Abstract
Nanoparticles may soon be used to transport diagnostic and therapeutic drugs to targeted sites not normally accessible, thereby improving treatment and reducing costs. Further research is still needed to establish the efficacy and safety of these nanomaterials.
This is the first in a series of three articles about nanomedicine. Part 2 will discuss the current and future clinical applications of nanomedicine. The third article in this series will focus on the regulatory and safety challenges presented by nanomedicine.
Introduction
Nanomedicine, the application of nanotechnology to medicine, is currently at an early stage but it is expected to have a revolutionary impact on health care.1 Nanomedical research is heavily supported by public policy and investment, and is progressing rapidly.1,2 The continued development of nanomedicines has the potential to provide numerous benefits, including improved efficacy, bioavailability, dose–response, targeting ability, personalization, and safety compared to conventional medicines.2–5 The most exciting concept in nanomedical research may be the design and development of multifunctional nanoparticle (NP) complexes that can simultaneously deliver diagnostic and therapeutic agents to targeted sites.5,6 These capabilities are unprecedented and represent tremendous progress toward improving patient diagnosis, treatment, and follow-up.6 However, despite these potential benefits, essential data regarding the pharmacokinetics, pharmacodynamics, and toxicity of many nanomaterials are currently lacking.5,7
Nanotechnology: Definitions and Trends
What Is Nanotechnology?
Nanotechnology is a rapidly advancing field that is expected to have a revolutionary impact on many industries, including medicine.8,9 Nanotechnology has been made possible through the convergence of many scientific fields, including chemistry, biology, physics, mathematics, and engineering.1,2,9
A nanometer (nm) is one billionth of a meter, and the prefix “nano-” comes from the Greek word for “dwarf.”4,10 Nanotechnology provides scientists with new tools for the investigation, manipulation, and control of atoms, molecules, and submicroscopic objects, generally ranging from 1 to 100 nm.1,6 Nanotechnology allows scientists to take advantage of naturally occurring quantum effects at the nanoscale level that influence biological, physical, chemical, mechanical, and optical properties.6,10,11 These unique effects often give nanoscale materials desirable chemical, physical, and biological properties that differ from those of their larger, or “bulk,” counterparts.12
What Is Nanomedicine?
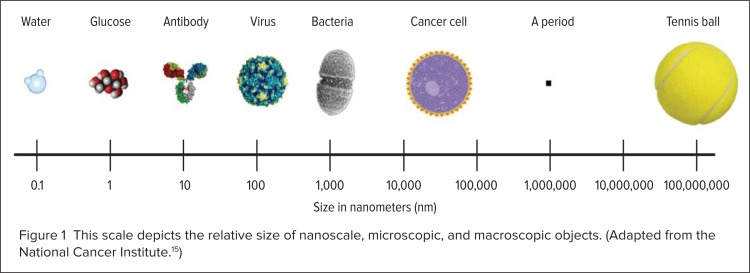
The convergence of nanotechnology and medicine has led to the interdisciplinary field of nanomedicine.6 Advances in genetics, proteomics, molecular and cellular biology, material science, and bioengineering have all contributed to this developing field, which deals with physiological processes on the nanoscale level.6,9 Many of the inner workings of a cell naturally occur on the nanoscale level, since the dimensions of many biologically significant molecules like water, glucose, antibodies, proteins, enzymes, receptors, and hemoglobin are already within the nanoscale range (see Fig. 1).6,11 Many researchers are currently working on medical treatments, devices, and instruments that use nanotechnology to increase efficacy, safety, sensitivity, and personalization.11 Potentially beneficial properties of nanotherapeutics include improved bioavailability, reduced toxicity, greater dose response, and enhanced solubility compared with conventional medicines.2
Figure 1.
