Abstract
Purpose
To evaluate microarray-based genotyping technology for the detection of mutations responsible for retinitis pigmentosa (RP) and to perform phenotypic characterization of patients with pathogenic mutations.
Methods
DNA from 336 patients with RP and 360 controls was analyzed using the GoldenGate assay with microbeads containing 95 previously reported disease-associated mutations from 28 RP genes. Mutations identified by microarray-based genotyping were confirmed by direct sequencing. Segregation analysis and phenotypic characterization were performed in patients with mutations. The disease severity was assessed by visual acuity, electroretinography, optical coherence tomography, and kinetic perimetry.
Results
Ten RP-related mutations of five RP genes (PRP3 pre-mRNA processing factor 3 homolog [PRPF3], rhodopsin [RHO], phosphodiesterase 6B [PDE6B], peripherin 2 [PRPH2], and retinitis pigmentosa 1 [RP1]) were identified in 26 of the 336 patients (7.7%) and in six of the 360 controls (1.7%). The p.H557Y mutation in PDE6B, which was homozygous in four patients and heterozygous in nine patients, was the most frequent mutation (2.5%). Mutation segregation was assessed in four families. Among the patients with missense mutations, the most severe phenotype occurred in patients with p.D984G in RP1; less severe phenotypes occurred in patients with p.R135W in RHO; a relatively moderate phenotype occurred in patients with p.T494M in PRPF3, p.H557Y in PDE6B, or p.W316G in PRPH2; and a mild phenotype was seen in a patient with p.D190N in RHO.
Conclusions
The results reveal that the GoldenGate assay may not be an efficient method for molecular diagnosis in RP patients with rare mutations, although it has proven to be reliable and efficient for high-throughput genotyping of single-nucleotide polymorphisms. The clinical features varied according to the mutations. Continuous effort to identify novel RP genes and mutations in a population is needed to improve the efficiency and accuracy of the genetic diagnosis of RP.
Introduction
Retinitis pigmentosa (RP, OMIM 268000) is the most common type of inherited retinal degeneration and is characterized by progressive degeneration of the photoreceptors. Epidemiological studies have consistently reported a frequency of approximately 1 in 3,000–5,000 individuals, without apparent ethnic or racial distinctions [1]. RP is both clinically and genetically heterogeneous. Clinically, symptoms usually emerge as night blindness during adolescence, progress to legal blindness by middle age and result in total loss of vision in later life. However, clinical features such as onset age and rate of progression have been documented to vary according to genetic background [2]. Genetically, RP can be transmitted in all inheritance patterns, and simplex cases represent 40%–50% of all RP patients [3]. To date, more than 40 genes causing nonsyndromic RP and 20 genes causing syndromic forms of RP have been identified. In addition to many RP genes, there are often several mutations at each locus, and different mutations within the same gene may cause different phenotypes [4]. Only a little more than 50% of RP cases can be explained by mutations in known genes [5]. This phenotypic and genetic heterogeneity of RP make its diagnosis highly complex and time consuming.
Mutation detection techniques that are exclusively PCR based are relatively inefficient, expensive, and labor intensive for genetic diagnosis. Therefore, different genotyping techniques such as microarray-based analysis and next-generation sequencing are useful for the detection of mutations associated with disorders showing high genetic and allelic heterogeneity. Recent mutation analysis studies using a genotyping microarray in patients with hereditary retinal diseases, including RP and Stargardt disease, have been reported for different ethnic groups [6-9]. However, there are no genotyping microarray data that establish the spectrum of RP mutations in the Asian population. Such data would be of great utility in providing effective genetic counseling and predicting disease severity for RP patients.
High-throughput, customized genotyping of targeted variants is desirable for biologically focused research [10]. For customized genotyping, assay reliability and productivity are considered the most important factors. The GoldenGate assay has proven to be reliable and efficient for high-throughput genotyping of single-nucleotide polymorphisms. In this study, we designed and used a GoldenGate assay with VeraCode microbeads to screen for mutations in Korean patients with RP. In addition, we describe the clinical characteristics of patients with RP mutations.
Methods
Subjects
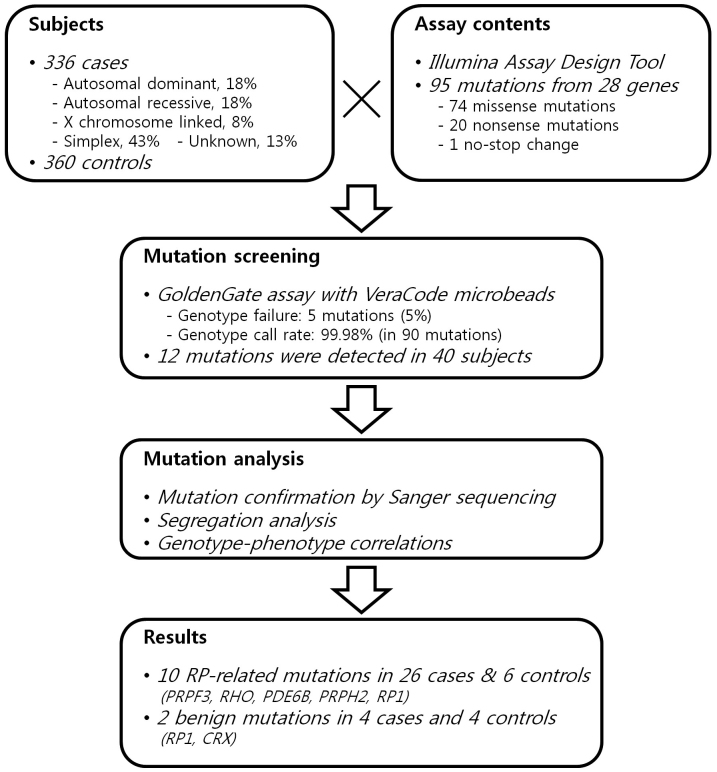
Informed consent was obtained from all participants before enrollment in the study, in accordance with the protocol approved by the Institutional Review Board at Seoul National University Hospital (SNUH) and at the Korea National Institute of Health. All protocols used in this study were also in full accordance with the tenets of the Declaration of Helsinki. A total of 696 subjects, 336 probands with nonsyndromic RP and 360 controls, were analyzed for 95 mutations of 28 RP genes. A summary of subjects’ data are represented in Table 1. Patients with RP and their family members were recruited during the period 2006–2009 from the clinic for hereditary retinal degeneration at the Department of Ophthalmology, SNUH, Seoul, Korea and the Korean Retinitis Pigmentosa Society, a nonprofit support network for Korean RP patients [11]. Pedigrees of all patients were obtained through interviews. Controls were recruited from two population-based cohort studies at the Korea National Institute of Health [12]. Briefly, both cohorts were initiated in 2001 as part of the Korean Genome Epidemiology Study and designed to allow longitudinal prospective study. Genomic DNA was extracted from peripheral blood leukocytes using the FlexiGene DNA Kit (Qiagen, Valencia, CA), in accordance with the manufacturer’s instructions.
