Abstract
Ipilimumab (Yervoy) for metastatic melanoma
INTRODUCTION
Melanoma, a cancer originating in melanocytes, is the most deadly form of skin cancer and the sixth leading type of cancer in the U.S.1 According to the World Health Organization, approximately 53,000 people die of melanoma each year worldwide.2 In 2011, an estimated 70,230 adults were diagnosed with melanoma in the U.S., and 8,790 died of the disease.3,4 This may be an underestimation of new cases, however, as superficial and in situ melanomas treated in the outpatient setting usually are not reported.5
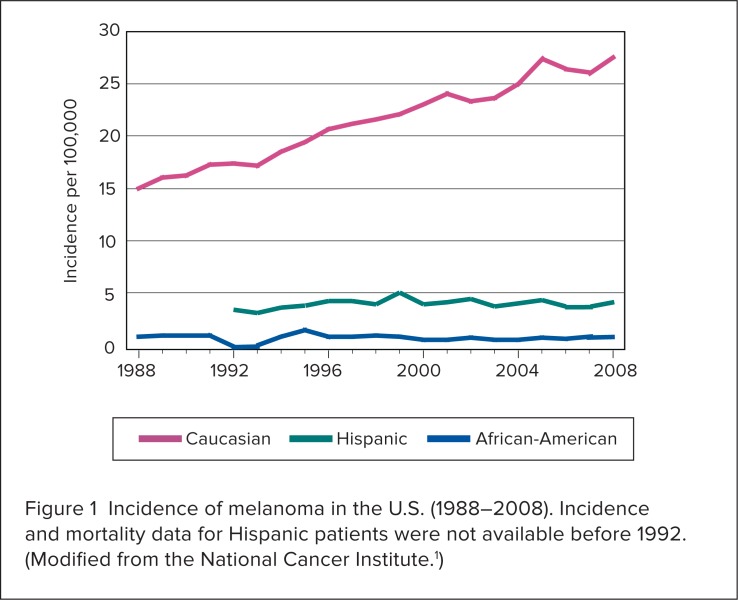
During the past 30 years, the annual incidence of melanoma in the U.S. has grown by more than 60%.1,5 The overall increase is attributed primarily to Caucasians (Figure 1).1 The median age at diagnosis is 59 years.4 In men, melanoma is increasing more than any other malignancy; in women, it is increasing more than any other malignancy except lung cancer.5 Men 65 years of age or older are more than twice as likely to develop melanoma as women in the same age group.1 Although melanoma is the rarest type of skin cancer, the estimated lifetime risk of developing the disease is 1 in 55 people.6 Each year, approximately $1.9 billion is spent on therapy for melanoma in the U.S.7
Figure 1.
Incidence of melanoma in the U.S. (1988–2008). Incidence and mortality data for Hispanic patients were not available before 1992. (Modified from the National Cancer Institute.1)
Risk factors include a family history, a history of melanoma, multiple clinically atypical moles or dysplastic nevi,8,9 and (rarely) inherited genetic mutations.5 Sun exposure and indoor tanning may also contribute to the development of melanoma from exposure to ultraviolet radiation.10,11
Clinical stages from 0 to IV are based on tumor thickness, tumor burden in lymph nodes, and metastatic involvement. The stages are as follows:12
Stage 0: melanoma in situ; no metastatic lymph nodes; no distant metastases
Stage Ia (localized disease): tumor thickness of 1 mm or less without ulceration and with mitosis less than one cell/mm2; no metastatic lymph nodes; no distant metastases
Stage Ib (localized disease): tumor thickness of 1 mm or less with ulceration or mitoses one cell/mm2 or more, or tumor thickness of 1 to 2 mm without ulceration; no metastatic lymph nodes; no distant metastases
Stage IIa (localized disease): tumor thickness of 1 to 2 mm with ulceration, or tumor thickness of 2 to 4 mm without ulceration; no metastatic lymph nodes; no distant metastases
Stage IIb (localized disease): tumor thickness of 2 to 4 mm with ulceration, or tumor thickness exceeding 4 mm without ulceration; no metastatic lymph nodes; no distant metastases
Stage III (regional disease): any tumor thickness and ulceration or mitosis status; one or more lymph nodes with metastatic involvement; no distant metastases
Stage IV (distant metastasis): any tumor thickness and ulceration or mitosis status; any lymph node metastasis; distant metastasis (i.e., distant skin, subcutaneous, or nodal metastases; lung metastases; any other visceral metastases; or any distant metastasis)
Most patients (84%) with melanoma initially present with stage I or II (localized) disease; 8% have stage III (regional) disease; and 4% have stage IV disease (distant metastases).3 Of the patients who present with localized melanoma, however, up to 12% will eventually develop distant metastases.13–15 The survival rate for patients with stage IV melanoma is low; from 2001 to 2007, 85% of patients with distant metastases were not expected to survive 5 years. 3 The median survival of patients with stage IV disease is less than 1 year.16,17
Surgical excision is the standard treatment for localized melanoma. Patients with regional (stage III) disease are also candidates for local excision of the primary tumor, in tandem with complete lymph node dissection; however, stage III disease is sometimes unresectable. Patients with unresectable stage III melanoma are typically referred to clinical trials of alternatives to complete lymph node dissection, such as careful observation with nodal ultrasound.4,5 Current guidelines recommend resection, observation, or systemic therapy for patients with resectable stage IV melanoma. Those with unresectable (disseminated) stage IV disease are treated with systemic therapy, radiation, and best supportive care.5 Unfortunately, melanoma is refractory to most standard systemic treatments.4
For more than 30 years, single-agent dacarbazine (DTIC, Dome/Bayer) has been the standard of care for advanced melanoma.18 High-dose aldesleukin (Proleukin, Prometheus), a human recombinant interleukin-2 (IL-2) product, is also approved for use in this setting.19,20 Neither agent, however, has demonstrated a significant effect on overall survival in randomized trials.4
Temozolomide (Temodar, Schering/Merck), an analogue of dacarbazine, was no more effective than dacarbazine in a phase 3 comparison study, but because of its oral availability, it is often used in an off-label fashion to treat metastatic melanoma. 18,21 There is currently no standard of care for melanoma patients who do not tolerate first-line treatments or who have progressive disease.22,23
Two monoclonal antibodies––tremelimumab and ipilimumab––were investigated as potential treatments for advanced melanoma. In April 2008, Pfizer announced that it had discontinued a phase 3 clinical trial of tremelimumab for patients with advanced melanoma after a review of interim data indicated that the study would not demonstrate superiority over standard chemotherapy. 24 Ipilimumab therapy, however, led to improved survival in a pivotal phase 3 study of patients with previously treated metastatic melanoma.25
In March 2011, ipilimumab (Yervoy, Bristol-Meyers Squibb) gained FDA approval for the treatment of metastatic melanoma, representing the first new treatment for advanced melanona in more than a decade.26,27 Ipilimumab was given a fast-track designation because of its potential to address an unmet medical need—prolonging survival. The time from submission to approval was 9 months.28
After the approval, ipilimumab was promptly added as a category 1 recommendation in the National Comprehensive Cancer Network (NCCN) guidelines of systemic therapy options for advanced or metastatic melanoma.5
CHEMICAL AND PHYSICAL PROPERTIES
Ipilimumab is an immunoglobulin G1 (IgG1)–kappa immunoglobulin produced in mammalian (Chinese hamster ovary) cell culture. It is supplied as a sterile, preservative-free solution for intravenous (IV) infusion in single-use vials of 50 mg/10 mL and 200 mg/40 mL.