This scale depicts the relative size of nanoscale, microscopic, and macroscopic objects. (Adapted from the National Cancer Institute.15)
Definitions of Nanotechnology
The National Nanotechnology Initiative (NNI), a federal research and development program, defines nanotechnology as the science of materials and phenomena in the range of 1 to 100 nm in diameter.2,4,10 Many federal agencies, including the FDA and the Patent and Trademark Office (PTO), continue to use this definition.2 However, some experts say that this size limitation is artificial and misleading, since nanomaterials can have unique properties even in sizes up to several hundred nanometers.2
The National Institutes of Health (NIH) has presented an alternative definition of nanotechnology that doesn’t rely on size; instead, it defines the field as (1) studies that use nanotechnology tools and concepts to study biology, (2) the engineering of biological molecules to have functions that differ from those that they have in nature, or (3) the manipulation of biological systems by methods more precise than standard molecular biological, synthetic, chemical, or biochemical approaches.2
Nanotechnology Products on the Market
Nanotechnology has the potential to be used in a wide range of products, including medicines, electronics, cosmetics, and foods.1,13–15 According to the Project for Emerging Nanotechnologies at The Woodrow Wilson International Center for Scholars, more than 800 nanotechnology-based products are already on the market.9 Nanotechnology has been used in laptop computers, cell phones, digital cameras, water-filtration systems, and cosmetics.14,15 Nanotechnology research is also under way to improve the bioavailability of food nutrients and to develop food packaging that detects and prevents spoilage.14,16
Nanotechnology has also been applied to improve a number of medical products and processes;14,15 these include drugs, medical imaging, antimicrobial materials, medical devices, sunscreens, burn and wound dressings, dental-bonding agents, sunscreens, and protective coatings for eyeglasses.14,15 Nanotechnology has improved drug targeting and bioavailability, diagnostic imaging, biomarker detection sensitivity, and drug-delivery efficiency.16 Some nanomedicines that are currently on the market include doxorubicin HCl liposome injection (Doxil, Ortho Biotech) for ovarian cancer; daunorubicin citrate liposome injection (DaunoXome, Diatos) for advanced AIDS-related Kaposi’s sarcoma; and amphotericin B liposome injection (AmBisome, Gilead) for fungal infections.3,5 In addition, paints containing silver NPs, which have antimicrobial properties, are being used in indoor medical settings, such as in hospitals.17
Projected Growth of Nanotechnology
Nanotechnology is a rapidly growing field. In 2008, nanotechnology was estimated to be a $10.5 billion industry in the U.S, mostly due to consumer product applications.17 It is estimated that the nanotechnology industry will grow to $1 trillion by 2015, representing an increase of about 100-fold in just 7 years.17
Nanomedicine has always been a major application for nanotechnology. 8 According to the National Science Foundation (NSF), by 2020, one-third of patents and start-up companies in the nanotechnology sector will involve biomedical applications. 8,18 The NSF also predicts that nearly half of future pharmaceuticals will have some nanotechnology components.4,18
Physical Features of Nanoparticles
The physical characteristics of NPs can differ in many ways that influence function.9 A discussion of several of these physical features follows.
Size
NPs are inherently small, with at least one dimension in the range of 1 to 100 nm, although they can also be micrometer (μm)-sized particles.6,9 NPs have novel structural, optical, and electronic properties that many larger molecules or bulk solids lack.9 They also have improved solubility, so they may be used to reinvestigate bulk drug counterparts that are known to have poor solubility.6 This property may provide the ability to convert insoluble or poorly soluble drugs into soluble aqueous suspensions, thus eliminating the need for toxic organic solvents.4 Another key benefit related to the small size of NPs is an increased bioavailability and circulation time.3 Studies have shown that particles under 200 nm have longer circulation times, compared with larger particles, irrespective of any surface modifications present.3
Shape
NPs come in a variety of shapes, including spheres, discs, hemispheres, cylinders, cones, tubes, and wires.6,9 NPs can also be hollow, porous, or solid.5 These characteristics of NPs can be selected on the basis of interactivity, loading capacity, and transport capabilities.6 For example, a hollow NP may be an attractive carrier for drug therapies or imaging contrast agents.6
Surface Area
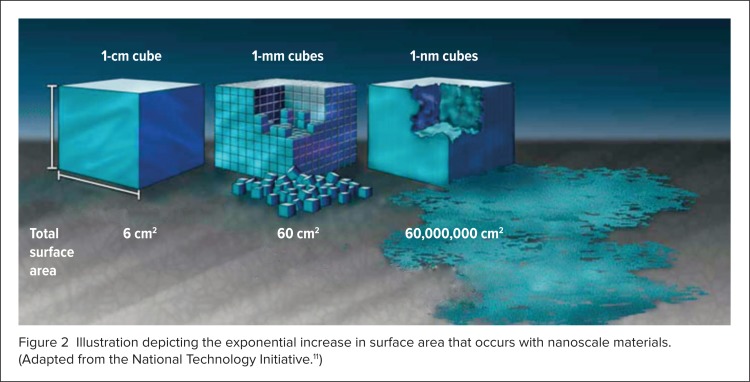
One feature of NPs that gives them unique physical properties is a large surface area relative to size.2 As particle size decreases, total surface area increases exponentially (Figure 2).2,11 An increase in surface area means that a greater proportion of atoms are located on the particle surface relative to the core.2 This phenomenon makes NPs more reactive compared with conventional larger molecules, or bulk solid counterparts.2 Increased surface area is also responsible for the enhanced water solubility and bioavailability that often occur with NPs.2
Figure 2.
