) serum C-peptide following a mixed meal tolerance test at two years. Secondary outcomes include difference between groups in incidence of loss of peak C-peptide to < 0·2 pmol/ml, slope of C-peptide over time, changes in HbA1c and insulin dose, and safety. This trial is registered in ClinicalTrials.gov (NCT00505375).
Findings
Adjusted C-peptide AUC was 59% (95% CI: 6·1%, 112%) higher at two years with abatacept (0·378 pmol/ml) versus placebo (0·238 pmol/ml) (p=0·0029). The difference between groups was present throughout the trial, with an estimated 9·6 months’ delay in decline with abatacept. There was lower HbA1c (p<0·002) but similar insulin use. There were few, clinically not significant infusion related adverse events and minimal overall adverse events. There was no increase in infections or neutropenia.
Interpretation
Co-stimulation modulation with abatacept slowed decline of beta cell function over two years. The beneficial effect suggests that T-lymphocyte activation still occurs around the time of clinical diagnosis of T1DM. Yet, despite continued administration of abatacept over 24 months, the decline in beta cell function with abatacept was parallel to that with placebo after six months of treatment, causing us to speculate that T-lymphocyte activation may lessen with time. Further observation will determine whether the beneficial effect continues after cessation of abatacept infusions.
Funding
National Institutes of Health.
Keywords: abatacept, type 1 diabetes, CTLA4-Ig, co-stimulation, T-lymphocyte, children
The most common form of type 1 diabetes mellitus (T1DM) is immune-mediated, in which insulin-producing beta cells are destroyed 1. Yet, at the time of diagnosis, a majority of patients still have appreciable amounts of insulin production as measured by C-peptide levels 2. Preservation of residual beta cell function (as measured by peak C-peptide ≥0·2 pmol/ml) is highly desirable as it may lead to reduction of the short- and long-term complications of the disease 3,4.
Hitherto, several clinical trials have been conducted attempting to arrest T1DM autoimmunity using immunomodulatory agents or antigen based therapies. Most notably, trials using anti-CD3 5,6, anti-CD20 7 and a GAD-65 antigen vaccine 8 have shown some efficacy in preserving beta cell function as evidenced by stimulated C-peptide secretion.
T-lymphocytes play a central role in T1DM autoimmunity. To become fully activated, these cells require two critical signals 9. The first signal is an interaction between an antigen in the groove of the major histocompatibility complex (MHC) molecule on antigen presenting cells (APC) with the T-lymphocyte receptor (TCR). The most significant second signal is the interaction between CD80/86 on the APCs and CD28 on the T-lymphocytes. This co-stimulatory second signal is needed for the full activation of T-lymphocytes, and without it, T-lymphocytes cannot function. Therefore, co-stimulation blockade has been proposed as a therapeutic modality for autoimmunity and transplantation 10. Abatacept (CTLA4-Ig) selectively binds to CD80/86 thereby blocking the interaction with CD28, and thereby interferes with the early phases of T-lymphocyte activation, proliferation, and survival. It inhibits naïve T cell activation, thus having the potential of selective inhibition of T cell response to specific antigens instead of broad immunosuppression. Effector memory T-lymphocyte responses are less dependent on CD28 costimulation, and presumably, less inhibited by costimulation blockade 11. Thus abatacept is expected to be mildly immunomodulatory, and most likely to impact disease at earlier stages of pathogenesis. Studies in both animals and human beings have shown that interruption of the co-stimulatory second signal beneficially alters autoimmunity. Lenschow and coworkers showed that co-stimulatory blockade with CTLA-4 Ig prevents diabetes in the NOD mice model of T1DM, when administered after insulitis developed but before frank diabetes ensues 12. Overall, the clinical success of co-stimulation blockade abatacept has been demonstrated to have clinical effectiveness in psoriasis 13 and psoriatic arthritis 14 and is approved for treatment of rheumatoid arthritis 15, including juvenile rheumatoid arthritis 16. In addition, co-stimulation blockade has been effective in controlling allograft rejection 17. Overall, the clinical success of co-stimulation blockade together with its good safety and tolerability profile, made this an attractive candidate for evaluation in recent-onset T1DM. Thus, we hypothesized that co-stimulation modulation with abatacept, by blocking the generation of autoagressive T-lymphocytes, would halt or slow autoimmune beta cell destruction leading to preservation of C-peptide secretion in recently diagnosed patients with T1DM.
Methods
Study Design and Patients
This Phase 2 clinical trial was registered with ClinicalTrials.gov (NCT00505375), and conformed to all applicable regulatory requirements. The protocol and consent documents were approved by appropriate Independent Ethics Committees or Institutional Review Boards. All participants (or parents) provided written, informed consent; in addition to their parents providing consent, participants younger than 18 years of age signed a study assent form.
We screened 149 patients (age 6–45 years) diagnosed with T1DM for less then 100 days. A total of 112 patients were enrolled (from March 2008 to February 2009) who had at least one diabetes related autoantibody (micro-assayed insulin antibodies [mIAA] {if duration of insulin therapy was less then 7 days}; glutamic acid decarboxylase-65 antibodies [GAD-65Ab]; islet cell antigen-512 antibodies [ICA-512Ab]; or islet cell autoantibodies [ICA]) and had stimulated C-peptide levels ≥ 0·2 pmol/ml measured during a mixed meal tolerance test (MMTT) conducted at least 21 days after diagnosis of diabetes and within 37 days of randomization.
The first author proposed the trial, which was conducted under the auspices of the Type 1 Diabetes TrialNet Study Group. Bristol-Myers Squibb (Princeton, NJ) provided abatacept but had no involvement with study management, data collection, data analysis, or manuscript preparation.