Each milliliter contains 5 mg of ipilimumab and the following inactive ingredients: diethylene triamine pentaacetic acid (DTPA), mannitol, polysorbate 80 (vegetable origin), sodium chloride, tris hydrochloride, and Water for Injection, USP at a pH of 7.29
MECHANISM OF ACTION
Because tumors express tumorassociated antigens, it is theoretically possible to mount an effective immune response via antibody therapy.23 Ipilimumab is a recombinant, human monoclonal antibody that binds to cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and blocks the interaction of CTLA-4 with its ligands, CD80 and CD86.29 The CTLA-4 molecule serves as an “immune checkpoint” that down-regulates pathways of T-cell activation and prevents autoimmunity.30 By blocking this function, ipilimumab potentiates the antitumor T-cell response, resulting in unrestrained T-cell proliferation.25,31–34 Thus, the mechanism of action of ipilimumab’s effect in patients with melanoma is indirect, possibly through T-cell mediated antitumor immune responses.29
PHARMACOKINETICS
The pharmacokinetic characteristics of ipilimumab were studied in 499 patients with unresectable or metastatic melanoma who received 0.3, 3, or 10 mg/kg once every 3 weeks for four doses. Peak plasma (Cmax), trough (Cmin), and area-under-the-curve (AUC) concentrations of ipilimumab were dose-proportional within the dose range examined. The steady-state concentration of ipilimumab was reached by the third dose.29
On average, the elimination half-life of ipilimumab is 14.7 days. The drug’s systemic clearance is 15.3 mL/hour, and the volume of distribution is low (7.21 L). The mean ipilimumab Cmin achieved at steady state with 3 mg/kg (the approved dose) was 21.8 mcg/mL.29
Specific Populations
Pharmacokinetic data were obtained from cross-study analyses of patients with a variety of conditions, including 420 patients with melanoma who received single or multiple infusions of ipilimumab at doses of 0.3, 3, or 10 mg/kg. In these analyses, ipilimumab clearance increased with increasing body weight; however, no dose adjustment of ipilimumab is required for body weight after administration on a milligram-per-kilogram basis. The following factors had no clinically meaningful effect on the drug’s clearance: age (range, 26–86 years), sex, concomitant use of budesonide (e.g., Pulmicort, AstraZeneca), performance status, human leukocyte antigen (HLA-A2*0201) status, positive anti-ipilimumab antibody status, previous use of systemic anticancer therapy, or baseline lactate dehydrogenase (LDH) levels. The effect of race on ipilimumab clearance was not evaluated.29
Renal Impairment
Creatinine clearance at baseline did not have a clinically important effect on the pharmacokinetics of ipilimumab in patients with serum creatinine clearance values of 29 mL/minute or greater.29
Hepatic Impairment
Baseline aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin levels did not have a clinically important effect on ipilimumab pharmacokinetics in patients with various degrees of hepatic impairment.29
SAFETY PROFILE
Because ipilimumab stimulates the unrestrained proliferation of T cells, it is associated with a substantial risk of immune-related adverse reactions.5,29 In clinical trials, more than 80% of patients treated with ipilimumab reported adverse events.25,35–39 Immune-related adverse reactions of grade 3 or higher occurred in 10% to 26% of treated patients.37–39 The NCCN guidelines note that ipilimumab should be used with extreme caution, if at all, in patients with serious underlying autoimmune disorders.5
Boxed Warning
The prescribing information for ipilimumab includes a boxed warning regarding the potential for severe and fatal immune-mediated adverse reactions resulting from T-cell activation and proliferation. These reactions may involve any organ system; however, the most common severe immune-mediated adverse reactions are enterocolitis, hepatitis, dermatitis (including potentially fatal toxic epidermal necrolysis [TEN]), neuropathy, and endocrinopathy. Most of these immune-mediated reactions are initially manifested during treatment, although a few can occur weeks to months after ipilimumab has been discontinued.29
Warnings and Precautions
Immune-mediated enterocolitis. In the pivotal phase 3 study of ipilimumab in patients with previously treated metastatic melanoma, severe, life-threatening, or fatal immune-mediated enterocolitis (grades 3 to 5) occurred in 34 (6.7%) of 511 patients treated with ipilimumab, either alone or with a vaccine containing glycoprotein 100 (gp100). Moderate enterocolitis (grade 2) occurred in 28 patients (5.5%). Among the 511 treated patients, 26 (5.1%) were hospitalized for severe enterocolitis; five (1%) developed intestinal perforation; and four (0.8%) died as a result of complications.29
Immune-mediated hepatitis. In the pivotal study, severe, life-threatening, or fatal hepatotoxicity (grades 3 to 5) occurred in eight (1.6%) of the 511 treated patients. Fatal hepatic failure and hospitalization occurred in one (0.2%) and two (0.4%) of these patients, respectively. An additional 13 patients (2.5%) experienced moderate hepatotoxicity (grade 2), as manifested by abnormal liver function test results. In some patients, the underlying pathology included immune-mediated hepatitis.29