Table 1. Summary of subjects.
| Group | Mode of inheritance | N (%) | Age* (range) | Male (%) |
|---|---|---|---|---|
| RP |
Autosomal dominant |
62 (18) |
41.5 (6–67) |
30 (48) |
| Autosomal recessive |
60 (18) |
41.0 (20–67) |
38 (63) |
|
| X-linked |
27 (8) |
37.3 (7–65) |
27 (100) |
|
| Simplex |
144 (43) |
35.9 (6–66) |
92 (64) |
|
| Unknown |
43 (13) |
35.6 (6–59) |
27 (63) |
|
| Subtotal |
336 (100) |
37.9 (6–67) |
212 (63) |
|
| Control | 360 (100) | 63.7 (60–70) | 180 (50) |
*mean age of subjects when they were recruited; N, total number tested
Assay design and genotyping
Twenty-eight genes carrying at least 350 previously described mutations associated with nonsyndromic RP were selected from the Human Gene Mutation Database (HGMD) [13] and the RetNet database in 2009. Mutations previously identified in patients with East Asian ancestry and/or reported in at least two independent research groups were preferred for the assay contents. A total of 95 assay probes for custom content were designed and optimized using the Illumina Assay Design Tool (Illumina, San Diego, CA; Appendix 1). A mutation with a high designability score was selected when multiple mutations were within 60 bp. The GoldenGate Genotyping Assay for VeraCode microbeads (Illumina) was used to screen mutations. Genotype calls were generated automatically using BeadStudio software version 3.3 (Illumina, San Diego, CA).
DNA sequencing
All variants detected in the GoldenGate assay were confirmed by sequence analysis. PCR primers were designed to amplify the regions of 12 mutations, using the reference sequences of PRP3 pre-mRNA processing factor 3 homolog (PRPF3; NM_004698.1), rhodopsin (RHO; NM_000539.2), phosphodiesterase 6B (PDE6B; NM_000283.2), peripherin 2 (PRPH2; NM_000322.3), retinitis pigmentosa 1 (RP1; NM_006269.1), and cone-rod homeobox containing gene (CRX; NM_000554.4). All primer sequences are available in Appendix 2. Direct sequencing was performed with a BigDye Terminator Sequencing Kit and an ABI 3730 DNA Analyzer (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocols.
Ophthalmic examinations
RP was diagnosed by retina specialists at SNUH when a patient showed bilateral retinal degeneration with typical pigmentation on fundoscopic examination, or rod dysfunction on a standard electroretinogram (ERG) with degenerative retina. Ophthalmic examination included the best-corrected visual acuity, refraction, intraocular pressure measurement, slit-lamp biomicroscopy, fundoscopy, Goldmann perimetry, ERG, and optical coherence tomography (OCT; Cirrus HD-OCT; Carl Zeiss Meditec, Dublin, CA). Refractive errors were measured with an autorefractometer and Goldmann perimetry with a V4e target was performed on all patients who were able to cooperate. ERG followed the guidelines of the International Society for Clinical Electrophysiology of Vision [14].
Mutation analysis
The frequency of a mutant allele was assessed in both patients and controls. Segregation analysis of mutations was performed by sequence analysis when other family members were available. Sequence changes at the DNA or protein level are described according to the recommendations of the Human Genome Variation Society. To predict the functional impact of missense mutations, PolyPhen-2 and SIFT software were used. The prediction result was classified into three types (probably damaging, possibly damaging, and benign) for PolyPhen-2 [15] and two types (damaging and tolerated) for SIFT software [16].
Results
Mutation detection and validation
We performed a mutation genotyping of RP genes using the GoldenGate assay for VeraCode microbeads. Twenty-eight genes carrying single-nucleotide mutations associated with nonsyndromic RP were selected to design the assay content in 2009. A total of 95 sequence variants (74 missense mutations, 20 nonsense mutations, and a nonstop change) were selected and analyzed in 336 unrelated patients with RP and 360 controls (Figure 1). Of these, 28 mutations (30% of 95 assay contents) were previously identified in patients with East Asian ancestry. Appendix 1 shows the list of mutations screened in the present study. Of 95 sequence variants tested, we failed to genotype five (5%) missense mutations (p.Y368H in retinal pigment epithelium-specific protein 65 kDa (RPE65); p.L12P in c-mer proto-oncogene tyrosine kinase (MERTK); p.G576D and p.L671P in PDE6B; and p.F1935L in RP1). The average call rate for 90 variants was 99.98%. Overall, 12 sequence variants of six genes (PRPF3, RHO, PDE6B, PRPH2, RP1, and CRX) were detected in 30 patients and 10 controls (Table 2). Of these, 11 mutations were previously found in Chinese, Japanese, or Korean RP patients. There was no sequence variation in the other 78 variants (87% of 90) tested in the Korean population, including the 360 controls.
Figure 1.
Schematic overview of the present study.