The study protocol is available at the Type 1 Diabetes TrialNet public website www.diabetestrialnet.org.
Randomisation and masking
This was a parallel group study, in which patients were randomly assigned in a 2:1 ratio, stratified by participating site, with 77 subjects randomised to receive experimental treatment with abatacept and 35 subjects randomised to receive placebo. Randomisation was conducted centrally at the TrialNet Coordinating Center, using computer-generated permuted block randomisation, with a block size of 3. Screening and subsequent study visits took place at 15 TrialNet sites in the United States and Canada (see On-Line Appendix).
Neither subjects nor clinical research personnel were aware of the treatment assignments. At each clinical site, only the pharmacists preparing the solutions were aware of treatment assignment. The abatacept and placebo solutions are indistinguishable in apppearance. An independent data and safety monitoring board (DSMB) reviewed interim data analyses every 6 months and conducted quarterly safety reviews. An independent medical monitor (masked to treatment assignment) reviewed all accruing safety data.
Procedures
Abatacept (CTLA4-Ig, Orencia, Bristol-Myers Squibb) was given on days 1, 14, 28, and then every 28 days with the last dose on day 700 (total 27 doses) as a 30 minute intravenous infusion at a dose of 10 mg/kg (maximum 1000 mg/dose) in a 100 ml 0.9% sodium chloride infusion. Normal saline infusion was used as placebo. Patients did not receive any premedication.
All subjects received intensive diabetes management. The goal was to achieve intensive glycemic control as recommended by the American Diabetes Association 18. The participants’ primary physician retained responsibility for their diabetes management. The research team at each study site played a supportive and advisory role with respect to the participant’s diabetes management. Patients used either multiple daily insulin injections or an insulin pump. Blood glucose monitoring was performed by means of frequent daily blood glucose monitoring. Usage of non-insulin pharmaceuticals that affect glycemic control was not allowed.
By March 2011, 103 out of 112 subjects (92%) had completed their 2-year visit MMTT and were included in the primary outcome assessment. After completion of the 2-year treatment phase, subjects entered a two-year follow-up phase to continue to assess safety and efficacy, including the performance of a MMTT every 6 months.
Beta cell function was evaluated by stimulated C-peptide secretion. The pre-specified primary outcome of this trial was a comparison of the area under the curve (AUC) of stimulated C-peptide response over the first 2 hours of a 4-hour MMTT 2,19 conducted at the 24 month visit. Four-hour MMTTs were performed at baseline and at 24 months; 2-hour MMTTs were obtained at 3, 6, 12 and 18 months. Pre-specified secondary outcomes included: slope of C-peptide over time, difference between groups in incidence of loss of peak C-peptide to < 0·2 pmol/ml, differences in HbA1c and insulin dose over time, and safety. Pre-specified subgroup factors included HLA type, baseline HbA1c, baseline insulin use, baseline C-peptide, race, gender, and age.
Laboratory Tests
Blood samples were sent to TrialNet core laboratories for analysis centrally. C-peptide levels were measured from frozen plasma using a two-site immunoenzymometeric assay (Tosoh Bioscience, South San Francisco, CA). HbA1c was measured using ion-exchange high performance liquid chromatography (Variant II, Bio-Rad Diagnostics). Reliability coefficients for each assay were above 0·99 from split duplicate samples.
Biochemical autoantibodies (mIAA, GAD-65Ab, ICA-512Ab) were measured using radio-immunobinding assays and ICA using indirect immunofluorescence. A routine chemistry panel was performed (Hitachi 917 with reagents from Roche Diagnostics).
Subjects who screened positive for serum antibodies to hepatitis-B surface antigen, hepatitis-C, or human immunodeficiency virus were excluded from participation. Subjects were also tested for Epstein-Barr virus (EBV). Individuals who had evidence of active EBV infection at the time of screening were ineligible. Subjects who showed evidence of active EBV infection after randomization did not receive additional study drug until resolution.
Human leukocyte antigen (HLA) class II alleles were measured using polymerase-chain-reaction amplification and sequence-specific hybridization.
Statistical Analyses
All analyses were based on the pre-specified intention-to-treat (ITT) cohort with known measurements. Missing values were assumed to be missing at random. The p-values associated with the ITT treatment comparisons of the primary and secondary endpoints are two-sided although the design of the trial was based on one-sided hypothesis test. Interim analysis for endpoint treatment effect was conducted and reported to the DSMB once in accordance to the method of Lan and DeMets with O’Brien-Fleming boundaries 20. The pre-specified analysis method for C-peptide mean AUC, HbA1c, and total daily insulin dose was an analysis of covariance model adjusting for age, gender, and baseline value of the dependent variable, and treatment assignment. The predicted means and associated 95% confidence intervals for each treatment group were determined at the means of the other covariates. The significance levels associated with the treatment effect are from the Wald test (from the fitted model). A normalizing transformation of log(XC−Pep + 1) was pre-specified for C-peptide AUC mean and normal plots of the residuals indicated that it was adequate. The C-peptide mean AUC equals the AUC divided by the two-hour interval (i.e. AUC/120). The AUC was computed using the trapezoidal rule from the timed measurements of C-peptide during the MMTT. The time to first stimulated peak C-peptide of less than 0·2 pmol/ml (a level above which was associated with decreased risk of complications in the DCCT) 3,4 was analyzed using standard survival methods (Cox Model 21 and Kaplan-Meier 22 method). Adverse event grades were analyzed using Wilcoxon Rank Sum Test 23. Mean rate of change of C-peptide mean AUC from 6 to 24 months was estimated using a mixed effects model with both random intercept and slope adjusting for age, gender, baseline C-peptide mean AUC, and treatment assignment. The initial fit included a fixed interaction effect of treatment and time but was removed due to the lack of any statistical evidence of it being other than zero. To assess the treatment effect over the entire time period, a similar mixed model was fitted to the data except time was defined without structure and grouped by six month intervals.