Illustration depicting the exponential increase in surface area that occurs with nanoscale materials. (Adapted from the National Technology Initiative.11)
The large surface area of NPs also allows them to be designed to include a broad range of surface characteristics, including conjugation with electrostatic charges or biomolecules.6 Such surface features can be strategically selected for targeting and other purposes and are therefore determined on that basis.9
Permeability
If NPs are properly designed, their small size can enable them to cross physiological barriers to deliver drugs to sites that are not normally accessible by traditional means.6 For example, the increased permeability of an NP may allow it to transport cancer drugs into tumors by passing through neovessel pores that are less than 1 μm in diameter.5 The increased permeability of NPs may also allow them to cross the blood–brain barrier through the use of different uptake mechanisms.6
Specific Nanoparticles and Materials
A wide variety of NPs and materials are used in nanomedicine, depending on the application.6 Among the most widely used are liposomes, polymers, quantum dots (QDs), iron oxide (IO) particles, and carbon nanotubes and nanoshells.6
Liposomes
A liposome is a spherical vesicle composed of a lipid bilayer membrane and an empty core that usually carries an aqueous solution.5 Liposomes are usually 90 to 150 nm in diameter and are thus slightly larger than conventional NPs.5 Liposomes are often designed to carry biomolecules (e.g., monoclonal antibodies, antigens) that are conjugated to the surface as ligands.5
Liposomes are often used in nanomedical research because they have many unique properties.5 The components of liposomes are similar to natural human cell membranes; thus, they confer liposomal drug delivery with several intrinsic benefits.5 Liposomes circulate in the bloodstream for an extended time, compared with non-liposomal drugs, providing a longer treatment effect. Liposomes also accumulate at the site of a tumor or infection, naturally locating and delivering higher drug levels to these targets.5 Liposomes can carry and deliver either hydrophilic or hydrophobic therapies, which can be stored in their empty cores.6 By using lipids of different fatty-acid-chain lengths, scientists can construct liposomes to be temperature-sensitive or pH-sensitive, thereby permitting the controlled release of their contents only when they are exposed to specific environmental conditions.5
Polymers
In contrast to other materials, data on the safety and efficacy of many polymers already exist; therefore, polymer NPs are widely used in nanomedical research.3 Polymer NPs can be fabricated in a wide range of varieties and sizes, ranging from 10 nm to 1 μm.3,5 Some polymer NPs can facilitate drug release for weeks and do not accumulate in the body.3,5,6 As such, polymeric NPs are considered promising carriers for numerous medications, including those used in cancer, cardiovascular disease, and diabetes treatments; bone-healing therapies; and vaccinations.3 Contrast agents can also be conjugated to the surface of polymeric NPs, allowing them to be used in diagnostic imaging.5
Biodegradable polymers are of particular interest, since they can be fully metabolized and removed from the body.6 Poly-lactic-co-glycolic acid (PLGA) is an especially intriguing example of a biodegradable polymer, since relative proportions of polylactic acid (PLA) and polyglycolic acid (PGA) can be used to fine-tune the biodegradability of PLGA.6
Quantum Dots
Quantum dots (QDs) are semiconductor nanocrystals that range in size from 2 to 10 nm and usually consist of 10 to 50 atoms.4,5 Although QDs have been used in electronics and optics for 20 years, they have only recently been applied to nanomedical research.5 The most commonly used QDs for biomedical applications contain cadmium selenide (CdSe) or cadmium telluride (CdTe).4 QDs containing indium phosphide (InP) and indium arsenide (InAs) are also frequently used.4
QDs have unique optical and electronic properties, making them valuable as luminescent probes and giving them tremendous potential in many biomedical applications.4,5 QDs are intrinsically fluorescent and emit light over a broad range, from the near-ultraviolet (UV) to mid-infrared spectrum.9 They have size-dependent optical properties, extraordinary photostability, and surface properties that can be fine-tuned, which make them ideal for optical imaging.4 QDs have molar extinction coefficients that are 10 to 50 times larger than those of organic dyes, making them much brighter in in vivo conditions.5 They have long blood circulation times and can fluoresce for several months in vivo.5