Table 2. Mutations identified in the present study.
|
Gene |
Amino acid |
Nucleotide |
Proband (336) |
Control (360) |
PolyPhen-2 |
SIFT |
Reference |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hetero | Homo | Frequency | Hetero | Homo | Frequency | ||||||
| PRPF3 |
p.T494M |
c.1481C>T |
1 |
0 |
0.001 |
0 |
0 |
0 |
prob/prob |
Damaging |
[17,38] |
| RHO |
p.T17M |
c.50C>T |
1 |
0 |
0.001 |
0 |
0 |
0 |
prob/poss |
*Damaging |
[18,39] |
| p.R135W |
c.403C>T |
1 |
0 |
0.001 |
0 |
0 |
0 |
prob/prob |
*Damaging |
[19,20] |
|
| p.D190N |
c.568G>A |
1 |
0 |
0.001 |
0 |
0 |
0 |
poss/poss |
*Damaging |
[21] |
|
| p.K296N |
c.888G>T |
1 |
0 |
0.001 |
0 |
0 |
0 |
prob/prob |
*Damaging |
[18,24] |
|
| p.P347L |
c.1040C>T |
2 |
0 |
0.003 |
0 |
0 |
0 |
poss/benign |
*Damaging |
[18,40] |
|
| PDE6B |
p.H557Y |
c.1669C>T |
9 |
4 |
0.025 |
2 |
0 |
0.003 |
prob/prob |
Damaging |
[20,22] |
| p.T604I |
c.1811C>T |
4 |
0 |
0.006 |
3 |
0 |
0.004 |
prob/prob |
Damaging |
[20] |
|
| PRPH2 |
p.W316G |
c.946T>G |
1 |
0 |
0.001 |
1 |
0 |
0.001 |
benign/benign |
Damaging |
[20] |
| RP1 |
p.G706R |
c.2116G>C |
1 |
0 |
0.001 |
1 |
0 |
0.001 |
benign/benign |
Tolerated |
[25] |
| p.D984G |
c.2951A>G |
1 |
0 |
0.001 |
0 |
0 |
0 |
poss/benign |
Tolerated |
[23] |
|
| CRX | p.G122D | c.365G>A | 3 | 0 | 0.004 | 3 | 0 | 0.004 | benign/benign | Tolerated | [20,24] |
Prediction by PolyPhen2 was performed using both HumDiv and HumVar data sets. *Damaging, low confidence predictions with Median conservation above 3.25. Hetero, heterozygous mutation; Homo, homozygous mutation; prob, probably damaging; poss, possibly damaging.
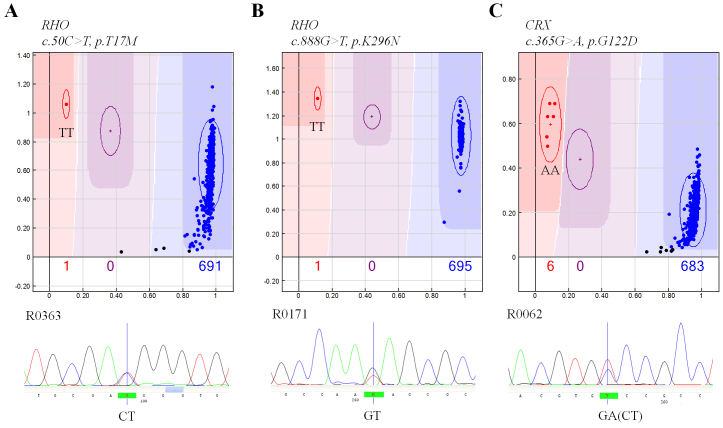
All sequence variants identified in the genotyping assay were confirmed by bidirectional sequence analysis in all subjects with variants. Of 12 variants identified in the GoldenGate assay, three showed genotype inconsistency with the results of sequence analysis (p.T17M and p.K296N in RHO; and p.G122D in CRX). All heterozygous samples with these mutations were assigned the homozygous mutant genotype in the GoldenGate assay (Figure 2).
Figure 2.
Genotype inconsistency between the GoldenGate assay (upper) and the sequence analysis (lower). In the GoldenGate assay, colored dots represent genotyped individuals (blue, a homozygote of reference allele; red, a homozygote of mutated allele; purple, a heterozygote; black, genotype fail).
Mutation description and segregation
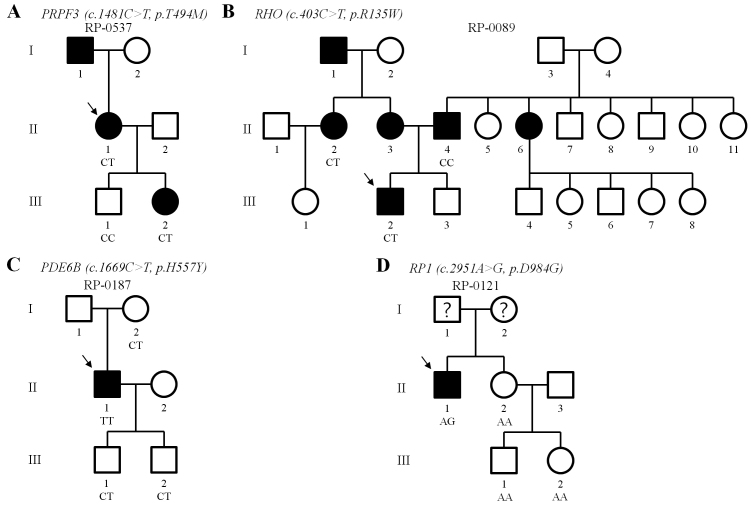
Based on the segregation analysis, mutation frequency, and previous reports, 10 mutations in five genes were considered to be associated with RP in the Korean population (p.T494M in PRPF3; p.T17M, p.R135W, p.D190N, p.K296N and p.P347L in RHO; p.H557Y and p.T604I in PDE6B; p.W316G in PRPH2; p.D984G in RP1). We were able to perform genetic analyses of additional members of four families. In these families, we performed segregation analysis to test whether the mutation was disease causing. The respective pedigrees and allele statuses are shown in Figure 3.