A sample size of 108 subjects was planned in order to provide 85% power to detect a 50% increase in geometric mean C-peptide relative to the placebo group using a test at the 0·05 level (one-sided), with 10% loss to follow-up and a 2:1 allocation to treatment versus control (based on the mean and standard deviation estimates, on the transformed scale, of 0·248 and 0·179, respectively) 2. Screening of new subjects was closed just after this target sample size was reached. However, subjects who had already begun screening were allowed to proceed. Thus, a total of 112 subjects were randomized.
Results
Baseline Characteristics
The baseline characteristics of the two groups are summarized in Table 1. The only noteworthy imbalances were more males in the placebo group and higher mean HbA1c in the placebo group.
Table 1.
Baseline Demographic and Laboratory Characteristics of Participants at Entry
| Abatacept N=77 |
Placebo N=35 |
|
|---|---|---|
| Age- yr | ||
| Mean | 13.9 ± 6.9 | 13.7 ± 5.3 |
| Median | 12 | 14 |
| Range | 6 – 36 | 7 – 34 |
| Male sex- number of patients (%) | 41 (53.2) | 25 (71.4) |
| Race* - number of patients (%) | ||
| White | 71 (93.4) | 32 (91.4) |
| Ethnicity – number of patients (%) | ||
| Non-Hispanic | 67 (87.0) | 31 (88.6) |
| Number of autoantibodies** - no. of patients (%) | ||
| 1 | 9 (11.7) | 4 (11.4) |
| 2 | 26 (33.8) | 9 (25.7) |
| 3 | 26 (33.8) | 15 (42.9) |
| 4 | 16 (20.8) | 7 (20.0) |
| Number of days from diagnosis to first infusion | 87.9 ± 14.1 | 83.2 ± 17.8 |
| Range | 51 – 108 | 38 – 107 |
| Weight (kg) | 52.6 ± 21.9 | 53.0 ± 19.7 |
| Body Mass Index (kg/m2) | 21.0 ± 4.48 | 20.5 ± 3.9 |
| Mean AUC for C-peptide (pmol/ml) | 0.743 ± 0.42 | 0.745 ± 0.31 |
| Glycated hemoglobin at baseline* (HbA1c -%) | 6.31 ± 0.80 | 6.74 ± 0.94 |
| Total daily insulin dose at baseline* – (U/kg) | 0.385 ± 0.24 | 0.339 ± 0.22 |
| Ketoacidosis at diagnosis – number of patients (%) | 25 (32.5) | 8 (22.9) |
| Diabetes associated HLA alleles present* - number of patients (%) | ||
| DR3 and DR4 | 25 (33.8) | 16 (48.5) |
| DR3 only | 11 (14.9) | 5 (15.2) |
| DR4 only | 30 (40.5) | 10 (30.3) |
| Neither | 8 (10.8) | 2 ( 6.1) |
Unless otherwise indicated statistics displayed are means with standard deviation.
excludes subjects with indicated variable missing (number missing: 1 – race not reported, 2 – glycated hemoglobin, 1 – insulin use, 4 – HLA allele status)
ICA not tested on 16 subjects (considered negative for count)
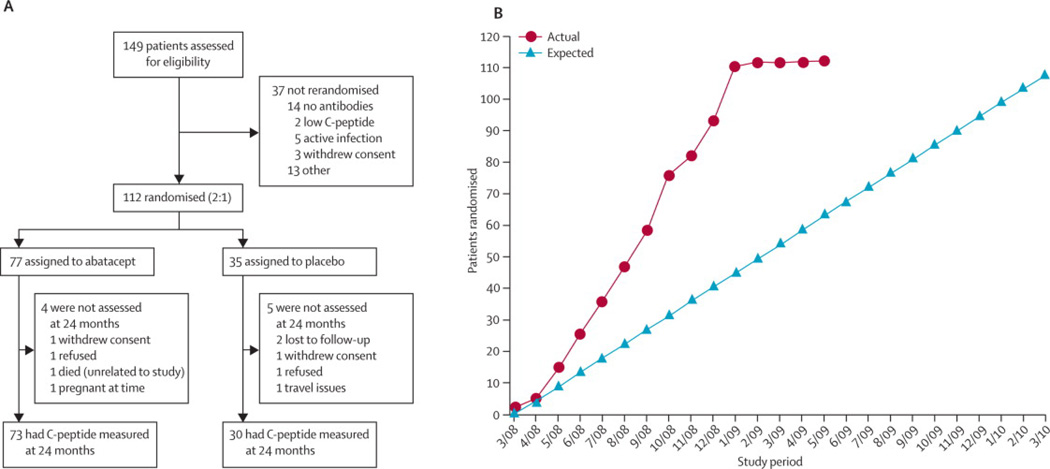
Figure 1 depicts the CONSORT diagram, showing randomization/enrollment and follow-up of subjects during the study. We compared the number of infusions actually administered by treament group using a Wilcoxon Rank Sum test; no significant difference was detected (p=0.61). Overall, 83% (2514 of 3024) of potential infusion were given, and many that were not given were per protocol (e.g. developed EBV infection, became pregnant). Also, 689 of 738 expected MMTTs (93%) were performed.
Figure 1.