Immune-mediated dermatitis. In the pivotal study, severe, life-threatening, or fatal immune-mediated dermatitis (grades 3 to 5) occurred in 13 (2.5%) of the 511 ipilimumab-treated patients. One patient (0.2%) died as a result of TEN, and one patient (0.2%) required hospitalization for severe dermatitis. Sixty-three patients (12%) had moderate (grade 2) dermatitis. In addition, one case of fatal Guillain-Barré syndrome and one case of severe (grade 3) peripheral motor neuropathy were reported.29
Immune-mediated endocrinopathies. In the pivotal trial, severe or life-threatening immune-mediated endocrinopathies (grade 3 or 4) occurred in nine (1.8%) of the 511 ipilimumab patients. Moderate endocrinopathies (grade 2) occurred in 12 patients (2.3%), including hypothyroidism, adrenal insufficiency, and hypopituitarism.29
Other immune-mediated adverse reactions. In the pivotal trial, nephritis, pneumonitis, meningitis, pericarditis, uveitis, iritis, and hemolytic anemia occurred in fewer than 1% of the 511 ipilimumab-treated patients.29
In the clinical development program for ipilimumab, the following potentially immune-mediated adverse reactions were reported with an incidence of less than 1%: myocarditis, angiopathy, temporal arteritis, vasculitis, polymyalgia rheumatica, conjunctivitis, blepharitis, episcleritis, scleritis, leukocytoclastic vasculitis, erythema multiforme, psoriasis, pancreatitis, arthritis, and autoimmune thyroiditis.29
Some reports have also associated ipilimumab with the occurrence of severe (grade 4) thrombocytopenia,40 pulmonary sarcoidosis,41 and hemophilia A42 in patients with metastatic melanoma.
Management of Immune-Mediated Side Effects
Most of the immune-mediated side effects associated with ipilimumab are reversible with early diagnosis and appropriate management (Table 1).29,43 The prescribing information for ipilimumab recommends that systemic corticosteroid therapy be initiated at a dosage of prednisone 1 to 2 mg/kg per day or equivalent for severe immune-mediated adverse reactions.29
Table 1.
Recommended Strategies for Managing the Immune-Mediated Adverse Effects of Ipilimumab
| Side Effect | Management |
|---|---|
| Immune-mediated enterocolitis |
|
| Immune-mediated hepatitis |
|
| Immune-mediated dermatitis |
|
| Immune-mediated neuropathies | |
| Immune-mediated endocrinopathies |
|
| Other immune-mediated adverse reactions |
|
Stevens–Johnson syndrome, toxic epidermal necrolysis, or rash complicated by full-thickness dermal ulceration, or necrotic, bullous, or hemorrhagic manifestations.
Not interfering with daily activities.
Interfering with daily activities (e.g., Guillain-Barré-like syndromes).
Including uveitis, iritis, and episcleritis.
Adapted from Yervoy (ipilimumab) prescribing information.29
In the phase 3 trial, immune-related diarrhea (grade 2 or higher) resolved within a median period of 2.3 weeks after the administration of corticosteroids in 14 of 15 patients who received ipilimumab monotherapy. In addition to corticosteroids, four patients with immune-related diarrhea of grade 3 or higher received infliximab (Remicade, Ortho Janssen), an anti-tumor necrosis factor–alpha antibody.25
Common Adverse Reactions
In studies of patients with unresectable or metastatic melanoma, the most common adverse reactions associated with ipilimumab 3 mg/kg in 5% or more patients were fatigue, diarrhea, pruritus, rash, and colitis.29
Eleven of 1,024 evaluable patients (1.1%) tested positive for binding antibodies against ipilimumab in an electrochemiluminescent (ECL)-based assay. Infusion-related or peri-infusional reactions consistent with hypersensitivity or anaphylaxis were not reported in these 11 patients, and neutralizing antibodies against ipilimumab were not detected.29
Because trough levels of ipilimumab interfere with the ECL assay results, a subset analysis was performed in the dose cohort with the lowest trough levels. In this analysis, four of 58 evaluable patients (6.9%) who received ipilimumab 0.3 mg/kg, tested positive for binding antibodies against the drug.29
Drug Interactions
No formal drug–drug interaction studies have been conducted with ipilimumab. 29 However, the drug’s metabolism of ipilimumab does not appear to involve the cytochrome P450 (CYP) enzyme system; therefore, the potential for drug–drug interactions is low.22 Interactions of ipilimumab with the gp100 vaccine have not been investigated.22
Safety in Specific Populations
Ipilimumab, a Pregnancy Category C drug, has not been studied extensively in pregnant women. Ipilimumab should be used during pregnancy only if the potential benefits justify the potential risks to the fetus.29
Human IgG1 crosses the placental barrier; therefore, ipilimumab has the potential to be transmitted from the mother to the developing fetus. It is not known whether ipilimumab is secreted in human milk.29
The safety and effectiveness of ipilimumab have not been established in pediatric patients.29
Of 511 patients treated with ipilimumab 3 mg/kg, 28% were 65 years of age and older. No overall differences in safety or efficacy were reported between patients 65 years of age and older and patients younger than 65 years of age.29
No formal studies of ipilimumab have been conducted in patients with renal or hepatic impairment.29
CLINICAL EFFICACY
Pivotal Phase 3 Trial
Pivotal efficacy data for ipilimumab in metastatic melanoma was provided by a randomized double-blind, double-dummy study that included 676 patients with unresectable stage III or IV melanoma. These patients had previously received one or more of the following agents: aldesleukin (Proleukin), dacarbazine (DTIC), temozolomide (Temodar), fotemustine (e.g., Muphoran, Servier; not available in the U.S.), or carboplatin (Paraplatin, Bristol-Myers Squibb).