Figure 3.
Familial segregations of mutated alleles. The arrows indicate probands. Question marks represent individuals whose disease status has not been determined.
PRPF3
Mutations in the PRPF3 gene have been associated with autosomal dominant RP (ADRP). In the present study, one missense mutation was observed in a family with ADRP. In family RP-0537, the heterozygous p.T494M mutation segregated with disease from the proband (II-1) to a daughter (III-2) and was not detected in a healthy son (III-1; Figure 3A). This heterozygous mutation was previously reported as causative mutation in a Japanese family with ADRP [17] and was predicted to be pathogenic by computational analysis (Table 2).
RHO
Rhodopsin mutations have been associated with ADRP or autosomal recessive RP (ARRP). In the present study, five missense mutations were detected in five different probands. Of these, three mutations (p.T17M, p.K296N, and p.P347L) previously identified in Korean RP patients [18] were included to verify the GoldenGate assay for mutation detection in the same subjects. The genetic and phenotypic characteristics of these three mutations were described in our previous report [18]. Except those patients, there were no subjects with these mutations in the present study.
The heterozygous p.R135W mutation was detected in a proband (III-2) of family RP-0089 (Figure 3B). This heterozygous mutation has been previously reported as a causative mutation of ADRP in other populations [19,20] and was predicted to be pathogenic by computational analysis (Table 2). Interestingly, both parents of the proband were patients with ADRP (mother, II-3) or ARRP (father, II-4). To confirm the mutation segregation, we analyzed the available affected relatives in the maternal and paternal pedigrees. Blood samples of the affected father (II-4) and an affected sister (II-2) of the mother were available. The heterozygous p.R135W mutation was identified in II-2 and not in his father (II-4) by direct sequencing. The disease-causing gene in the paternal pedigree is unknown to date.
The heterozygous p.D190N mutation was observed in a patient from an ADRP family but was not detected in the other subjects tested. This heterozygous mutation was previously reported as causative mutation in Sardinian families with ADRP [21] and was expected to be pathogenic in computational analysis (Table 2). As far as we know, this is the first report of the p.D190N mutation in an East Asian population.
PDE6B
Variants in the phosphodiesterase 6B gene have been mainly associated with ARRP. This mutation has been previously reported as a causative mutation of ARRP [20,22], and was predicted to be pathogenic in computational analysis (Table 2). In the present study, p.H557Y was the most frequent mutation, including four homozygous mutations in different patients (Table 2). The heterozygous carrier state was found in nine patients and two controls. In family RP-0187 with ARRP, a homozygous patient (II-1) had inherited mutations from both heterozygous carrier parents (Figure 3C). The patient’s two sons (III-1 and III-2) also carried the heterozygous p.H557Y mutation without disease.
Another missense mutation of PDE6B, p.T604I, was detected in four patients and three controls in the heterozygous state. No homozygous patient with the p.T604I mutation was observed in our study. A digenic pattern of RP with the heterozygous p.T604I mutation in the recessive PDE6B gene and the heterozygous p.G167S mutation in the dominant PRPH2 gene was previously reported in Japanese patients [20]. However, there was no subject that exhibited these two heterozygous mutations simultaneously in our study.
PRPH2
Some variants in the peripherin 2 gene have been associated with ADRP or digenic RP. In the present study, the heterozygous p.W316G mutation was observed in a patient and a control (Table 2). This heterozygous mutation was previously identified in a Japanese patient and was expected to be pathogenic from computational analysis [20]. The association of this mutation with RP could not be assessed in our study because the family history and segregation analysis were not available in subjects with this mutation.
RP1
Variants in the RP1 gene have been mostly associated with ADRP or ARRP. In the present study, the heterozygous p.D984G mutation was observed in one patient, but not in control chromosomes. The genes from three healthy relatives of the patient were sequenced and did not have the mutation (Figure 3D). This mutation was previously reported in a Chinese ADRP family and was expected to be pathogenic [23].
Two missense mutations expected to be benign were detected in this study (Table 2). The p.G706R mutation in the RP1 gene and p.G122D in the CRX gene were found in both patients and controls in the heterozygous state (Table 2). Although RP1 and CRX are associated with ADRP, these mutations have been previously reported in other ethnic groups and were not expected to be pathogenic [20,23-25].
Clinical evaluation in retinitis pigmentosa patients with missense mutations
The mutations and phenotypes are summarized in Table 3. The patients ranged in age from 22 to 51 years.
Table 3. Phenotypic characterization of patients with missense mutation.
| Gene | Mutation | Family | Patient | Age | BCVA: | Lens opacities: | Visual field | ERG: | OCT (CFT, µm): |
|---|---|---|---|---|---|---|---|---|---|
| ID |
ID |
/Sex |
RE/LE |
RE/LE |
Cone/Rod |
RE/LE |
|||
| PRPF3 |
p.T494M |
RP-0537 |
R0537, II-1 |
49/F |
0.02/0.02 |
Clear |
Central<5° |
ND/ND |
184,CME/355,VMT |
| p.T494M |
RP-0537 |
R0553, III-2 |
27/F |
0.6/0.6 |
Clear |
Central 10° |
ND/ND |
― |
|
| ? |
? |
RP-0089 |
R0089, II-4 |
49/M |
0.2/0.2 |
NS |
Central 5°~10° |
ND/ND |
― |
| RHO |
p.R135W |
RP-0089 |
R0352, II-2 |
45/F |
HM/HM |
IOL |
Failure |
ND/ND |
178/140 |
| p.R135W |
RP-0089 |
R0119, III-2 |
22/M |
0.4/0.6 |
Clear |
Pph.constriction |
ND/ND |
― |
|
| p.D190N |
RP-0513 |
R0513 |
59/M |
0.2/0.4 |
PSC |
Central 5°~10° |
↓↓/ND |
256/347,ERM |
|
| PDE6B |
p.H557Y |
RP-0187 |
R0187, II-1 |
51/M |
0.02/0.04 |
IOL/NS |
Central 5°~10° |
ND/ND |
201/187 |
| p.H557Y |
RP-0143 |
R0143 |
26/F |
0.9/0.4 |
Clear |
Central 10° |
― |
263,ERM/244,CME |
|
| p.H557Y |
RP-0295 |
R0295 |
28/F |
0.7/0.4 |
Clear |
― |
― |
― |
|
| p.H557Y |
RP-0310 |
R0310 |
32/F |
0.5/0.5 |
ASC |
Pph.constriction |
― |
― |
|
| PRPH2 |
p.W316G |
RP-0030 |
R0030 |
33/M |
0.2/0.2 |
Clear |
Central<5° |
ND/ND |
― |
| RP1 | p.D984G | RP-0121 | R0121, II-1 | 29/M | 0.02/0.04 | IOL/PSC | ― | ND/ND | 181/109 |
BCVA, best corrected visual acuity (decimal); CFT, central foveal thickness (μm); CME, cystoid macular edema; ERM, epiretinal membrane; ERG, electroretinogram; HM, hand motion; IOL, intraocular lens; LE, left eye; ND, non-detectable; NS, nuclear sclerosis; OCT, optical coherence tomography; PSC, posterior subcapsular opacity; RE, right eye; VMT, vitreomacular traction.