QDs also have sufficient surface area to attach agents for simultaneous targeted drug delivery and in vivo imaging or for tissue engineering.4 Many uses of QDs for in vivo imaging have already been reported, including lymph node and angiogenic vessel mapping and cell subtype isolation.5 QDs are very efficient agents for cancer diagnosis in vivo, because the extremely small size of the QDs allows unimpeded access to systemic circulation and surface modifications can target them to neoplastic sites.4 Additional potential uses for QDs include image-guided surgery, light-activated therapies, and diagnostic tests.19
Surface coatings have been found to enhance the surface fine-tunability and increase the fluorescent yield of QDs.4 They may also reduce the adverse effects that can be elicited by QDs containing Cd, Se, and As, which are toxic materials.4 At present, the investigation of QDs is restricted to in vitro and animal studies because of toxicity concerns regarding these heavy metals.5,19 Novel methods to produce new generations of QDs in which toxic materials are reduced or absent are being pursued for future applications in humans.5
Superparamagnetic Iron Oxide Nanoparticles
Superparamagnetic NPs, like iron oxide (SPIO) and magnetite, have been used for years as nontargeted contrast agents for magnetic resonance imaging (MRI).1,5,17 However, these NPs do have superparamagnetic properties that allow them to be directed in situ with the use of a magnetic field.17 They also have a long retention time in circulation, are usually biodegradable, and have low toxicity.5 They are therefore excellent candidates for producing imageable therapeutic nanodevices.5
In addition to possessing other desirable properties, SPIO NPs can also be “functionalized” (designed) to achieve specific tumor targeting.5 SPIO NPs are increasingly being used for the development of target-specific MRI contrast agents.5 To date, SPIO NPs have been used for many applications, such as the delivery of antibiotics and drugs with simultaneous enhancement of MRI contrast and for the separation of bacteria from biomolecules.17
Carbon Nanotubes
Carbon nanotubes are composed of a distinct molecular form of carbon atoms that give them unusual thermal, mechanical, and electrical properties.5 For example, they are 100 times stronger than six times their weight in steel.5 Carbon nanotubes modified with polyethylene glycol (PEG) are surprisingly stable in vivo, with long circulation times and low uptake by the reticuloendothelial system (RES).5 Carbon nanotubes have been used for the delivery of imaging and therapeutic agents and in the transport of DNA molecules into cells.5 The nanoscale dimensions of single-walled and multiwalled carbon nanotubes, along with their electrocatalytic properties and high surface area, have compelled researchers to utilize them as nanoelectrodes.20
Carbon Nanoshells
Carbon nanoshells are composed of a silica core that is covered by a thin metallic shell, usually composed of gold.5 Carbon nanoshells have an ability to scatter light, a feature that is useful for cancer imaging.5 However, their primary use continues to be in thermal ablation therapy.5 Alternatively, focused lasers have been useful for cancer thermotherapy, but they cannot discriminate between diseased and healthy tissue.1 However, when carbon nanoshells are used for targeting in thermal ablation therapy, thermal energy passes through healthy tissue without causing harm, killing only the targeted tumor cells.5 In mice, carbon nanoshells and near-infrared spectroscopy (NIRS) thermal ablation therapy completely eliminated colon carcinoma cell tumors in vivo.5
The Multifunctional Nanoparticle Complex
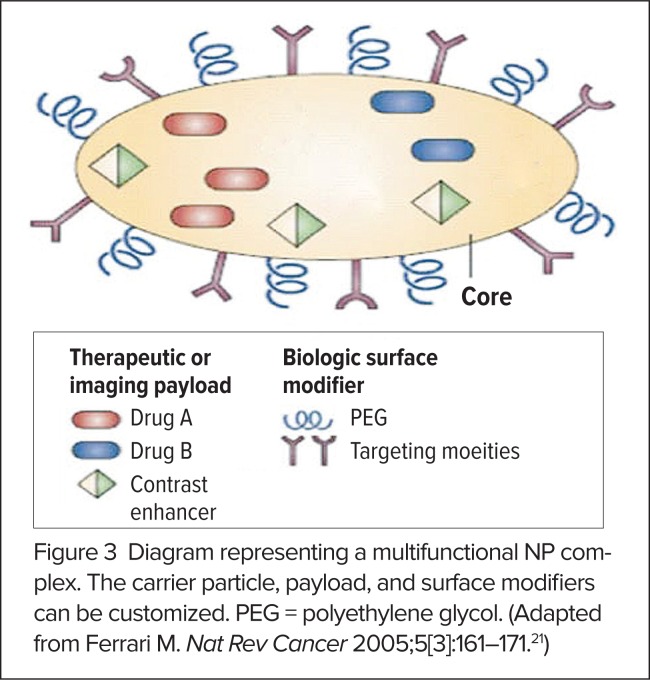
The aforementioned, and other, NPs are used to construct multifunctional NP complexes that mix and match different features, or “functionalizations,” in order to achieve an intended purpose.17 A multifunctional NP complex may be designed to include the following components (Figure 3):3,5,21
surface ligands that target the attachment of NPs to specific locations (e.g., organs, cells, or tissues).