A total of 403 patients were randomly assigned to receive ipilimumab 3 mg/kg in combination with an investigational vaccine consisting of HLA-A*0201–restricted gp100 peptides with incomplete Freund’s adjuvant. The remaining patients received either ipilimumab 3 mg/kg alone (n = 137) or the gp100 vaccine alone (n = 136).25,29
Antigenic gp100 peptides are present in melanoma cells and are recognized by T lymphocytes.44 In a meta-analysis of vaccine trials conducted by the National Cancer Institute, monotherapy with the investigational gp100 vaccine induced immune responses in patients with metastatic melanoma, but antitumor activity was virtually nonexistent.45 Another study suggested that concomitant use of the gp100 vaccine may increase the antitumor efficacy of high-dose interleukin-2 in patients with metastatic melanoma.46 Because no standard of care exists for treating advanced melanoma in previously treated patients, the investigational gp100 vaccine served as the active control in the pivotal trial of ipilimumab.25,29 This study did not include an observation arm or comparison with conventional chemotherapy. 44
Moreover, the pivotal phase 3 trial of ipilimumab enrolled only patients with a positive status for the HLA-A2* 0201genotype, as this genotype facilitates the immune presentation of the gp100 vaccine.25,29 Ipilimumab’s ability to block CTLA-4, however, appears to be independent of a patient’s HLA status.44 Approximately 50% of Caucasian populations express the HLA-A*0201 allele.47
Patients with active autoimmune disease and those receiving systemic immunosuppression for organ transplantation were excluded from the study.29
Ipilimumab plus gp100 placebo was administered at 3 mg/kg as an IV infusion every 3 weeks for four doses. The gp100 vaccine plus ipilimumab placebo was administered at a dose of 2 mg peptide by deep subcutaneous injection every 3 weeks for four doses.25,29 Tumor responses were assessed at weeks 12 and 24, and every 3 months thereafter. Patients with evidence of an objective tumor response at 12 or 24 weeks were assessed for confirmation of durability of response at 16 or 28 weeks, respectively.25,29
The primary efficacy endpoint was overall survival in patients receiving ipilimumab plus the gp100 vaccine compared with patients receiving only the vaccine. The original primary endpoint of this study was the best overall objective response rate (i.e., the proportion of patients with complete or partial reduction of tumor masses). Prior to unblinding, this endpoint was changed to reflect phase 2 data, which had identified potential limitations to traditional response-based endpoints in studies involving the use of immunotherapies48,49 and to align the trial with another ongoing phase 3 study of ipilimumab in patients with previously untreated metastatic melanoma (see page 508).25,50,51
Secondary efficacy outcome measures included overall survival in the ipilimumab/gp100 arm versus the ipilimumab arm; overall survival in the ipilimumab arm versus the gp100 arm; the best over-all response rate at week 24 between each of the study arms; and the duration of response.25,29
Of the 676 enrolled patients, 56% were men; 29% were 65 years of age or older (mean age, 56 years). Of the patients, 71% had stage M1c disease; 12% had a history of previously treated brain metastasis; 98% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; 23% had received aldesleukin; and 38% had elevated LDH levels. Sixty-one percent of patients randomized to either of the ipilimumab-containing arms received all four planned doses.25,29
The median duration of follow-up was 8.9 months.29
The median overall survival in the ipilimumab + gp100 arm was 10.0 months (95% confidence interval [CI], 8.5–11.5) compared with 6.4 months (95% CI, 5.5–8.7) in the gp100 arm (hazard ratio [HR] for death, 0.68; P = 0.0004) (Table 2).
Table 2.
Overall Survival Results in Pivotal Phase 3 Trial of Ipilimumab in Patients With Previously Treated, Unresectable Advanced Melanoma
| Ipilimumab Alone (n = 137) | Ipilimumab + gp100 Vaccine (n = 403) | gp100 Vaccine Alone (n = 136) | |
|---|---|---|---|
| HR vs. gp100 vaccine (95% CI) | 0.66 (0.51–0.87) P = 0.0026* |
0.68 (0.55–0.85) P = 0.0004 |
|
| HR vs. ipilimumab (95% CI) | 1.04 (0.83–1.30) P = 0.76 |
||
| Median, months (95% CI) | 10.1 (8.3–13.8) | 10.0 (8.5–11.5) | 6.4 (5.5–8.7) |
The median overall survival in the ipilimumab arm was 10.1 months (95% CI, 8.0–13.8) (HR for death with ipilimumab alone compared with gp100 alone, 0.66; P = 0.0026). No difference in overall survival was observed between the two ipilimumab arms (HR for death with ipilimumab + gp100, 1.04; P = 0.76).25,29 The rates of overall survival are listed by assessment time points in Table 3.
Table 3.
Overall Survival Rates (%) in Pivotal Phase 3 Trial of Ipilimumab in Patients With Previously Treated, Unresectable Advanced Melanoma
| Time Points | Ipilimumab Alone (n = 137) | Ipilimumab + gp100 Vaccine (n = 403) | gp100 Vaccine Alone (n = 136) |
|---|---|---|---|
| 12 months | 45.6 | 43.6 | 25.3 |
| 18 months | 33.2 | 30.0 | 16.3 |
| 24 months | 23.5 | 21.6 | 13.7 |
gp100 = glycoprotein 100.
Data based on text from Hodi FS, O’Day SJ, McDermott DF, et al. N Engl J Med 2010;363:211–223.25
At week 12, the rates of progression-free survival were 49.1% with ipilimumab/gp100, 57.7% with ipilimumab, and 48.5% with gp100. Ipilimumab monotherapy reduced the risk of disease progression by 36% compared with gp100 alone (HR, 0.64; P < 0.001), and ipilimumab/gp100 reduced the risk of progression by 19% compared with gp100 alone (HR, 0.81; P < 0.05).25
At the end of the study, the best overall response rates were 5.7% in the ipilimumab/gp100 arm, 10.9% in the ipilimumab arm, and 1.5% in the gp100 arm. The median duration of response was 11.5 months in the ipilimumab/gp100 arm and had not been reached in the ipilimumab or the gp100 arm.25,29
The highest percentage of patients with an objective response or stable disease was the group that had received ipilimumab monotherapy; these patients had a disease control rate (the proportion of patients with a partial or complete response or stable disease) of 28.5% compared with 20.0% in the ipilimumab/gp100 group and 11.1% in the gp100 group (Table 4).