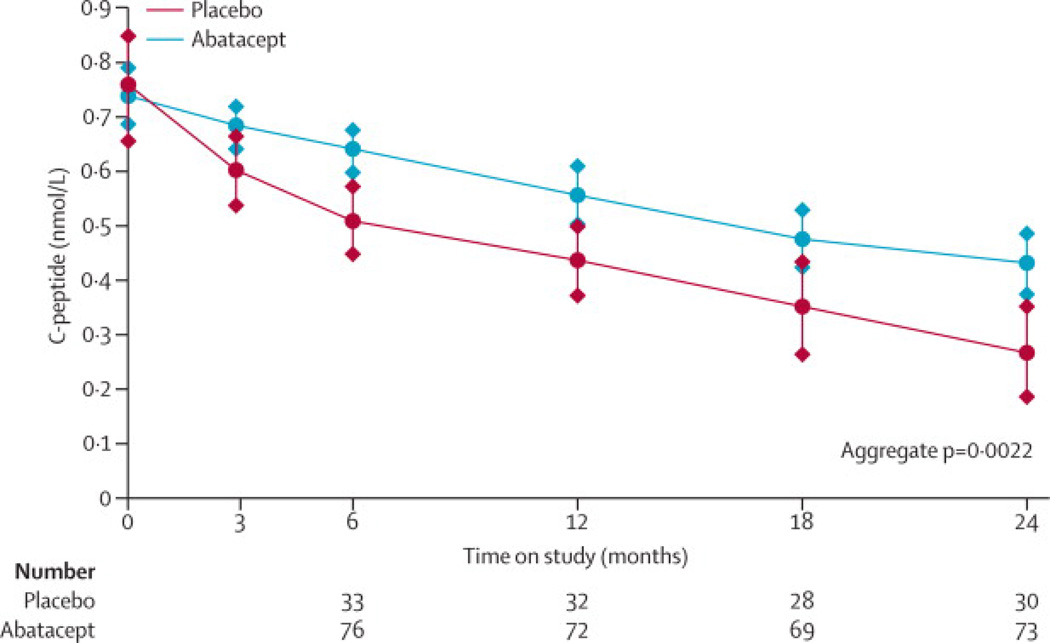
In the primary analysis at two years, those assigned to abatacept had a geometric mean stimulated C-peptide 2-hour AUC of 0·375 pmol/ml (95% CI: 0·290, 0·465), versus 0·266 pmol/ml (95% CI: 0·171, 0·368) for those assigned to placebo. The adjusted population C-peptide mean 2-hour AUC at two years was 0·378 pmol/ml and 0·238 pmol/ml for the abatacept and placebo groups, respectively; thus, C-peptide AUC at two years was 59% (95% confidence interval [6·1%, 112%]) higher with abatacept (p=0·0029). The result remains unchanged and significant (p=0·0028) when baseline HbA1c is added as a covariate. To address the difference in C-peptide levels from baseline to the two year assessments (primary endpoint), the C-peptide levels for 3, 6, 12 and 18 months were separately modeled. Figure 2A displays the adjusted population C-peptide mean 2-hour AUC over two years. Subjects who received abatacept had a significantly higher mean AUC at 6, 12, and 18 month versus placebo subjects, and over all the time points in aggregate (p=0·0022).
Figure 2.
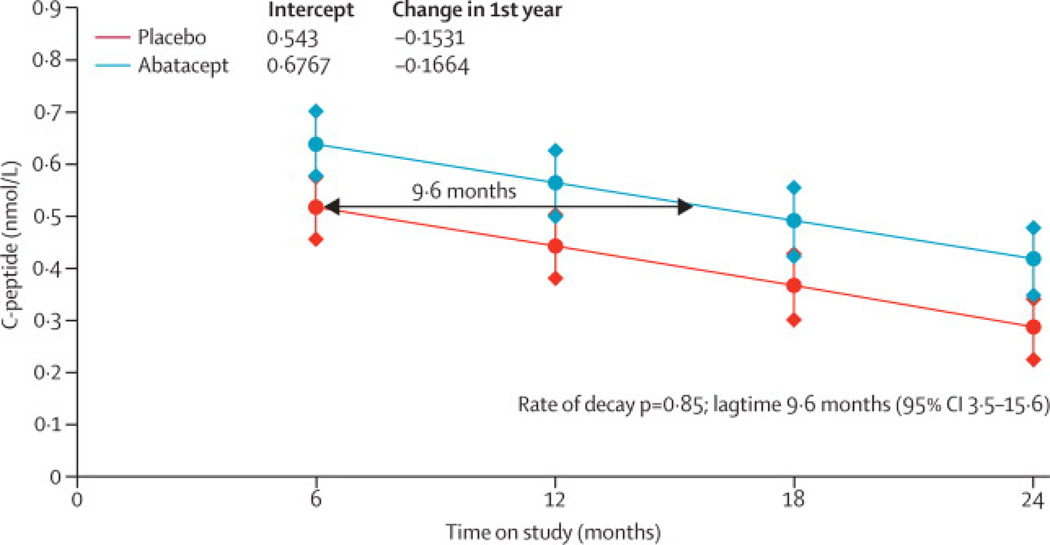
In order to calculate the impact of treatment on delaying the decline of C-peptide, the predicted population mean of C-peptide AUC mean by treatment group over time was calculated (Figure 2B). The lines are based on the fitting of a mixed linear model using all available data from 6, 12, 18, and 24 months MMTT's. When testing for the improvement in the fit for the interaction term of slope and treatment (i.e., testing the evidence that the 2 treatment groups have differing C-peptide decay rates), this was not significant (p = 0·85). Consequently, the simpler model assuming identical slopes was used and the graph represents the results. Thus, estimated lag time in the means of the abatacept group to drop to the same level as the placebo group is 9·6 months (95% confidence interval [3·47, 15·6]).