linker molecules that release the cargo carried by the NP at the target site in response to a remote trigger or specific environmental cues.
a core that encapsulates targeting or imaging cargo or has optical or magnetic properties (gold, SPIO) that can localize the NP at the target site.
one or more therapeutic or diagnostic cargoes that are encapsulated in the NP core or attached to its surface.
a coating, such as PEG, that improves biocompatibility and/or enhances bioavailability by increasing circulation times and slowing clearance from the body.
Figure 3.
Diagram representing a multifunctional NP complex. The carrier particle, payload, and surface modifiers can be customized. PEG = polyethylene glycol. (Adapted from Ferrari M. Nat Rev Cancer 2005;5[3]:161–171.21)
Nanoparticle Functionalization
One of the most interesting capabilities in nanomedicine is the functionalization of NPs.7 Functionalization involves altering properties of an NP through chemical or physical modifications that are applied to achieve a desired effect.7 This process can provide local or directed delivery, prolong drug effects, facilitate transport into target cells, locate a tumor or area of infection, provide feedback regarding efficacy or drug delivery, or reduce blood flow shear effects.9 A discussion of the various approaches to functionalizing NPs follows.
Targeting
NPs can be administered locally or can be actively targeted using cell-specific ligands, magnetic localization, and/or size-based selectivity.3 Many factors need to be considered when constructing targeted NPs, including size, biocompatibility, target affinity, avoidance of the RES, and stability in the blood, as well as the ability to facilitate controlled drug release.7
Magnetic polymer nanocomposites or magnetoliposomes grafted with drug molecules have great potential for targeted drug delivery.3 These NPs have potentially favorable biodistribution and pharmacokinetic profiles, which can be enhanced by the external application of a static magnetic field at the site of action.3 For example, in one study, MRI confirmed that magnetic NPs had migrated toward neodymium/iron/boron (NdFeB) magnets that had been placed outside the peritoneal cavity, above grafts of a human ovarian carcinoma.3
NPs can be engineered to incorporate a wide variety of chemotherapeutic agents that can be targeted directly and specifically to the tumor site for better efficacy and safety.4 NPs can also be filled with contrast agents for imaging purposes.6 In comparison to small-molecule contrast agents, multifunctional NP complexes or NPs used in diagnostic imaging have the advantage of a large surface area that allows targeting through surface modifications and the ability to simultaneously deliver therapeutic agents.1
Surface Conjugation
One way in which NPs can be functionalized for specific applications is through surface conjugation.17 Nanoparticle surfaces can be conjugated with a wide range of diagnostic or therapeutic agents.1 Some candidate biomolecules for NP surface conjugation are cell-penetrating peptides (CPPs) that enhance intracellular delivery, fluorescent dyes for imaging, and agents for genetic therapy such as small inhibitory RNA (siRNA).7 Nanoparticle surfaces, conjugated with a targeting molecule that binds to highly expressed tumor cell receptors, can also facilitate the transport of imaging contrast agents that provide increased sensitivity and specificity, which aid in tumor detection.5
The surfaces of NPs can also be conjugated with drug therapies. 3 Surface conjugation with ligands that specifically bind to the target site can enhance the efficacy of NP drug-delivery systems while significantly reducing toxicity.4 In cancer treatment, tumor targeting can be achieved by conjugating a molecule or biomarker (such as a peptide, protein, or nucleic acid) that is known to bind to tumor cell receptors on the NP surface.5
Improved Bioavailability
NPs are generally cleared from circulation by immune system proteins called opsonins, which activate the immune complement system and mark the NPs for destruction by macrophages and other phagocytes.3 Neutral NPs are opsonized to a lesser extent than charged particles, and hydrophobic particles are cleared from circulation faster than hydrophilic particles.3 NPs can therefore be designed to be neutral or conjugated with hydrophilic polymers (such as PEG) to prolong circulation time.3 The bioavailability of liposomal NPs can also be increased by functionalizing them with a PEG coating in order to avoid uptake by the RES.5 Liposomes functionalized in this way are called “stealth liposomes.”5