Table 4.
Best Overall Response (%) in Pivotal Phase 3 Trial of Ipilimumab in Patients With Previously Treated, Unresectable Advanced Melanoma
| Ipilimumab Alone (n = 137) | Ipilimumab + gp100 Vaccine (n = 403) | gp100 Vaccine Alone (n = 136) | |
|---|---|---|---|
| Complete response | 1.5 | 0.2 | 0 |
| Partial response | 9.5 | 5.5 | 1.5 |
| Stable disease | 17.5 | 14.4 | 9.6 |
| Progressive disease | 51.1 | 59.3 | 65.4 |
| Not evaluated | 20.4 | 20.6 | 23.5 |
gp100 = glycoprotein 100.
Data based on text from Hodi FS, O’Day SJ, McDermott DF, et al. N Engl J Med 2010;363:211–223.25
In the group receiving ipilimumab alone, nine of 15 patients (60%) maintained an objective response for at least 2 years. In the ipilimumab/gp100 group, four of 23 patients (17.4%) maintained the response for at least 2 years.25
This study is significant as the first randomized trial to show a survival benefit in patients with advanced melanoma.43 Based on these results, the FDA approved ipilimumab at a dose of 3 mg/kg for patients with previously treated, unresectable advanced melanoma.29 Because the gp100 vaccine did not appear to improve the efficacy of ipilimumab, the FDA approved ipilimumab alone, thereby avoiding the additional costs and possible toxicities of the vaccine.22
Ipilimumab Plus Dacarbazine For Metastatic Melanoma
A second randomized phase 3 study investigated ipilimumab plus dacarbazine in patients with previously untreated disease. A total of 502 patients received ipilimumab 10 mg/kg plus dacarbazine 850 mg/m2 or dacarbazine plus placebo, administered at weeks 1, 4, 7, and 10, followed by dacarbazine alone every 3 weeks through week 22. Patients who achieved stable disease or an objective response without dose-limiting toxicities were given ipilimumab or placebo every 12 weeks thereafter as maintenance therapy.50
As in the pivotal phase 3 study, the primary endpoint of this trial was amended from progression-free survival to overall survival before unblinding to reflect phase 2 data, which had indicated that traditional response-based endpoints might not be applicable to immunotherapy studies.48,49
The estimated rates of overall survival for ipilimumab plus dacarbazine versus dacarbazine plus placebo were 47.3% vs. 36.3% at 1 year; 28.5% vs. 17.9% at 2 years; and 20.8% vs. 12.2% at 3 years (HR for death with ipilimumab/dacarbazine, 0.72; P < 0.001).
The risk of disease progression was reduced by 24% in the ipilimumab/dacarbazine patients compared with those receiving dacarbazine/placebo (HR for progression, 0.76; P = 0.006). Rates of disease control were similar between the two groups (33.2% for ipilimumab/dacarbazine and 30.2% for dacarbazine/placebo; P = 0.41).50
Immune-mediated adverse events occurred in 192 of 247 patients (77.7%) who received ipilimumab plus dacarbazine, compared with 96 of 251 (38.2%) of those treated with dacarbazine plus placebo. In the ipilimumab/dacarbazine group, immune-related adverse events were grade 3 in 31.6% of patients and grade 4 in 10.1% of patients. The most common immune-related adverse events in the ipilimumab/dacarbazine patients included diarrhea (32.8%), elevated ALT levels (29.1%), elevated AST levels (26.7%), pruritus (26.7%), and rash (22.3%).50
This study was an important first step in positioning ipilimumab as a viable candidate for first-line therapy in patients with previously untreated advanced melanoma. However, the patients in this study were treated with the higher 10-mg/kg dose of ipilimumab, not the currently approved dose of 3 mg/kg.43
DOSAGE AND ADMINISTRATION
The recommended regimen for ipilimumab is 3 mg/kg administered intravenously over 90 minutes every 3 weeks for a total of four doses.29
The current prescribing information recommends that ipilimumab be permanently discontinued if (1) persistent moderate adverse reactions occur; (2) the corticosteroid dosage cannot be reduced to 7.5 mg of prednisone or equivalent per day; (3) the patient fails to complete the full treatment course within 16 weeks after receiving the first dose; or (4) severe or life-threatening reactions occur. 29
Clinicians should be aware that responses to ipilimumab may be delayed for months5 and that they can even occur after the initial progression or development of new lesions.52 Patients should be initially evaluated for response after 12 weeks of therapy (at the end of the treatment cycle).22 Repeated doses are not recommended in the current product labeling.29
COST ANALYSIS
Bristol-Myers Squibb is pricing ipilimumab at $30,000 per injection.22,23 This translates to a cost of $120,000 for a course of therapy, based on the approved dosing regimen of 3 mg/kg every 3 weeks for four doses. The company’s patient-assistance program, however, may reduce net pricing to $80,000.23 Nevertheless, ipilimumab has been criticized in the media as an overly expensive new drug,53,54 especially at a time when all eyes are on health care spending.
In the pivotal phase 3 trial, the median survival for patients receiving 3 mg/kg was 10.1 months compared with 6.4 months for those receiving the investigational gp100 vaccine––a difference of 3.7 months in favor of ipilimumab.25,29 Thus, at the full price of $120,000 for a course of therapy, the average cost per month of added survival would have been approximately $32,432. This is approximately 40% higher than the cost of the only other immunotherapy for cancer, sipuleucel-T (Provenge, Dendreon Corp.),53 which was approved for men with metastatic castrate-resistant prostate cancer in April 2010.55 Sipuleucel-T is administered in three cycles, compared with four cycles for ipilimumab.28,56
Ipilimumab’s hefty price tag has raised eyebrows in the United Kingdom as well as in the U.S. In September 2011, after reviewing data submitted by Bristol-Myers Squibb, Britain’s National Center for Pharmacoeconomics stated:57
We believe the Company has failed to demonstrate the cost-effectiveness of ipilimumab for the treatment of advanced melanoma in adult patients who received prior therapy. We cannot recommend reimbursement at the submitted price.