PRPF3, p.T494M
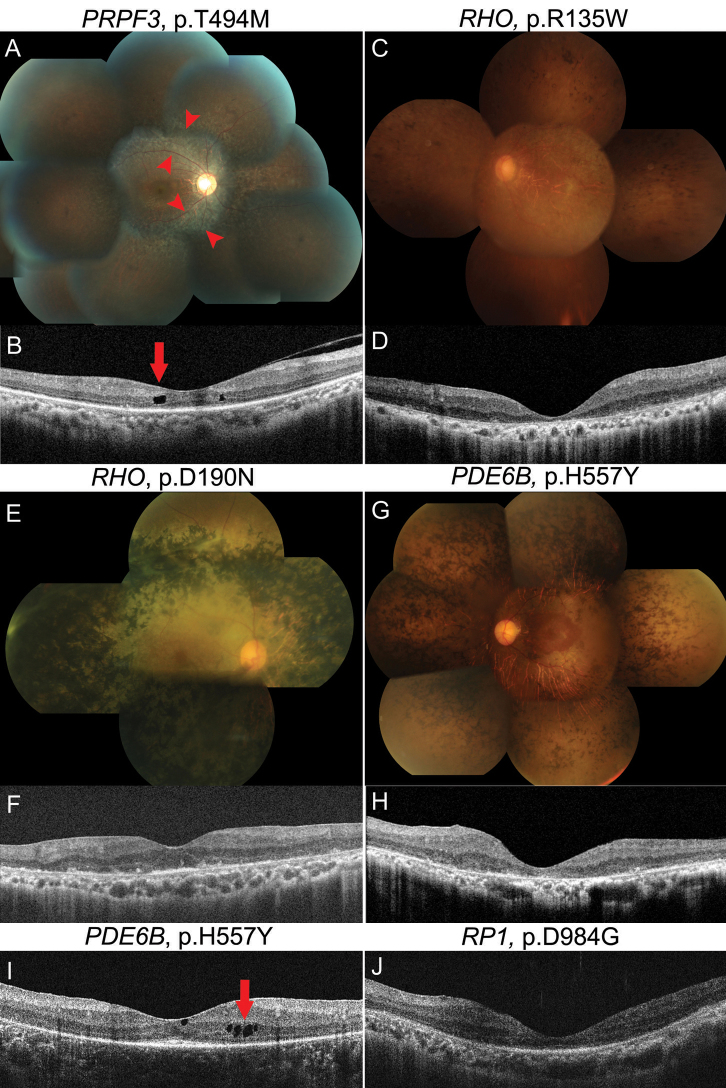
No lens opacities were found in a family with this mutation. Fundus findings of a 27-year-old daughter showed areas of atrophy along the arcades (Figure 4A). OCT images from the mother showed cystoid macular edema (CME) in the right eye (RE) and vitreotraction membrane in the left eye (LE; Figure 4B).
Figure 4.
Fundus photographs (A, C: E, G: and spectral domain optical coherence tomography images B: D: F: H-J: in retinitis pigmentosa patients with missense mutations. In fundus images, areas of atrophy along the arcades (A; red arrowheads) and marked pigmentary changes E: were noted. In optical coherence tomography (OCT) images, intraretinal cyst or cystoid macular edema (CME; B: I:; red arrow), severe foveal atrophy D: H: J: and normal range of foveal thickness F: were observed.
RHO, p.R135W
A 45-year-old woman showed poor visual acuity of hand motion in both eyes, although she had already undergone cataract surgery. The fundus examination showed severe retinal pigmentary degeneration involving the macula (Figure 4C). OCT revealed severe foveal atrophy in both eyes, a 178 μm in the RE and 140 μm in the LE (Figure 4D). The patient’s cousin, aged 22 years, had decreased visual acuity of 0.4 in the RE and 0.6 in the LE, and showed peripheral constriction of the visual field.
RHO, p.D190N
In spite of being the oldest subject and having cataracts, especially of the posterior subcapsular type, a 59-year-old patient maintained relatively good visual acuity of 0.2 in the RE and 0.4 in the LE. Moreover, the cone response in ERG was not yet extinguished. Marked pigmentary changes were seen in the whole retina, except the macula (Figure 4E). OCT images showed a normal range of foveal thickness in the RE (Figure 4F) and mild epiretinal membrane in the LE.
PDE6B, p.H557Y
A 51-year-old patient had undergone cataract surgery in the RE and had foveal atrophy in both eyes (Figure 4G,H). OCT images from a 26-year-old patient showed CME in the LE (Figure 4I). Mild cataract, particularly anterior subcapsular opacity, was observed in a 32-year-old patient.
PRPH2, p.W316G
A 33-year-old patient had decreased visual acuity of 0.2 and a severely constricted visual field, with less than the central 5° of radius remaining in each eye.