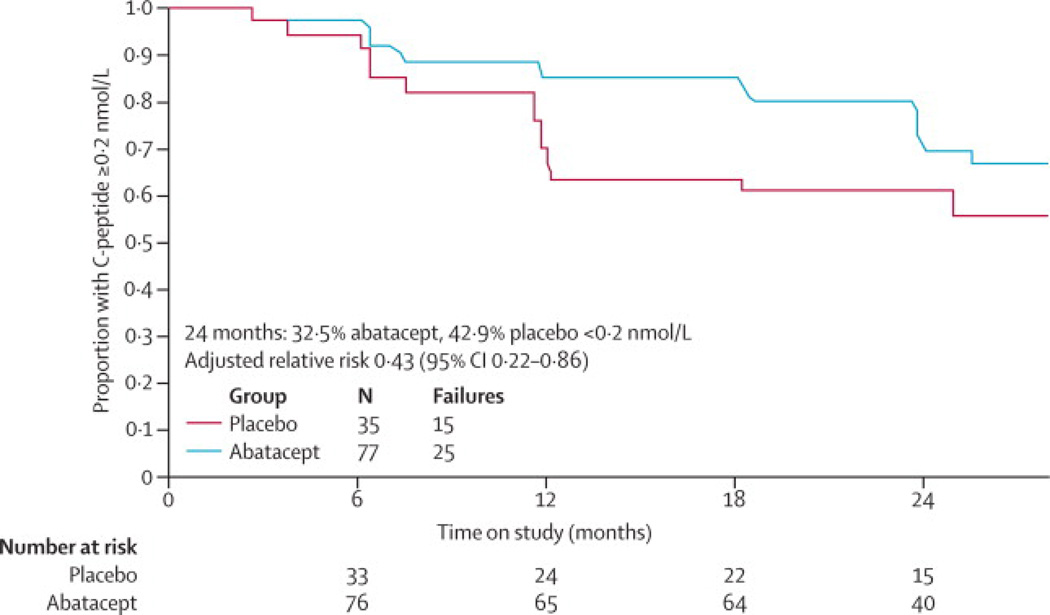
By the 24 month assessment, 25 (32·5%) in the abatacept group had an AUC peak stimulated C-peptide < 0·2 pmol/ml compared to 15 (42·9%) placebo subjects (Figure 2C). The adjusted relative (abatacept to placebo groups) risk of peak C-peptide falling below 0·2 pmol/ml level was 0·433 (95% confidence interval [0·218, 0·861]).
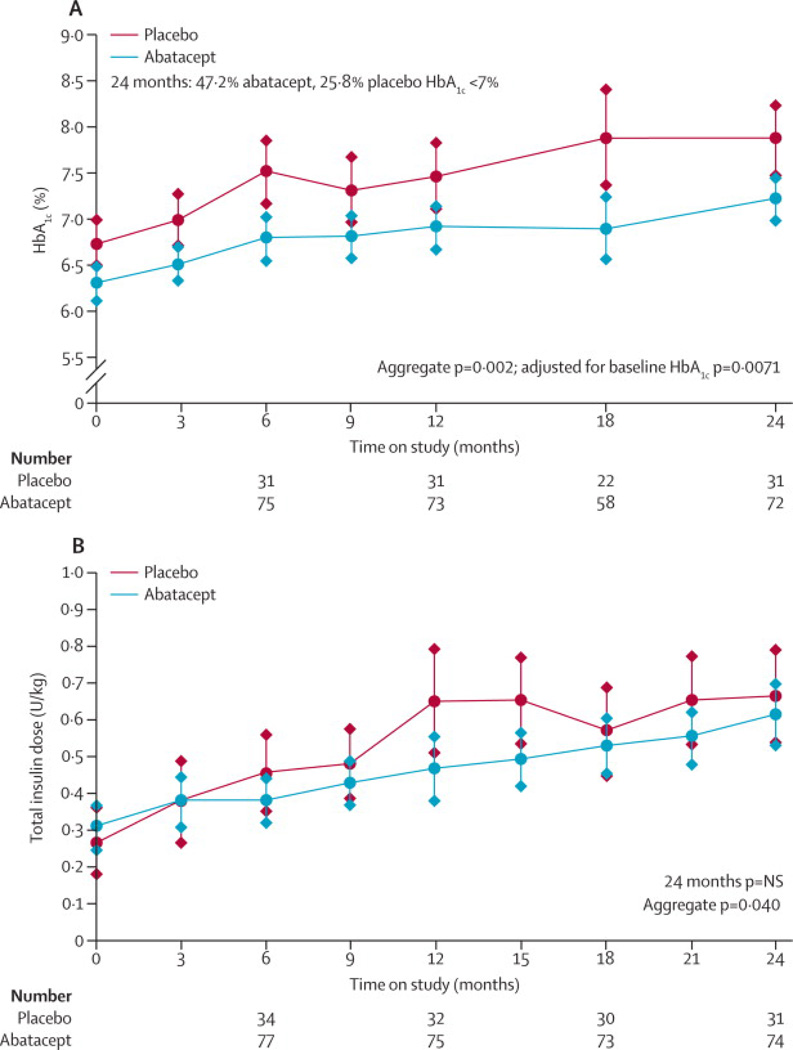
During the 24 months of follow-up, the abatacept group had a lower adjusted mean HbA1c (Figure 3A) than the placebo group (for all time points in the aggregate, p=0·002), although HbA1c was also lower at baseline. Nonetheless, even after adjusting for the difference at baseline, the treatment group difference over 24 months persists (p=0.0071). At study end, 34 (47.2%) abatacept patients had HbA1c <7%, compared to 8 (25.8%) placebo subjects. Subjects in the abatacept group had lower insulin doses at some time points (6 and 12 months) during the study, but at 24 months, insulin doses in the two groups were similar (Figure 3B) (p=NS at 24 months, but due to differences at the earlier time points, for all time points in the aggregate p=0.040).