NPs are often covered with a PEG coating as a general means of preventing opsonization, reducing RES uptake, enhancing biocompatibility, and/or increasing circulation time.5 SPIO NPs can also be made water-soluble if they are coated with a hydrophilic polymer (such as PEG or dextran), or they can be made amphophilic or hydrophobic if they are coated with aliphatic surfactants or liposomes to produce magnetoliposomes. 5 Lipid coatings can also improve the biocompatibility of other particles.3
Controlled Release
NPs can also be designed so that they can be activated to release therapeutic or diagnostic cargo in response to a site-specific or remote trigger.3 Properties that can be used to functionalize NPs for controlled release include pH, temperature, magnetic field, enzymatic activity, or other features such as light or radiofrequency signals.6 NPs constructed with pH-responsive materials can be designed to trigger drug release at a target site upon detecting a change in pH.3 For example, the mildly acidic environment inside inflammatory and tumor tissues (pH 6.8) and cellular vesicles, such as endosomes (pH 5.5–6.0) and lysosomes (pH 4.5–5.0), can be exploited to trigger drug release.3
Thermally responsive linkers, consisting of nucleic acids, peptides, proteins, lipids, carbohydrates, or polymers, can also be used to attach one or more agents for controlled release from the NP.3 When the thermally responsive linker is exposed to a specific temperature or temperature range (the “trigger temperature”), the linker is disrupted and the agent is released.3 For example, DNA molecules with heat-labile hydrogen bonding between complementary strands can act as a heat-sensitive linker.5 An NP can also be designed to include several thermally responsive linkers that are designed to disrupt at different temperatures, enabling drug delivery to occur in a specific order over varied periods of time.3
The release of agents from NPs can also be achieved through the incorporation of bonds that degrade under other specific conditions at the target site.5 For example, tumor-specific processes may be exploited to break a bond and trigger the release of a therapeutic agent.5 Tumor site-specific conditions that could be used to trigger release might include abnormal oxygen levels, unique biomarkers, or exposure to proteolytic enzymes that are overexpressed in tumors.5
Tunability
The tunability of NP properties is an important and powerful concept.20 NPs have a broad range of tunable biologic, optical, magnetic, electric, and mechanical features that differ dramatically from the same materials in larger forms because of modified quantum mechanics occurring at the nanoscale level.6 By changing the size of an NP, researchers can fine-tune many different properties of nanomaterials.11 For example, they can achieve different colors of fluorescence by changing the size of an NP, allowing a means of color coding or labeling during diagnostic imaging applications.11
Potential Advantages of Nanomedicine
Nanomedicines might someday provide answers to longstanding problems in medical research, ranging from poor drug solubility to a lack of target specificity for therapeutic compounds.2 Nanomedicine also has tremendous promise as a noninvasive tool for diagnostic imaging, tumor detection, and drug delivery because of the unique optical, magnetic, and structural properties of NPs that other tools do not possess.1
Nanomedicine presents new opportunities to improve the safety and efficacy of conventional therapeutics.5 Drugs with low bioavailability can now be targeted directly to the site required.3,5 The large surface area and greater reactivity of NPs may allow dose reduction of a drug, which can improve toxicity profiles and patient compliance.2,3 The large surface area of NPs can also increase the dissolution rate, saturation solubility, and intracellular uptake of drugs, improving in vivo performance.2,3 Combining encapsulation, release modalities, and surface modifications to improve therapeutic targeting or bioavailability could improve the efficacy of NP formulations several-fold compared with bulk counterparts.4 Targeted NPs can also transport large doses of therapeutic agents into malignant cells while sparing normal, healthy cells.4,5
One of the most exciting applications of nanomedicine is the use of multifunctional NP complexes for simultaneous non-invasive targeting, imaging, and treatment.1,4,5 Multifunctional NPs for cancer treatment can potentially include a variety of tumor targeting ligands as well as imaging and therapeutic agents that allow noninvasive monitoring and treatment.5 Multifunctional NPs that include fluorescent dyes can also provide in vivo imaging of biologic events during drug administration as well as potential diagnostic labels for the early detection and localization of tumors.7