RP1, p.D347G
A 29-year-old patient had very poor visual acuity of 0.02 in the RE and 0.04 in the LE, and slit-lamp examination showed an intraocular lens in the RE and cataract in the LE. Marked foveal atrophy was noticed in OCT images, although the patient was of young age, at 181 μm in the RE and 109 μm in the LE (Figure 4J). When we compared OCT findings among similarly aged RP patients with missense mutations, foveal atrophy was more severe in patients with p.D347G in RP1 or p.R135W in RHO than in those with p.T494M in PRPF3 or p.H557Y in PDE6B. CME was observed in patients with p.T494M in PRPF3 and p.H557Y in PDE6B.
Discussion
Owing to the high genetic heterogeneity of RP, identification of its genetic cause is expensive and time consuming. To develop an efficient screening method, various genotyping platforms have been evaluated. However, as a result of the large number of RP genes identified to date, the fraction of patients in whom a mutation could be found by screening the known genes has often been low.
The purposes of this project were to evaluate a high-throughput genotyping assay, the GoldenGate assay with VeraCode microbeads, for mutation genotyping, and to find the frequent disease-causing mutations in Korean patients with RP. The genotyping platform with VeraCode microbeads is known to be flexible in assay content and multiplexing analytes, and can serve medium- to high-throughput applications [26]. With this technology, researchers have an optimal combination of assay content for each population. However, the platform used in this study also shows a limitation in the selection of mutations for the 96-well format, which may introduce strong selection bias in assay design. In addition, of 95 sequence variants that were selected to screen disease-causing mutations in a Korean population and to verify the assay platform, five mutations failed to detect genotypes because of the poor separation of clusters. A total of 12 mutations in six RP genes were identified and validated in this study. As independent verification of the ability of the GoldenGate assay, four DNA samples with known RHO mutations (p.T17M, p.K296N, and p.P347L) were analyzed. Genotype inconsistency between the GoldenGate assay and sequence analysis was observed in three mutations (Figure 2), although the GoldenGate assay detected all previously known mutations in samples for validation. For rare variants, genotyping technologies must accurately perform two-cluster calling. Miscalling is usually characterized by heterozygous samples being assigned a homozygous mutant genotype for rare variants and vice versa for X-linked variants [27]. In the present study, incorrect two-cluster calling was observed in 3/11 (27%) rare autosomal variants. Customized genotyping assays are being used for mutation screening, frequency determination, and validation in investigations of disease-causing rare variants. For these applications, genotyping technologies, including the GoldenGate assay with VeraCode microbeads, should be improved in two-cluster calling for rare variants as well as in design flexibility of the assay probe.
In the RP-0089 family, a proband (III-2) inherited an RHO mutation (p.R135W) from the mother with ADRP and a mutant allele from the father with ARRP. Although the disease-causing gene of paternal pedigree is unknown, the mutant allele from the father may play a role as a modifier, either by directly influencing the function of the unknown gene or by interacting with the environment. Therefore, the phenotypic analysis and prognostic follow-up study of the patient (III-2) who received an ARRP mutation as well as an ADRP mutation would be meaningful.
Although Jin et al. have previously reported the p.T604I mutation of the PDE6B gene in Japanese patients and it was expected to be pathogenic [20], we could not confirm the association of p.T604I with RP in our samples due to absence of any patient with a homozygous mutation. Sequence analysis of the whole PDE6B gene in the heterozygote for the p.T604I mutation is necessary to determine whether the mutation is associated with compound heterozygous ARRP.
The characterization of genotype–phenotype correlations in patients with mutations was also one of the main purposes of the present study. The mode of inheritance is believed to play an important role in determining the prognosis of the disease. In general, ADRP has the slowest progression, and X-linked RP tends to induce the most severe form of the disease [23,28]. Most mutations identified in our study are known to be related to ADRP or ARRP heritance. OCT is widely used to study retinal diseases and has been used to diagnose and monitor RP. Several studies using OCT have revealed that the retinal thickness and the status of inner and outer segments of the photoreceptor layer are correlated with visual acuity, and are significant factors for visual acuity in RP patients [29,30]. In our study, comparing the phenotypes in patients with other mutations by foveal thickness and visual acuity after correcting for age, a patient with p.D984G in RP1 had the most severe foveal atrophy with subsequent severe deterioration of visual acuity, despite this patient’s young age of 29 years. In contrast, a patient with p.D190N in RHO had a normal range of foveal thickness and relatively good visual acuity, although this patient was the oldest at 59 years. Although the mutation of p.D984G in RP1 was the first to be reported in Chinese patients, the clinical features were not described at that time [23]. Therefore, the severe phenotype associated with this mutation is first reported in our study. The mild phenotype of p.D190N in RHO is consistent with that in a previous study [31]. However, another mutation in the same RHO gene, p.R135W, caused more severe and more rapidly progressive RP than p.D190N, which is comparable to the observations in a previous study [32]. Interestingly, fundus findings in patients with p.T494M in PRPF3 showed areas of atrophy along the arcades, which is similar to the results of a previous study in a Swiss family [33].
Although CME has been frequently described in RP patients and its prevalence has been reported to be 13%–49% using OCT [34,35], the risk factors, including the specific genotype associated with CME in RP, have not been previously elucidated. In the present study, using OCT, we found CME in patients with p.T494M in PRPF3 and p.H557Y in PDE6B. In our previous study, OCT revealed bilateral CME in all four children of a family with p.P347L in RHO [18]. These results indicate that CME in RP patients may be associated with a specific genotype. Further investigations of the risk factors for RP-associated CME in a larger cohort are required.
