Abstract
N-linked glycosylation is a critical determinant of protein structure and function, regulating processes such as protein folding, stability and localization, ligand–receptor binding and intracellular signalling. TβRII [type II TGF-β (transforming growth factor β) receptor] plays a crucial role in the TGF-β signalling pathway. Although N-linked glycosylation of TβRII was first demonstrated over a decade ago, it was unclear how this modification influenced TβRII biology. In the present study, we show that inhibiting the N-linked glycosylation process successfully hinders binding of TGF-β1 to TβRII and subsequently renders cells resistant to TGF-β signalling. The lung cancer cell line A549, the gastric carcinoma cell line MKN1 and the immortal cell line HEK (human embryonic kidney)-293 exhibit reduced TGF-β signalling when either treated with two inhibitors, including tunicamycin (a potent N-linked glycosylation inhibitor) and kifunensine [an inhibitor of ER (endoplasmic reticulum) and Golgi mannosidase I family members], or introduced with a non-glycosylated mutant version of TβRII. We demonstrate that defective N-linked glycosylation prevents TβRII proteins from being transported to the cell surface. Moreover, we clearly show that not only the complex type, but also a high-mannose type, of TβRII can be localized on the cell surface. Collectively, these findings demonstrate that N-linked glycosylation is essentially required for the successful cell surface transportation of TβRII, suggesting a novel mechanism by which the TGF-β sensitivity can be regulated by N-linked glycosylation levels of TβRII.
Keywords: cell surface transport, N-linked glycosylation, transforming growth factor β sensitivity (TGF-β sensitivity), transforming growth factor β signalling (TGF-β signalling), type II transforming growth factor β receptor (TβRII)
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; dNG, deficient N-linked glycosylation; Endo H, endoglycosidase H; ER, endoplasmic reticulum; FBS, fetal bovine serum; GlcNAc, N-acetylglucosamine; HEK, human embryonic kidney; KIF, kifunensine; PDI, protein disulfide-isomerase; PEI, polyethyleneimine; PNGase F, peptide N-glycosidase F; RT, room temperature; TGF-β, transforming growth factor β; TβRI, type I TGF-β receptor; TβRII, type II TGF-β receptor; Tun, tunicamycin; WT, wild-type
INTRODUCTION
The TGF-β (transforming growth factor β) signalling pathway regulates a diverse set of cellular processes, including proliferation, differentiation, migration and apoptosis, in metazoan biology [1]. TGF-β ligand family members signal through heteromeric receptor complexes of TβRI (type I TGF-β receptor) and TβRII (type II TGF-β receptor) serine/threonine kinases. The ligand–receptor binding on the cell surface allows the constitutively active TβRII to phosphorylate the intracellular kinase domain of TβRI, which then propagates the signal through phosphorylation of the downstream signal transducers, the Smad proteins. R-Smads (receptor-regulated Smads), such as Smad2 and Smad3, are directly phosphorylated on two serine residues at their extreme C-terminal SSXS (Ser-Ser-Xaa-Ser) motif by TβRI, and subsequently form complexes with the Co-Smad (co-mediator Smad) Smad4. The activated Smad complexes translocate into the nucleus, where Smad4 complexes are directly involved in positively or negatively regulating the transcription of target genes, such as Pai1, Smad7, p15, p21 and c-myc [1–5].
Given that the TGF-β signalling pathway has been implicated in various diseases including tumours, TGF-β resistance or TGF-β sensitivity has been regarded as an essential determinant in the development of diseases [6]. It has been reported that genetic alterations can influence TGF-β responsiveness by inactivating essential components of the TGF-β signalling pathway, such as the p15INK4B locus or TβRII [7–10]. However, such alterations account for only a small portion of the loss of TGF-β responsiveness. Consequently, TGF-β resistance must also be attained by other unknown mechanisms. In the present study, we suggest one novel mechanism, different N-linked glycosylation levels of TβRII, which alters TGF-β responsiveness even without certain genetic mutations hampering normal TGF-β signalling.