Recent research efforts are also focused on developing magnetic NPs for the targeted delivery of various therapeutic or diagnostic agents.4 Interest in magnetic NP targeting applications is inspired by the possibility of detecting the particles by MRI and then correlating the results with histologic findings after treatment.3 Polymer/SPIO composites are the most common NPs used for theranostics (diagnostics).3 More than one cancer drug can also be incorporated on a polymer/IO conjugate backbone.3 The drugs can be released at the tumor site, allowing them to act together synergistically, potentially achieving higher efficacy.3 Because SPIO NPs generate heat when exposed to an alternating field, electromagnetic fields can also be applied externally for remote activation of SPIO NPs for thermal ablation therapy.5
Nanotechnologies have already transformed genetic and biological analysis through devices that examine molecular biomarkers.1 Compared with conventional modalities, these tests can be conducted more rapidly, reliably, and cost effectively via in vitro and in vivo diagnostic technologies that, for example, might use nanochips or QDs.1 Nanotechnologies can also produce diagnostic devices that are more sensitive and can detect earlier signs of metabolic imbalances, which can assist in the prevention of diseases like diabetes and obesity.20 The continued application of nanotechnologies to produce better and more cost-effective means of detecting molecular biomarkers will also open the way to the more routine practice of personalized medicine.1
Challenges for Nanomedicine
Despite the benefits that nanomedicine has to offer, much research is still required to evaluate the safety and toxicity associated with many NPs.3 Much of nanomedical research has concentrated on drug delivery, with relatively few studies focusing on the pharmacokinetics or toxicity of NPs.7 Investigating NP pharmacokinetics, pharmacodynamics, and potential long-term toxicity in vivo is essential to monitoring the effects of NPs on patient populations.5 Validating every nanotherapeutic agent for safety and efficacy, whether drug, device, biologic, or combination product, presents an enormous challenge for researchers and the FDA, which is currently struggling to formulate testing criteria and accumulate safety data.2,3
Studies are also needed to assess the immunogenicity of NPs.20 Nanotherapeutics and diagnostics may present unexpected toxic effects because of increased reactivity compared with their bulk counterparts.2 The most frequently reported side effect after injection of a nanotherapeutic agent seems to be a hypersensitivity reaction, which may be caused by activation of the immune complement system.6 The main molecular mechanism for in vivo NP toxicity is thought to be the induction of oxidative stress through the formation of free radicals.3 In excess, free radicals can cause damage to lipids, protein, DNA, and other biological components through oxidation. Several authors have reported that intrinsic characteristics of NPs, such as aspect ratio and surface area, can be pro-oxidant and pro-inflammatory.7 However, the formation of free radicals in response to an NP can also have other causes, such as the reaction of phagocytic cells to foreign material, insufficient antioxidants, the presence of transition metals, environmental factors, and other intrinsic chemical or physical properties.3
Research to evaluate the size and surface properties of NPs may also help to identify the critical dimensions at which they tend to significantly accumulate in the body.20 NPs have an increased ability to cross biological barriers and therefore have the potential to accumulate in tissues and cells because of their small size.2 The possible tissue accumulation, storage, and slow clearance of these potentially free radical–producing particles, as well as the prevalence of numerous phagocytes in the RES, may make organs such as the liver and spleen the main targets of oxidative stress.3,6
This lack of data about potential toxicity issues forces nanomedical research to focus predominantly on polymer NPs, for which safety and efficacy data already exist.3 In fact, several nanomedicines containing polymer NPs are already approved by FDA.3 Unlike other materials that may become toxic in NP form, the lipid NPs are also considered to be biocompatible and tolerable.3 Consequently, biodegradable, soluble, nontoxic NPs, such as polymers, liposomes, and IO particles, are much more desirable to use in nanomedicines than biopersistent components are.5 The use of NPs like carbon nanotubes, QDs, and some metallic nanocarriers that are not biodegradable might be more problematic.1,7 This characteristic need not discourage nanomedical research with these NPs but should reinforce efforts to identify additional biodegradable shapes, materials, and surface treatments.7