We carefully selected the assay content thought probable to occur in Korean RP patients. Nevertheless, only 10 RP-related mutations were identified in 26 (7.7%) of 336 patients. The most mutations identified in the study were previously detected in RP patients with East Asian ancestry, with the exception of p.D190N in RHO (Table 2). This suggests that the spectrum of mutations causing RP is more different than we expected in a population-specific manner. In previous studies, screening known genes in ADRP families led to the identification of a disease-causing mutation in 60% of cases among Americans of European ancestry and less frequently among other populations [36,37]. Furthermore, less than 50% of RP patients had known inheritance patterns in the present study (Table 1). These factors likely explain why a small number of mutations were identified in the present study. Continuous effort to identify novel RP genes and mutations in a population would improve the efficiency and accuracy of the genetic diagnosis of RP. As a rapid and efficient way of finding disease-causing mutations, next-generation sequencing technologies could be useful in designing the best assay content for genetic diagnosis of RP in different populations. A rapid and reliable diagnostic tool for RP and its complete phenotypic characterization may enable patients and their families to receive appropriate genetic counseling and may contribute to the development of gene- or mutation-specific treatments.
Acknowledgments
We thank all of the subjects and their relatives for participating, as well as the Korean RP Society for cooperation in patient recruitment. This work was supported by an intramural grant from the Korea National Institute of Health, Korea Center for Disease Control and Prevention (4845–301), and the Mid-career Researcher Program through NRF grant (No. 20,110,027,478) funded by the MEST, Republic of Korea.
Appendix 1. The assay content for mutation detection.
Variants previously reported in east Asians are underlined. Chr, chromosome; n, number of mutations. To access these data, click or select the words “Appendix 1.” This will initiate the download of a compressed (pdf) archive that contains the file.
Appendix 2. Primers.
To access these data, click or select the words “Appendix 2.” This will initiate the download of a compressed (pdf) archive that contains the file.
References
- 1.Rivolta C, Sharon D, DeAngelis MM, Dryja TP. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002;11:1219–27. doi: 10.1093/hmg/11.10.1219. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan J, Bonneau D, Frezal J, Munnich A, Dufier JL. Clinical and genetic heterogeneity in retinitis pigmentosa. Hum Genet. 1990;85:635–42. doi: 10.1007/BF00193589. [DOI] [PubMed] [Google Scholar]
- 3.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 4.Daiger SP. RetNet. The Retinal Information Network. 2012 https://sph.uth.tmc.edu/Retnet/
- 5.Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–84. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 6.Ávila-Fernandez A, Cantalapiedra D, Aller E, Vallespin E, Aguirre-Lamban J, Blanco-Kelly F, Corton M, Riveiro-Alvarez R, Allikmets R, Trujillo-Tiebas MJ, Millan JM, Cremers FP, Ayuso C. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol Vis. 2010;16:2550–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Ernest PJ, Boon CJ, Klevering BJ, Hoefsloot LH, Hoyng CB. Outcome of ABCA4 microarray screening in routine clinical practice. Mol Vis. 2009;15:2841–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Klevering BJ, Yzer S, Rohrschneider K, Zonneveld M, Allikmets R, van den Born LI, Maugeri A, Hoyng CB, Cremers FP. Microarray-based mutation analysis of the ABCA4 (ABCR) gene in autosomal recessive cone-rod dystrophy and retinitis pigmentosa. Eur J Hum Genet. 2004;12:1024–32. doi: 10.1038/sj.ejhg.5201258. [DOI] [PubMed] [Google Scholar]
- 9.Vallespin E, Cantalapiedra D, Riveiro-Alvarez R, Wilke R, Aguirre-Lamban J, Avila-Fernandez A, Lopez-Martinez MA, Gimenez A, Trujillo-Tiebas MJ, Ramos C, Ayuso C. Mutation screening of 299 Spanish families with retinal dystrophies by Leber congenital amaurosis genotyping microarray. Invest Ophthalmol Vis Sci. 2007;48:5653–61. doi: 10.1167/iovs.07-0007. [DOI] [PubMed] [Google Scholar]
- 10.Simpson DA, Clark GR, Alexander S, Silvestri G, Willoughby CE. Molecular diagnosis for heterogeneous genetic diseases with targeted high-throughput DNA sequencing applied to retinitis pigmentosa. J Med Genet. 2011;48:145–51. doi: 10.1136/jmg.2010.083568. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Yu HG, Seo JM, Moon SW, Moon JW, Kim SJ, Chung H. Hereditary and clinical features of retinitis pigmentosa in Koreans. J Korean Med Sci. 2010;25:918–23. doi: 10.3346/jkms.2010.25.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Oh B, Kim HL. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–34. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 13.Stenson PD, Mort M, Ball EV, Howells K, Phillips AD, Thomas NS, Cooper DN. The Human Gene Mutation Database: 2008 update. Genome Med. 2009;1:13. doi: 10.1186/gm13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update). Doc Ophthalmol. 2004;108:107–14. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 15.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng PC, Henidoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada Y, Itabashi T, Sato H, Tamai M. Clinical features of a Japanese family with autosomal dominant retinitis pigmentosa associated with a Thr494Met mutation in the HPRP3 gene. Graefes Arch Clin Exp Ophthalmol. 2004;242:956–61. doi: 10.1007/s00417-004-0923-x. [DOI] [PubMed] [Google Scholar]
- 18.Kim KJ, Kim C, Bok J, Kim KS, Lee EJ, Park SP, Chung H, Han BG, Kim HL, Kimm K, Yu HG, Lee JY. Spectrum of rhodopsin mutations in Korean patients with retinitis pigmentosa. Mol Vis. 2011;17:844–53. [PMC free article] [PubMed] [Google Scholar]