TGF-β signalling is regulated by other signalling pathways and post-translational modifications, such as phosphorylation, acetyl-ation, ubiquitination and SUMOylation [13,14]. Less is known about the regulation of TGF-β receptors by post-translational modifications. To date, phosphorylation, ubiquitination and SUMOylation have been shown to modify the receptors post-translationally [13–15]. The core fucosylation (which is found on most N-glycans owing to the activity of Fut8 fucosyltransferase) of TβRI has been shown to be essential in ligand binding [16]. However, despite previous studies of N-linked glycosylation on the extracellular domain of TβRII, N-linked glycosylation of TβRII has not yet been precisely reported [17,18]. Given that many membrane-associated and secreted proteins in eukaryotic cells are known to be modified by N-linked glycosylation, the N-linked glycosylation of TβRII, a protein that plays a pivotal role in the TGF-β signalling pathway, is worthy of examination [19].
N-linked glycosylation begins with the formation of an amide linkage between GlcNAc (N-acetylglucosamine) and an asparagine residue, specifically involving a subset residing in the Asn-Xaa-Ser/Thr motif on target proteins [20]. Three major types of N-linked oligosaccharides have been reported: high-mannose oligosaccharides, hybrid oligosaccharides and complex oligosaccharides. High-mannose oligosaccharides contain five to nine mannose residues in addition to the two GlcNAc molecules, whereas the hybrid and complex type of N-linked glycans can contain as many and various types of saccharides besides two original GlcNAcs. Nearly all proteins that travel through the ER (endoplasmic reticulum)–Golgi complex undergo N-linked glycosylation, and this modification can act as a determinant of protein folding, stability, trafficking, localization and oligomerization, with important implications for cell–cell interactions, ligand–receptor binding affinity and intracellular signalling [21–27]. The importance of N-linked glycosylation is evident by the fact that a lack of all N-glycans is lethal in species ranging from yeast to mammals [24].
N-linked glycosylation of TβRII has been expected, owing to the fact that TβRII is consistently detected with a broad range of molecular masses on Western blot analysis. In the present study, we have demonstrated that different N-linked glycosylation levels of TβRII determined TGF-β sensitivity, revealing the essential role of N-linked glycosylation of TβRII in the TGF-β signalling pathway. Moreover, we found that the high molecular mass of TβRII was engendered by N-linked glycosylation on the two conserved asparagine residues on the extracellular domain of TβRII. This was demonstrated by using a PNGase F (peptide N-glycosidase F), Endo H (endoglycosidase H) and by generating a dNG (deficient N-linked glycosylation) mutant in which both of the conserved asparagine residues were changed to glutamine. Defective N-linked glycosylation of TβRII blocked its transportation to the cell surface membrane, followed by impaired TGF-β-mediated intracellular signalling.
EXPERIMENTAL
Plasmids
The expression plasmid for C-terminally FLAG-tagged TβRII was a gift from Dr S.T. Hong (Lee Gil Ya Cancer and Diabetes Institute, Incheon, South Korea). N70Q, N94Q and N70/94Q mutants were generated by a DpnI site-directed mutagenesis method using mutagenic oligonucleotide primers that were perfectly complementary to each other [27a]. Primer information is available upon request.
Cell culture and transient transfection
A549, HepG2, HeLa and HEK (human embryonic kidney)-293T cell lines were maintained in Dulbecco's modified Eagle's medium containing high glucose (WelGENE), supplemented with 10% (v/v) heat-inactivated FBS (fetal bovine serum) (WelGENE); MKN1, MKN28 and MKN45 cell lines were maintained in RPMI 1640 medium (WelGENE) containing 25 mM Hepes, supplemented with 10% (v/v) heat-inactivated FBS, at 37°C in a humidified 5% CO2 incubator. A549, MKN1, MKN28, MKN45 and AGS cell lines were transiently transfected using Lipofectamine™ LTX with PLUS reagent (Invitrogen); HepG2 and HeLa cell lines were transiently transfected using FuGENE HD (Promega), according to the manufacturer's instructions. The HEK-293T cell line was transiently transfected using PEI (polyethyleneimine; Polysciences), 25 kDa, using 3 μg of PEI per 1 μg of DNA. For KIF (kifunensine; Sigma–Aldrich) or Tun (tunicamycin; Sigma–Aldrich) treatment, KIF (10 μg/ml) or Tun (1 μg/ml) was added into the A549 cell line for 24 h or 12 h respectively before cell harvest.