The following month, ipilimumab was turned down by the taxpayer-funded National Health Service. In addition, the influential National Institute for Health and Clinical Excellence (NICE) declined to recommend the drug on the grounds that its longer-term benefits were unclear.58
SUPPLY CONSTRAINTS
Because of the severe and potentially fatal immune-mediated adverse reactions associated with ipilimumab, a Risk Evaluation and Mitigation Strategy (REMS) was mandated by the FDA to inform health care professionals about these serious risks.59,60 A medication guide is also provided to patients to inform them about the drug’s potential side effects.29
To ensure compliance with REMS and the safe use of ipilimumab, Bristol-Myers Squibb has instituted a restricted distribution model for this drug.54,61 For oncology and office-based settings, ipilimumab, as of this writing, is available only from Cardinal Health, McKesson Specialty Care, and Oncology Supply. Hospitals and infusion centers may obtain the drug only from Cardinal Health and McKesson Plasma & Biologics.61
There has been concern that these supply constraints may place an undue financial burden on hospital pharmacies, which might lose millions of dollars in volume discounts that they would otherwise earn through existing wholesalers.54 The company, however, states that the system for obtaining ipilimumab is not a “closed” distribution model and that it is seeking to expand its list of distributors.54
POTENTIAL COMPETITORS
As the first immunotherapy to improve survival in patients with advanced melanoma, ipilimumab currently has the stage all to itself––but potential competition is waiting in the wings. One immunotherapeutic drug for advanced melanoma has been evaluated in a pivotal phase 3 trial, and three others are in active phase 3 development (Table 5).54,62–72
Table 5.
Immunotherapies Currently in Phase 3 Development for Advanced Melanoma
| Company | Product | Type |
|---|---|---|
| Vical/AnGes MG | Allovectin-7 | Allogeneic, plasmid DNA/lipid complex |
| GlaxoSmithKline | MAGE-A3 ASCI | Allogeneic, peptide |
| Avax Technologies | M-Vax | Autologous, whole-cell, hapten-modified |
| Amgen (through acquisition of BioVex Group) | T-VEC (formerly OncovexGM-CSF; talimogene laherparepvec) | Allogeneic, oncolytic herpes simplex virus encoding GM–CSF |
MAGE-A3. Mitogen-associated antigen-3 (MAGE-A3) is a cancer germline gene that encodes tumor-specific antigens recognized by T cells.62 It is present in 76% of metastatic melanoma and in many other tumors.62 GlaxoSmithKline has developed an antigen-specific cancer immunotherapeutic (ASCI) agent that combines the tumor-specific MAGE-A3 antigen, delivered as a recombinant protein, with a potent immunostimulant (AS15).63 A maximum of 13 doses are given by intramuscular injection over a period of 27 months.64
The phase 3 DERMA trial evaluated MAGE-A3 ASCI in 832 patients with unresectable MAGE-A3–positive melanoma with lymph node involvement (stage IIIb/c disease).63,64 The study was completed in January 2011,65 and a preliminary report was presented at the American Society of Clinical Oncology (ASCO) 2011 Annual Meeting.64
Allovectin-7. Another pivotal phase 3 trial is comparing Allovectin-7 (Vical/AnGes MG), a plasmid DNA/lipid complex,66 with dacarbazine and temozolomide in 375 patients with chemotherapy-naive, recurrent stage III or IV melanoma.67,68 The study is expected to be completed in late 2012.67 Allovectin-7 is injected directly into accessible melanoma lesions.66
T-VEC. Amgen’s talimogene laherparepvec (T-VEC, formerly OncovexGM-CSF) is an oncolytic herpes virus that encodes granulocyte macrophage–colony-stimulating factor (GM–CSF). Like Allovectin-7, it is injected directly into the tumor.69 The ongoing phase 3 OncovexGM-CSF Pivotal Trial in Melanoma (OPTiM) is comparing T-VEC with subcutaneously administered GM–CSF in 430 patients with unresectable advanced melanoma (stage IIIb/c or stage IV).69,70 Enrollment is completed, and results are anticipated in 2013.70
M-Vax vaccine. M-Vax (Avax Technologies), a therapeutic melanoma vaccine, consists of autologous melanoma cells that have been irradiated and modified with the hapten dinitrophenyl (DNP).71,72 M-Vax is administered via subcutaneous injection. A phase 3 trial is being conducted to evaluate M-Vax in 387 patients with stage IV melanoma and lung and/or soft-tissue metastasis. To increase the vaccine’s effectiveness, administration of M-Vax is being followed by low doses of IL-2. The study’s expected completion date is January 2014.72
CLINICAL CONSIDERATIONS
As the first drug to demonstrate a survival benefit in advanced melanoma in a randomized trial and the first to be approved by the FDA for the treatment of advanced melanona in more than a decade, ipilimumab represents a major step forward in cancer immunotherapy. The initial enthusiasm surrounding the drug’s introduction, however, has been tempered by concerns about its severe immune-mediated adverse effects,29 high cost,53,54 and restricted distribution.54,61
Moreover, in the pivotal phase 3 trial conducted in previously treated patients with advanced melanoma, the best overall response rate was only 11% for ipilimumab. 25 This was consistent with best overall response rates of 4% to 16% in phase 2 studies of ipilimumab monotherapy (Table 6).36–39
Table 6.
Results of Phase 2 Clinical Trials of Ipilimumab Monotherapy in Patients With Unresectable Stage III or IV Melanoma
Ipilimumab, therefore, appears to be clinically ineffective in a high proportion of treated patients. Considering the drug’s daunting cost and problematic safety profile, predictive biomarkers are needed to allow the pretreatment selection of patients who are most likely to derive clinical benefit from ipilimumab. Unfortunately, such biomarkers have yet to be identified.43
CONCLUSION
Despite these limitations, it is likely that oncologists will embrace ipilimumab as a bright light in an area of oncology that has been darkened by failed treatments and a dearth of innovation for more than a decade. At the very least, the door has been opened to a new era of melanoma therapy based on molecular targets.
REFERENCES
- 1.National Cancer Institute A snapshot of melanoma. Oct, 2011. Available at: www.cancer.gov. Accessed January 16, 2012.