Conclusion
Although nanomedicine is still at an early stage of development, several drugs that utilize nanotechnology have been approved and marketed, and many others are being studied.2,3 Nanomedicines potentially offer a means of earlier diagnosis; more effective, safer, and personalized treatments; as well as reduced health care costs.1 Many experts agree that nanomedicine will create a paradigm shift that revolutionizes health care within the next 10 years.2,8 However, for significant progress to be made toward this goal, much more work is needed to establish testing criteria, validate efficacy, and accumulate safety data for various nanotherapeutic agents and materials.2,3
References
- 1.Poirot-Mazères I. Legal aspects of the risks raised by nanotechnologies in the field of medicine. J Int Bioethique. 2011;22(1):99–118. 212. doi: 10.3917/jib.221.0099. [DOI] [PubMed] [Google Scholar]
- 2.Bawa R. Regulating nanomedicine—can the FDA handle it? Curr Drug Deliv. 2011;8(3):227–234. doi: 10.2174/156720111795256156. [DOI] [PubMed] [Google Scholar]
- 3.Galvin P, Thompson D, Ryan KB, et al. Nanoparticle-based drug delivery: Case studies for cancer and cardiovascular applications. Cell Mol Life Sci. 2012;69(3):389–404. doi: 10.1007/s00018-011-0856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: Current perspective and future promise. Pharmacol Ther. 2010;128(2):324–335. doi: 10.1016/j.pharmthera.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Sajja HK, East MP, Mao H, et al. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr Drug Discov Technol. 2009;6(1):43–51. doi: 10.2174/157016309787581066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seigneuric R, Markey L, Nuyten DS, et al. From nanotechnology to nanomedicine: Applications to cancer research. Curr Mol Med. 2010;10(7):640–652. doi: 10.2174/156652410792630634. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskar S, Tian F, Stoeger T, et al. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood–brain barrier: Perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7:3. doi: 10.1186/1743-8977-7-3. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrady E, Conger S, Blanke S, Landry BJ. Emerging technologies in healthcare: Navigating risks, evaluating rewards. J Healthcare Manag. 2010;55(5):353–364. [PubMed] [Google Scholar]
- 9.Godin B, Sakamoto JH, Serda RE, et al. Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol Sci. 2010;31(5):199–205. doi: 10.1016/j.tips.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.What is nanotechnology? National Nanotechnology Initiative. Available at: www.nano.gov/nanotech-101/what/definition. Accessed June 7, 2012.
- 11.What’s so special about the nanoscale? National Nanotechnology Initiative. Available at: www.nano.gov/nanotech-101/special. Accessed June 7, 2012.
- 12.Nanotechnology. FDA Science & Research. Available at: www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/default.htm. Accessed June 7, 2012.
- 13.Fact Sheet: Nanotechnology. FDA Guidance, Compliance, & Regulatory Information. Available at: www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/ucm300914.htm. Accessed June 7, 2012.
- 14.FDA continues dialogue on ‘nano’ regulation. FDA for Consumers. Available at: www.fda.gov/ForConsumers/ConsumerUpdates/ucm258462.htm. Accessed June 7, 2012.
- 15.FDA readies for more ‘Nanoscale’ challenges. FDA Science & Research, July 25, 2007. Available at: www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/ucm153723.htm. Accessed June 7, 2012.
- 16.FDA Nanotechnology Task Force Report outlines scientific, regulatory challenges. Available at: www.fda.gov/ForConsumers/ConsumerUpdates/ucm110934.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=nanomedicine&utm_content=1. Accessed June 7, 2012.
- 17.Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomed. 2011;6:1463–1473. doi: 10.2147/IJN.S22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanotechnology Task Force FDA Science & Research. Available at: www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/NanotechnologyTaskForce/default.htm. Accessed June 7, 2012.
- 19.Good news for nanomedicine: Quantum dots appear safe in pioneering study on primates. Science News. 2012 May 20; Available at: www.sciencedaily.com/releases/2012/05/120520133919.htm. Accessed June 7, 2012. [Google Scholar]
- 20.Vaddiraju S, Tomazos I, Burgess DJ, et al. Emerging synergy between nanotechnology and implantable biosensors: A review. Biosens Bioelectron. 2010;25(7):1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]