Production of cell extracts
Cells were harvested and lysed in a buffer containing 20 mM Hepes (pH 7.5), 150 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM EDTA and Complete protease inhibitor cocktail (Roche). N-linked glycosylation was enzymatically removed from the denatured proteins in the extracts through incubation with PNGase F and Endo H (both from New England Biolabs), according to the manufacturer's instructions.
Western blot analysis
After cells were transiently transfected with the indicated plasmids, extracts were separated by SDS/PAGE (10% gel) followed by electrotransfer on to PVDF membranes and probed with the following antibodies: anti-TBRII (E6; Santa Cruz Biotechnology); anti-FLAG (M2), anti-β-actin (AC15) and anti-α-tubulin (B-5-1-2; Sigma–Aldrich); and anti-phospho-Smad2 (138D4) and anti-(total Smad2) (86F7; Cell Signaling Technology).
Microscopic analysis
HeLa cells plated on LabTeK II four-well glass slides (Nalge Nunc International) were rinsed in PBS, fixed in 4% (w/v) paraformaldehyde for 30 min at RT (room temperature; 25°C), rinsed in PBS, and permeabilized with 0.5% Triton X-100 for 10 min at RT. Blocking was performed with 5% (w/v) non-fat dried skimmed milk powder in PBS for 1 h at RT. The cells were then incubated with mouse anti-FLAG (M2; 1:500 dilution) and rabbit anti-PDI (protein disulfide-isomerase) (Abcam; 1:500 dilution) primary antibodies overnight at 4°C. For phalloidin staining, the cells were incubated with Alexa Fluor® 488-conjugated phalloidin (Invitrogen; 1:40 dilution) for 30 min at RT before probing with the secondary antibody. The secondary antibodies, Alexa Fluor® 488-conjugated goat anti-(rabbit IgG) and Alexa Fluor® 594-conjugated goat anti-(mouse IgG) (Invitrogen) for green and red colour respectively, were used at a 1:400 dilution for 1 h at RT. Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen; 1:1000 dilution) and then coverslips were mounted on to slide glasses using a ProLong Antifade Kit (Invitrogen). Slides were viewed with a confocal laser-scanning microscope (LSM-710; Carl Zeiss) for bright-field and fluorescence applications. Images were acquired using an AxioCam MRc digital camera (Carl Zeiss) and were processed with ZEN software (Carl Zeiss).
Flow cytometry
The numbers of biotinylated TGF-β1-bound TβRII molecules were quantified using biotinylated human TGF-β1 (R&D Systems), according to the manufacturer's instructions. Briefly, various amounts of biotinylated TGF-β1 (2.5–60 ng) were added to 105 HEK-293T cells that were transiently transfected with mock, wild-type or N70/94Q versions of TβRII. After 1 h of incubation at 4°C, avidin–FITC reagent was added to each sample and incubated for 30 min at 4°C in the dark. Before flow cytometric analysis, cells were washed twice and treated with 7-AAD (7-amino-actinomycin D) to exclude dead cells.