- 19.Sung CH, Davenport CM, Hennessey JC, Maumenee IH, Jacobson SG, Heckenlively JR, Nowakowski R, Fishman G, Gouras P, Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991;88:6481–5. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin ZB, Mandai M, Yokota T, Higuchi K, Ohmori K, Ohtsuki F, Takakura S, Itabashi T, Wada Y, Akimoto M, Ooto S, Suzuki T, Hirami Y, Ikeda H, Kawagoe N, Oishi A, Ichiyama S, Takahashi M, Yoshimura N, Kosugi S. Identifying pathogenic genetic background of simplex or multiplex retinitis pigmentosa patients: a large scale mutation screening study. J Med Genet. 2008;45:465–72. doi: 10.1136/jmg.2007.056416. [DOI] [PubMed] [Google Scholar]
- 21.Keen TJ, Inglehearn CF, Lester DH, Bashir R, Jay M, Bird AC, Jay B, Bhattacharya SS. Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site. Genomics. 1991;11:199–205. doi: 10.1016/0888-7543(91)90119-y. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin ME, Ehrhart TL, Berson EL, Dryja TP. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA. 1995;92:3249–53. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang SW, Wang DY, Chan WM, Tam PO, Chong KK, Lam DS, Pang CP. A novel missense RP1 mutation in retinitis pigmentosa. Eye (Lond) 2006;20:602–5. doi: 10.1038/sj.eye.6701944. [DOI] [PubMed] [Google Scholar]
- 24.Sohocki MM, Daiger SP, Bowne SJ, Rodriquez JA, Northrup H, Heckenlively JR, Birch DG, Mintz-Hittner H, Ruiz RS, Lewis RA, Saperstein DA, Sullivan LS. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat. 2001;17:42–51. doi: 10.1002/1098-1004(2001)17:1<42::AID-HUMU5>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum L, Chan WM, Yeung KY, Lam DS, Kwok AK, Pang CP. RP1 in Chinese: Eight novel variants and evidence that truncation of the extreme C-terminal does not cause retinitis pigmentosa. Hum Mutat. 2001;17:436. doi: 10.1002/humu.1127. [DOI] [PubMed] [Google Scholar]
- 26.Lin CH, Yeakley JM, McDaniel TK, Shen R. Medium- to high-throughput SNP genotyping using VeraCode microbeads. In: Bugert P, editor. DNA and RNA profiling in human blood: Methods and protocols. Vol 496: Humana press; 2009. p. 129–42. [DOI] [PubMed] [Google Scholar]
- 27.Tindall EA, Petersen DC, Nikolaysen S, Miller W, Schuster SC, Hayes VM. Interpretation of custom designed Illumina genotype cluster plots for targeted association studies and next-generation sequence validation. BMC Res Notes. 2010;3:39. doi: 10.1186/1756-0500-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim KP, Yip SP, Cheung SC, Leung KW, Lam ST, To CH. Novel PRPF31 and PRPH2 mutations and co-occurrence of PRPF31 and RHO mutations in Chinese patients with retinitis pigmentosa. Arch Ophthalmol. 2009;127:784–90. doi: 10.1001/archophthalmol.2009.112. [DOI] [PubMed] [Google Scholar]
- 29.Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye (Lond) 2009;23:304–8. doi: 10.1038/sj.eye.6703076. [DOI] [PubMed] [Google Scholar]
- 30.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2005;46:3349–54. doi: 10.1167/iovs.04-1383. [DOI] [PubMed] [Google Scholar]
- 31.Tsui I, Chou CL, Palmer N, Lin CS, Tsang SH. Phenotype-genotype correlations in autosomal dominant retinitis pigmentosa caused by RHO, D190N. Curr Eye Res. 2008;33:1014–22. doi: 10.1080/02713680802484645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iannaccone A, Man D, Waseem N, Jennings BJ, Ganapathiraju M, Gallaher K, Reese E, Bhattacharya SS, Klein-Seetharaman J. Retinitis pigmentosa associated with rhodopsin mutations: Correlation between phenotypic variability and molecular effects. Vision Res. 2006;46:4556–67. doi: 10.1016/j.visres.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Vaclavik V, Gaillard MC, Tiab L, Schorderet DF, Munier FL. Variable phenotypic expressivity in a Swiss family with autosomal dominant retinitis pigmentosa due to a T494M mutation in the PRPF3 gene. Mol Vis. 2010;16:467–75. [PMC free article] [PubMed] [Google Scholar]
- 34.Adackapara CA, Sunness JS, Dibernardo CW, Melia BM, Dagnelie G. Prevalence of cystoid macular edema and stability in oct retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina. 2008;28:103–10. doi: 10.1097/IAE.0b013e31809862aa. [DOI] [PubMed] [Google Scholar]
- 35.Hirakawa H, Iijima H, Gohdo T, Tsukahara S. Optical coherence tomography of cystoid macular edema associated with retinitis pigmentosa. Am J Ophthalmol. 1999;128:185–91. doi: 10.1016/s0002-9394(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan LS, Bowne SJ, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, Lewis RA, Garcia CA, Ruiz RS, Blanton SH, Northrup H, Gire AI, Seaman R, Duzkale H, Spellicy CJ, Zhu J, Shankar SP, Daiger SP. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Invest Ophthalmol Vis Sci. 2006;47:3052–64. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daiger SP, Sullivan LS, Bowne SJ, Birch DG, Heckenlively JR, Pierce EA, Weinstock GM. Targeted high-throughput DNA sequencing for gene discovery in retinitis pigmentosa. Adv Exp Med Biol. 2010;664:325–31. doi: 10.1007/978-1-4419-1399-9_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakarova CF, Hims MM, Bolz H, Abu-Safieh L, Patel RJ, Papaioannou MG, Inglehearn CF, Keen TJ, Willis C, Moore AT, Rosenberg T, Webster AR, Bird AC, Gal A, Hunt D, Vithana EN, Bhattacharya SS. Mutations in HPRP3, a third member of pre-mRNA splicing factor genes, implicated in autosomal dominant retinitis pigmentosa. Hum Mol Genet. 2002;11:87–92. doi: 10.1093/hmg/11.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Sheffield VC, Fishman GA, Beck JS, Kimura AE, Stone EM. Identification of novel rhodopsin mutations associated with retinitis pigmentosa by GC-clamped denaturing gradient gel electrophoresis. Am J Hum Genet. 1991;49:699–706. [PMC free article] [PubMed] [Google Scholar]
- 40.Dryja TP, McGee TL, Hahn LB, Cowley GS, Olsson JE, Reichel E.Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med 19903231302–7.Sandberg MABerson EL [DOI] [PubMed] [Google Scholar]