Figure 3.
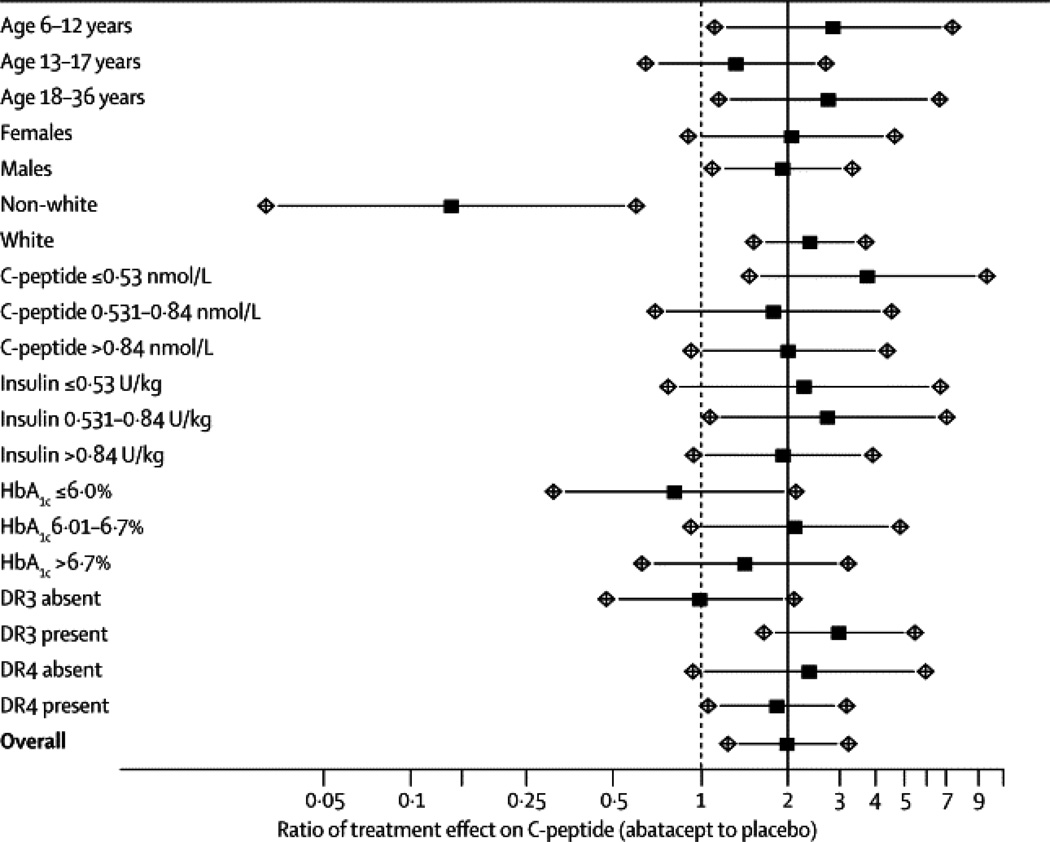
Predefined subgroup analyses were conducted; thus a homogeneity test of treatment effect was conducted on HLA type, HbA1c, insulin use, baseline C-peptide, race, gender, and age (groups: 6 – 12, 13 – 17, and 18+). Results are displayed in Figure 4.
Figure 4.
Safety and adverse events are summarized in Table 2. Abatacept therapy was well tolerated. Infusion related adverse events occurred with low frequency (47 of 2514 infusions [1·9%] involving 27 subjects) and were clinically not significant. Of these, 36 reactions occurred in 17 of 77 (22.1%) abatcept subjects and 11 reactions in 6 of 35 (17.1%) placebo subjects, p=0.62 for proportion of subjects by Fisher’s exact test. Overall adverse event rate (including laboratory abnormalities) was low with no difference between the two groups. Specifically, there was no increase in infection (including EBV) or in neutropenia. There were 8 episodes of hypoglycemia reported as an adverse event, 2 of which were severe hypoglycemia, one in each group.
Table 2.
The number of subjects by worst grade of adverse effects and number of events and subjects by adverse events type. Worst grade by treatment group was not statistically different using a Wilcoxon Rank Sum Test. Adverse effect category by treatment group was tested using a one-sided (alternative of higher frequency in Abatacept Group) Fisher’s Exact Test; only Constitutional Symptoms was significant (p = 0.049).