RESULTS
Inhibition of N-linked glycosylation leads to reduced TGF-β signalling
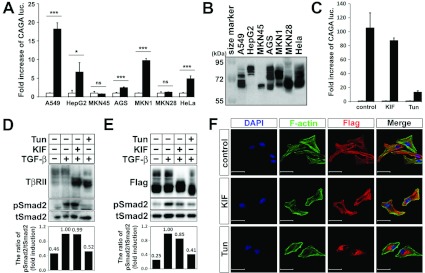
TGF-β1 responsiveness was examined through a TGF-β1-responsive (CAGA)12-luciferase reporter assay in six randomly chosen different human cancer cell lines: the lung cancer cell line A549, the liver cancer cell line HepG2, gastric cancer cell lines MKN45, AGS, MKN1 and MKN28, and one immortalized cell line HeLa. A549, HepG2, AGS, MKN1 and HeLa cells responded significantly to TGF-β1 with different induction ratios (Figure 1A). In contrast, MKN45 and MKN28 cells did not show a significant response to TGF-β1 (Figure 1A). Regarding TβRII as the first protein in ligand binding and subsequent downstream signal transduction, protein expression of TβRII was examined by using several commercial antibodies. Unfortunately, no antibody could efficiently detect the reliable expression patterns of TβRII in the cell lines (results not shown). However, we observed that TβRII exhibited different expression patterns, with different multiple bands in the broad range of molecular masses in the cell lines, even though the same plasmid DNA construct of TβRII was transfected into those cells (Figure 1B). Although the transfection efficiencies were variable for each cell line, it was evident that a major portion of each transfected TβRII protein was differently expressed in various molecular masses. Of note, A549, HepG2, AGS, MKN1 and HeLa cells exhibited their expression with high molecular mass, >72 kDa, compared with MKN45 and MKN28. This observation suggested that the high-molecular-mass portions were probably responsible for the TGF-β sensitivity shown in Figure 1(A). Since TβRII protein has been known to be modified by N-linked glycosylation, we assumed that the TGF-β responsiveness could be dependent on N-linked glycosylation of TβRII. By using two inhibitors, we then examined the hypothesis in the A549 cell line, which was the most responsive to TGF-β1 among the seven cell lines. KIF, an inhibitor of ER and Golgi mannosidase I family members, which stops the processing of N-glycans at the Man9-GlcNAc2-Asn stage [28], and Tun, a potent inhibitor of endogenous N-linked glycosylation, which prevents the attachment of N-glycans to nascent polypeptides [29], were used for the examination. Although KIF treatment did not significantly decrease the (CAGA)12-luciferase reporter gene induction level, Tun treatment significantly blocked the gene induction upon TGF-β1 stimulation (Figure 1C). To verify the efficiency of each inhibitor, we examined endogenous TβRII protein expression by Western blot analysis. The fact that diffused multiple bands were disrupted by KIF or Tun treatment implied that they were likely to represent endogenous TβRII and that both inhibitors were functionally active (Figure 1D). The effect of the different N-linked glycosylation levels of TβRII on TGF-β signalling induced by the two inhibitors was further evaluated by the phosphorylation level of Smad2 after TGF-β1 stimulation. Consistent with the result of the (CAGA)12-luciferase reporter assay, Smad2 phosphorylation was significantly reduced in the Tun-treated cells (Figure 1D). To exclude the imprecise endogenous TβRII expression pattern, we transiently overexpressed FLAG-tagged TβRII and obtained the same result showing distinctive patterns of N-linked glycosylated TβRII (Figure 1E). As seen in Figures 1(D) and 1(E), it was more efficient and convincing to detect the N-linked glycosylation levels of the transfected TβRII than that of endogenous TβRII. Besides, the transient transfection generated the same effect upon TGF-β1, KIF and Tun treatment. Therefore we thereafter employed the transfection system for further evaluation. KIF led to the accumulation of TβRII of ~68 kDa, whereas Tun resulted in a great shift of the molecular mass of TβRII to ~64 kDa (Figure 1E). Since KIF blocked N-glycan maturation from high-mannose to complex type structures, this result suggested that the high-mannose type of TβRII could still bind to TGF-β1 and signal downstream. The phenomenon that Tun-treated TβRII was still responsive to TGF-β1, to a lesser extent, might be explained by that the residual TβRII proteins, which escaped from the Tun effect, could bind to TGF-β1 and trigger the signal. Taken together, these results indicated that the different N-linked glycosylation levels of TβRII could regulate the interaction between TβRII and TGF-β1, suggesting that TGF-β sensitivity might be controlled by the different N-linked glycosylation levels of TβRII. In addition, we observed the same phenomena in other cell lines, such as the gastric cancer cell line MKN1 and the immortal cell line HEK-293, suggesting that this mechanism might be universal in cellular systems (Supplementary Figure S1 at http://www.BiochemJ.org/bj/445/bj4450403add.htm).
Figure 1. N-linked glycosylation levels of TβRII regulate TGF-β signalling activity and subcellular localization of TβRII.