- 2.World Health Organization Skin cancers. Available at: www.who.int/uv/faq/skincancer/en/index1.html. Accessed January 16, 2012.
- 3.National Cancer Institute SEER stat fact sheets: Melanoma of the skin. Available at: http://seer.cancer.gov/statfacts/html/melan.html. Accessed January 16, 2012.
- 4.National Cancer Institute Melanoma treatment (PDQ) Aug, 2011. Available at: www.cancer.gov. Accessed January 17, 2012.
- 5.National Comprehensive Cancer Network NCCN practice guidelines in oncology: Melanoma (version 3.2012) Dec 1, 2011. Available at: www.nccn.com. Accessed January 16, 2012.
- 6.American Cancer Society Melanoma skin cancer. Available at: www.cancer.org. Accessed January 16, 2012.
- 7.Cancer Trends Progress Report. Available at: http://progressreport.cancer.gov. Accessed January 16, 2012.
- 8.Naeyaert JM, Brochez L. Clinical practice: Dysplastic nevi. N Engl J Med. 2003;349:2233–2240. doi: 10.1056/NEJMcp023017. [DOI] [PubMed] [Google Scholar]
- 9.Rigel DS, Rivers JK, Kopf AW, et al. Dysplastic nevi: Markers for increased risk for melanoma. Cancer. 1989;63:386–389. doi: 10.1002/1097-0142(19890115)63:2<386::aid-cncr2820630231>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Ivry GB, Ogle CA, Shim EK. Role of sun exposure in melanoma. Dermatol Surg. 2006;32:481–492. doi: 10.1111/j.1524-4725.2006.32101.x. [DOI] [PubMed] [Google Scholar]
- 11.FDA Consumer Health Information Indoor tanning: The risks of ultraviolet rays. May, 2010. Available at: www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM190664.pdf. Accessed January 16, 2012.
- 12.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretschmer L, Beckmann I, Thoms KM, et al. Factors predicting the risk of intransit recurrence after sentinel lymphonodectomy in patients with cutaneous malignant melanoma. Ann Surg Oncol. 2006;13:1105–1112. doi: 10.1245/ASO.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy WH, Shaw HM, Thompson JF, Milton GW. Time and frequency of recurrence of cutaneous stage I malignant melanoma with guidelines for follow-up study. Surg Gynecol Obstet. 1988;166:497–502. [PubMed] [Google Scholar]
- 15.Roses DF, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision for primary cutaneous malignant melanoma. Ann Surg. 1983;198:65–69. doi: 10.1097/00000658-198307000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 17.Agarwala SS. Current systemic therapy for metastatic melanoma. Exp Rev Anticancer Ther. 2009;9:587–595. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- 18.Fricker J. New era in metastatic melanoma. Mol Oncol. 2010;4:91–97. [Google Scholar]
- 19.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 20.Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: Long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 21.Quirbt I, Verma S, Petrella T, et al. Temozolomide for the treatment of metastatic melanoma. Curr Oncol. 2007;14:27–33. doi: 10.3747/co.2007.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culver ME, Gatesman ML, Mancl EE, Lowe DK. Ipilimumab: A novel treatment for metastatic melanoma. Ann Pharmacother. 2011;45:510–519. doi: 10.1345/aph.1P651. [DOI] [PubMed] [Google Scholar]
- 23.Sondak VK, Smalley KSM, Kuchadkar R, et al. Ipilimumab. Nat Rev Drug Discov. 2011;10:411–412. doi: 10.1038/nrd3463. [DOI] [PubMed] [Google Scholar]
- 24.Pfizer announces discontinuation of phase III clinical trial for patients with metastatic melanoma. Apr 1, 2008. Available at: www.biospace.com/News/pfizer-inc-announces-discontinuation-of-phase-iii/91279. Accessed August 8, 2012.
- 25.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:211–223. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA approves new treatment for a type of late-stage skin cancer. Mar 25, 2011. Available at: www.fda.gov/newsevents/newsroom/pressannouncements/ucm1193237.htm. Accessed January 18, 2011.
- 27.Melanoma Research Foundation Approval of new melanoma therapy changes landscape for patients. Mar 25, 2011. Available at: www.melanoma.org. Accessed August 8, 2012.
- 28.FDA . FY 2011 Innovative Drug Approvals. Silver Spring; Md: Nov, 2011. Section A4: ipilimumab. Available at: www.fda.gov/downloads/aboutfda/reports-manualsforms/reports/ucm278358.pdf. Accessed January 25, 2012. [Google Scholar]
- 29.Yervoy (ipilimumab) Injection, prescribing information. Princeton, N.J: Bristol-Myers Squibb; Mar, 2011. Available at: http://packageinserts.bms.com. Accessed January 18, 2012. [Google Scholar]
- 30.Melero I, Hervas-Stubbs S, Glennie M, et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 31.Hwu P. Treating cancer by targeting the immune system. N Engl J Med. 2010;363:779–781. doi: 10.1056/NEJMe1006416. [DOI] [PubMed] [Google Scholar]
- 32.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): A novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–2627. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 33.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: The first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 34.Robert C, Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist. 2009;14:848–861. doi: 10.1634/theoncologist.2009-0028. [DOI] [PubMed] [Google Scholar]
- 35.Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 36.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomized, double-blind, multicentre, phase 2, dose ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 37.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 38.Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 39.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad S, Lewis M, Corrie P, Iddawela M. Ipilimumab-induced thrombocytopenia in a patient with metastatic melanoma. J Oncol Pharm Pract. 2012;18(2):287–292. doi: 10.1177/1078155211411001. [DOI] [PubMed] [Google Scholar]
- 41.Vogel WV, Guislain E, Kvistborg P, et al. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol. 2012;30:e7–e10. doi: 10.1200/JCO.2011.37.9693. [DOI] [PubMed] [Google Scholar]
- 42.Delyon J, Mateus C, Lambert T. Hemophilia A induced by ipilimumab (Letter) N Engl J Med. 2011;365:1747–1748. doi: 10.1056/NEJMc1110923. [DOI] [PubMed] [Google Scholar]
- 43.Lipson EJ, Drake CG. Ipilimumab: An anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Retsas S. Latest developments in the treatment of melanoma: ‘A penicillin moment for cancer’? J R Soc Med. 2011;104:269–272. doi: 10.1258/jrsm.2011.100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartzentruber DJ, Lawson D, Richards J, et al. A phase 3 multi-institutional randomized study of immunization with the gp100:209–217 (210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma (Abstract) J Clin Oncol. 2009;27(Suppl):463s. [Google Scholar]
- 47.Sun Y, Song M, Stevamovic S, et al. Identification of a new HLA-A*0201-restricted T-cell epitope from the tyrosinase related protein 2 (TRP2) melanoma antigen. Int J Cancer. 2000;87:399–404. doi: 10.1002/1097-0215(20000801)87:3<399::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 49.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 51.Robert C, Wolchok JD. Ipilimumab plus dacarbazine in melanoma (authors’ reply) N Engl J Med. 2011;365:1257. doi: 10.1056/NEJMc1108661. [DOI] [PubMed] [Google Scholar]
- 52.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: Patient cases. Cancer Immun. 2008;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 53.Similarities between two immune therapies in cancer. Life Sci Digest. Mar 30, 2012. Available at: http://lifesciencedigest.com. Accessed August 8, 2012.