| Grade | Treatment Group | |||
|---|---|---|---|---|
| Abatacept | Placebo | |||
| No. of subjects (%*) | No. of subjects (%*) | |||
| 0 | 14 (18.2) | 8 (22.9) | ||
| 1 | 1 (1.3) | 1 (2.9) | ||
| 2 | 44 (57.1) | 17 (48.6) | ||
| 3 | 12 (15.6) | 7 (20.0) | ||
| 4 | 5 (6.5) | 2 (5.7) | ||
| 5** | 1 (1.3) | 0 (0.0) | ||
| Total | 77 | 35 | ||
| Adverse Effect Category |
No. of events |
No. of subjects (%*) |
No. of events |
No. of subjects (%*) |
| Allergy/Immunology | 3 | 2 (2.6) | 0 | 0 (0.0) |
| Auditory/Ear | 3 | 3 (3.9) | 0 | 0 (0.0) |
| Blood/Bone Marrow | 16 | 11 (14.3) | 18 | 6 (17.1) |
| Cardiac Arrhythmia | 1 | 1 (1.3) | 1 | 1 (2.9) |
| Cardiac General | 2 | 2 (2.6) | 0 | 0 (0.0) |
| Constitutional Symptoms | 19 | 15 (19.5) | 2 | 2 (5.7) |
| Death** | 1 | 1 (1.3) | 0 | 0 (0.0) |
| Dermatology/Skin | 15 | 13 (16.9) | 5 | 4 (11.4) |
| Endocrine | 4 | 4 (5.2) | 2 | 2 (5.7) |
| Gastrointestinal | 30 | 18 (23.4) | 11 | 7 (20.0) |
| Infection | 63 | 32 (41.6) | 31 | 15 (42.9) |
| Hypoglycemia | 5 | 3 (3.9) | 2 | 1 (2.9) |
| Metabolic/Laboratory♦ | 8 | 6 (7.8) | 4 | 2 (5.7) |
| Musculoskeletal/Soft Tissue | 13 | 11 (14.3) | 7 | 6 (17.1) |
| Neurology | 13 | 8 (10.4) | 3 | 2 (5.7) |
| Ocular/Visual | 3 | 3 (3.9) | 1 | 1 (2.9) |
| Pain | 7 | 6 (7.8) | 5 | 4 (11.4) |
| Pulmonary/Upper Respiratory | 20 | 10 (13.0) | 7 | 4 (11.4) |
| Renal/Genitourinary | 0 | 0 (0.0) | 1 | 1 (2.9) |
| Secondary Malignancy | 1 | 1 (1.3) | 0 | 0 (0.0) |
| Sexual/Reproductive Function | 1 | 1 (1.3) | 0 | 0 (0.0) |
| Surgery/Intra-Operative Injury | 2 | 2 (2.6) | 0 | 0 (0.0) |
| Syndromes | 9 | 9 (11.7) | 5 | 5 (14.3) |
| Total | 239 | -- | 105 | -- |
Denominator in percent calculation is number in respective treatment groups
Accidental death, unrelated to study
Other than hypoglycemia
Discussion
The results of this study demonstrate that over two years co-stimulation modulation with abatacept slows the decline of beta cell function in recent-onset T1DM by 9·6 months. The early beneficial effect suggests that T-lymphocyte activation still occurs around the time of clinical diagnosis of T1DM, even though the disease course has presumably been going on for several years 1. However, despite continued administration of abatacept over 24 months, the decline in beta cell function in the abatacept group parallels that in the placebo group based on the mixed model results that included the time interval from 6 to 24 months. This subsequent decline in beta cell function causes us to speculate that continuing T-lymphocyte activation may subside as the clinical course of the disease progresses. Nevertheless, the difference from the placebo group is mantained during the drug administration. Further observation will determine whether the beneficial effect continues after cessation of monthly abatacept infusions.
It is important to note that abatacept was well tolerated, with no difference between the two groups in adverse events. However, a potential limitation to clinical applicability is that live vaccines cannot be used within 3 months of abatacept therapy. This may be important in view of the young age of the target population.
The main effect appears to be early after initiation of treatment with subsequent resumption of decline in beta cell function. This is reminiscent of the effects of anti-CD3 5,6, anti-CD20 7 and a GAD-65 vaccine 8, all of which showed some efficacy followed later by a decline in beta cell function parallel to that in the control group. This is consistent with our notion that there is an early window of opportunity after diagnosis in which T-lymphocyte activation is prominent. The 59% (0·378/0·238) higher mean AUC C-peptide than placebo at 24 months in our study is similar to that seen with those other interventions, although it is difficult to make direct comparisons of different studies. This is because the studies have differed in important baseline characteristics, including age, disease duration at time of randomisation, and baseline HbA1c. Moreover, our study differs from those studies in that abatacept was administered continuously throughout the study, whereas in the case of anti-CD3, anti-CD20, and GAD-65 vaccine, administration of drug was completed within 2–4 weeks after randomization.
It is crucial to note that our study was not designed to answer the questions whether a shorter treatment protocol would be sufficient to maintain improved C-peptide secretion over two years or whether a continuation of therapy is needed beyond two years. With all patients having completed their course of abatacept, the ongoing follow-up phase of the study will investigate whether the improved C-peptide secretion is sustained after discontinuation of abatacept. Long term follow up of patients in one anti-CD3 trial showed diminishing difference in C-peptide secretion between the treated and the placebo group after 3 years 24.
In the abatacept group mean HbA1c was lower than the placebo group throughout the trial, although it was also lower at baseline. The maintenance of HbA1c below 7% for 18 months in the abatacept treated group is noteworthy as 96 (86%) study participants were 18 years or younger. The clinical importance of HbA1c at this level has been well documented 25. Insulin usage was similar in the two groups, and thus did not contribute to the difference in HbA1c.
In this phase II clinical trial, abatacept treated patients with recent-onset T1DM had more endogenous insulin production, measured by C-peptide, during the two years of study drug administration. The duration of these effects after discontinuation of abatacept is being tested in ongoing follow up of these patients.
Abatacept administered over two years showed an excellent safety profile in patients with T1DM. Its main effect seems to be early after the initiation of treatment. TrialNet uses the paradigm of studying interventions in recently diagnosed T1DM not only to evaluate its safety and effectiveness in that setting but also to qualify interventions that might be useful to evaluate in a prevention study in individuals at-risk for T1DM, or to be used in combination with other agents. In this regard, abatacept has characteristics to support it as a potential candidate to be tested in a prevention trial. Abatacept would also seem to be a candidate as a useful component of a combination therapy protocol in recent-onset T1DM 26. These approaches might be more easily tested using a subcutaneous version of abatacept, which is currently in development.
Panel: Research in context
Systematic review
We searched the PubMed database for articles published up to March 31, 2011, with the search terms “immune intervention” AND “type 1 diabetes”. A comprehensive review by Luo et al summarised immune intervention studies performed in people with type 1 diabetes 27. There have been four recent randomised trials 5–8 with adequate sample size that have demonstrated some preservation of beta cell function in T1DM as evidenced by stimulated C-peptide secretion. These trials used anti-CD3 5,6, anti-CD20 7, and a GAD-65 antigen vaccine 8. Our trial is the first trial in which patients were randomised to abatacept or any co-stimulation modulating agent.
Interpretation
In this study, abatacept was superior to placebo with respect to the preservation of beta cell function in T1DM as evidenced by stimulated C-peptide secretion, with minimal adverse events. However, the effect diminished with time. Abatacept has characteristics to support it as a potential candidate to be tested in a prevention trial, or to be a candidate as a useful component of a combination therapy protocol in recent-onset T1DM. Until further studies are conducted, it is not appropriate to use abatacept in T1DM in clinical practice.
Supplementary Material
Acknowledgements
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. TrialNet is a clinical trials network funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Research Resources; the Juvenile Diabetes Research Foundation International; and the American Diabetes Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: The trial was proposed to TrialNet by Tihamer Orban, who served as study chair, and who wrote the first draft of this manuscript. Other members of the manuscript writing group included Jay S. Skyler, Brian Bundy, and Jeffrey P. Krischer. All of the authors were involved in the conduct of the study and the collection and review of study data. The writing group had full access to all of the data and made the decision to publish the paper. The other authors reviewed and commented on various versions of the paper, and suggested revisions. The members of the writing group assume responsibility for the overall content and integrity of the article.
Bristol-Myers Squibb (Princeton, NJ) provided abatacept (Orencia®), but had no involvement with study design, conduct, or management; data collection, analysis or interpretation; or manuscript preparation. There are no agreements concerning confidentiality of the data between the sponsor and the authors or the institutions named in the credit lines. The authors did provide Bristol-Myers Squibb a copy of the original manuscript prior to submission.
Lifescan Division of Johnson and Johnson provided blood glucose monitoring meters and strips to research subjects.
Dualities:
Dr. Orban reports serving on the Data Safety Monitoring Board for Osiris Therapeutics, and being a founder of Orban Biotech LLC; Dr. Becker reports receiving a grant from Diamyd; Dr. Gitelman reports serving on an advisory board for Genentech; Dr. Golaand reports receiving grants from Diamyd and Tolerx; Dr. Gottlieb reports serving on advisory boards for Genentech, Eli Lilly, Sanofi-Aventis, and Tolerx, and reports receiving grants from Bayhill Therapeutics, Diamyd, Macrogenics, Omni BioTherapeutics, and Tolerx; Dr. Greenbaum reports receiving grants from Bayhill Therapeutics, Diamyd, and Tolerx; Dr. Marks reports serving on an advisory board for Amgen; Dr. Moran reports serving on an advisory board for Pfizer, and receiving grants from Tolerx, Merck, and Osiris Therapeutics; Dr. Raskin reports serving on advisory boards for Amgen, AstraZeneca, MannKind, and Novo-Nordisk, serving on speakers bureaus for Merck and Novo-Nordisk, and receiving grants from Aegera, Andromeda Biotech, Bayhill Therapeutics, Biodel, Boehringer Ingelheim, Calibra, CPEX, Generex, Hoffman-LaRoche, MannKind, Novo-Nordisk, Osiris Therapeutics, and Reata; Dr. Rodriguez reports serving on an advisory board for Marcadia Biotech, serving as a consultant to Eli Lilly, Genentech, Bayer, EMD Serono, and Merck, being on the speakers bureau of Eli Lilly and Novo-Nordisk, and receiving grant support from Macrogenics and Eli Lilly; Dr. Schatz reports serving on advisory boards for Eli Lilly and GlaxoSmithKline, and receiving a grant from Diamyd; Dr. Wherrett reports receiving lecture fees from Eli Lilly and Medtronic; Dr. Wilson reports serving on an advisory boards for DexCom and Genentech, and receiving grants support from Genentech, Diamyd, and Osiris Therapeutics; Dr. Skyler reports serving on boards for Amylin Pharmaceuticals, DexCom, and Sanofi-Aventis, and reports receiving grants from Bayhill Therapeutics, Halozyme, Intuity, and Osiris Therapeutics. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7:988–994. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- 2.Palmer JP, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial: a randomized, controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 5.Herold KC, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. 2002. [DOI] [PubMed] [Google Scholar]
- 6.Keymeulen B, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 7.Pescovitz MD, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludvigsson J, et al. GAD Treatment and Insulin Secretion in Recent-Onset Type 1 Diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 9.Marelli-Berg FM, Okkenhaug K, Mirenda V. A two-signal model for T cell trafficking. Trends Immunol. 2007;28:267–273. doi: 10.1016/j.it.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Bluestone JA, St. Clair EW, Turka LA. CTLA4Ig: Bridging the Basic Immunology with Clinical Application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11:22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenschow DJ, et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrams JR, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mease, et al. Abatacept in the treatment of patients with psoriatic arthritis: Results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939–948. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 15.Genant HK, et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann Rheum Dis. 2008;67:1084–1089. doi: 10.1136/ard.2007.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruperto N, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 17.Vincenti F, Larsen C, Durrbach A. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum CJ, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31:1966–1971. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 21.Cox DR. Regression model and Life Tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Agresti A. Categorical Data Analysis. John Wiley & Sons; 1990. [Google Scholar]
- 24.Keymeulen B, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 25.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 26.Skyler JS, Ricordi C. Stopping Type 1 Diabetes: Attempts to prevent or cure type 1 diabetes in man. Diabetes. 2011;60:1–8. doi: 10.2337/db10-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32:488–499. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.