- 54.Bronstein D. Who benefits the most from ipilimumab restricted distribution? Some question safety/REMS rationale. Pharm Pract News. 2011;38(8) [Google Scholar]
- 55.FDA approval letter for Provenge Apr 29, 2010. Available at: www.fda.gov/BiologicsBloodVaccines/CellularGene-TherapyProducts/ApprovedProducts/ucm210215.htm. Accessed January 24, 2012.
- 56.Provenge (sipuleucel-T), prescribing information. Seattle, Wash.: Dendreon Corp.; June 2011. Available at: www.dendreon.com. Accessed January 24, 2012.
- 57.National Centre for Pharmacoeconomics Pharmacoeconomic evaluation of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adult patients who have received prior therapy. Sep, 2011. Available at: www.ncpe.ie/u_docs/doc_216.pdf. Accessed January 24, 2012.
- 58.Kelland K. UPDATE 1. UK cost agency rejects Bristol-Myers’ skin cancer drug. Oct 14, 2011. Available at: wwwreuterscom/article/2011/10/14/bmsyervoy-nice-idUSL5E7LE06A20111014. Accessed January 24, 2012.
- 59.FDA BLA 125377 Yervoy (ipilimumab) injection, for intravenous infusion. Human cytotoxic T-lymphocyte antigen-4 (CTLA-4)-blocking monoclonal antibody. Risk Evaluation and Mitigation Strategy (REMS) Mar, 2011. Available at: www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationfor-PatientsandProviders/UCM249435.pdf. Accessed January 26, 2012.
- 60.FDA Yervoy (ipilimumab): Risk Evaluation and Mitigation Strategy (REMS): Severe immune-mediated adverse reactions. Apr 6, 2011. Available at: www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm249770.htm. Accessed January 26, 2012.
- 61.Bristol-Myers Squibb. How to order Yervoy (ipilimumab). Available at: www.hcp.yervoy.com/pages/practice-resources/order-yervoy. Accessed August 8, 2012.
- 62.Francois V, Ottaviani S, Renkvist N, et al. The CD4+ T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res. 2009;69(10):4335–4345. doi: 10.1158/0008-5472.CAN-08-3726. [DOI] [PubMed] [Google Scholar]
- 63.Trefzer U, Jouary T, Robert C, et al. FC13 DERMA Phase III trial of MAGE-A3 immunotherapy as adjuvant treatment in stage III melanoma: MAGE-A3 gene expression frequency and baseline demographics (Abstract) Melanoma Res. 2010;20(Suppl A):e34–e35. [Google Scholar]
- 64.Kirkwood JM, Dreno B, Hauschild A, et al. DERMA phase III trial of MAGE-A3 antigen-specific cancer immunotherapeutic (ASCI) as adjuvant therapy in patients with MAGE-A3–positive resected stage III melanoma (Abstract TPS232) J Clin Oncol. 2011;29(Suppl) [Google Scholar]
- 65.National Institutes of Health MAGE-A3 antigen-specific cancer immunotherapeutic in patients with progressive metastatic cutaneous melanoma. Available at: http://clinicaltrials.gov/ct2/show/NCT00706238. Accessed January 30, 2012.
- 66.Stopeck AT, Jones A, Hersh EM, et al. Phase II study of direct intralesional gene transfer of Allovectin-7, an HLA-B7/β2-microglobulin DNA-liposome complex, in patients with metastatic melanoma. Clin Cancer Res. 2001;7:2285–2291. [PubMed] [Google Scholar]
- 67.National Institutes of Health A phase 3 pivotal trial comparing Allovectin-7 alone vs. chemotherapy alone in patients with stage 3 or stage 4 melanoma. Available at: http://clinicaltrials.gov/ct2/show/NCT00395070. Accessed January 27, 2012.
- 68.Vical’s Allovectin-7 phase 3 trial receives positive review from safety monitoring board. Feb 14, 2011. Available at: www.vical.com. Accessed January 27, 2012.
- 69.Kaufman HL, Bines SD. OPTIM trial: A phase III trial of an oncolytic herpes virus encoding GM–CSF for unresectable stage III or IV melanoma. Fut Oncol. 2010;6:941–949. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- 70.National Institutes of Health Efficacy and safety study of OncoVEXGM-CSF compared to GM-CSF in melanoma. Available at: http://clinicaltrials.gov/ct2/show/NCT00769704. Accessed January 27, 2012.
- 71.Melanoma vaccine, AVAX Technologies: DNP-VACC, M-Vax. Biodrugs. 2003;17:69–72. doi: 10.2165/00063030-200317010-00007. Available at http://adisonline.com. [DOI] [PubMed] [Google Scholar]
- 72.National Institutes of Health M-Vax + low dose interleukin-2 versus placebo vaccine in metastatic melanoma in patients with stage IV melanoma. Available at: http://clinicaltrials.gov/ct2/show/NCT00477906. Accessed January 30, 2012.