(A) Cells were transfected with the pGL3-(CAGA)12-luciferase reporter gene and pCMV-βgal. At 12 h after transfection, TGF-β1 (5 ng/ml) was added to the medium for 16 h. Cells were then collected for luciferase (luc.) and β-galatosidase assays. Results represent luciferase activity relative to β-galactosidase activity and are means±S.D. for experiments in triplicate. ***P<0.001; *P<0.05; ns, not significant. (B) FLAG–TβRII was transfected into various cell lines. After 30 h of transfection, cells were harvested and immunoblotted with an anti-FLAG antibody. (C) (CAGA)12 and β-galatosidase were transfected into the A549 cell line. At 6 h after transfection, A549 was treated with KIF (10 μg/ml for 24 h) or Tun (1 μg/ml for 12 h), followed by incubation with or without TGF-β1 (5 ng/ml for 16 h). Cells were then collected for luciferase and β-galatosidase assays. Results are means±S.D. for experiments in triplicate. Note that inhibiting N-linked glycosylation of TβRII by KIF or Tun treatment led to decreased (CAGA)12-luciferase transcriptional activity. (D and E) Untransfected (D) or FLAG–TβRII-transfected (E) A549 was treated with KIF or Tun, followed by TGF-β1 treatment (5 ng/ml for 30 min). Cell extracts were immunoblotted with anti-FLAG, anti-phospho-Smad2 or anti-Smad2 antibody. Band intensities representing phospho-Smad2 and Smad2 expression levels were converted into densitometry using ImageJ software in the ratio of phospho-Smad2 to Smad2. Note that KIF or Tun treatment reduced or inhibited the N-linked glycosylation level of TβRII as well as Smad2 phosphorylation. (F) Fluorescence micrographs of HeLa cells that were transiently transfected with FLAG-tagged TβRII and untreated or treated with KIF or Tun. Cells were stained with an anti-FLAG antibody (red) and phalloidin (F-actin; green). Note that TβRII proteins are mainly localized on the cell surface in the untreated and KIF-treated HeLa cells, whereas they accumulated mostly in the perinuclear region in the Tun-treated cells. DAPI was used for nuclear staining (blue). Scale bars, 50 μm.
N-linked glycosylation controls cell surface transport of TβRII
Since it has been demonstrated that N-linked glycosylation could determine or influence the cell surface transport of certain membrane proteins [25,30], we examined the effect of N-glycosylation on the cell surface transport of TβRII. Fluorescence confocal microscopy was used to determine the subcellular localization of TβRII proteins transiently overexpressed in the HeLa cell line, which had a relatively large cell size compared with A549 cells, to distinguish the cell surface from the perinuclear region. The transiently transfected WT (wild-type) TβRII proteins were mostly localized on the cell surface. Although KIF treatment seemed not to influence the cell surface localization of WT TβRII, Tun treatment led to the accumulation of TβRII proteins in the perinuclear region. The subcellular localization was more evident when the cells were co-stained with phalloidin, which showed the cell morphology by binding to actin filaments (Figure 1F). The comparable localization of KIF-treated TβRII on the cell surface corresponded to the fact that KIF-treated TβRII proteins were still able to transduce TGF-β signalling (Figures 1C–1E). The fact that Tun-treated TβRII hardly transduced the signal was probably due to the predominant perinuclear localization of TβRII proteins. Collectively, these results suggested that N-linked glycosylation played an indispensable role for the cell surface transport of TβRII proteins, providing evidence that the high-mannose type of TβRII could be transported to the cell surface, similar to fully glycosylated TβRII, whereas the deglycosylated type of TβRII could not be localized on the cell surface.
Two conserved asparagine residues for N-linked glycosylation of TβRII
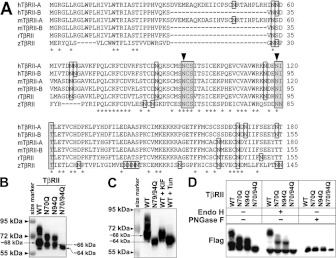
Although TβRII has been known to be N-linked glycosylated, the exact sites have not been identified [17,18,34]. Given that N-linked glycosylation occurs on asparagine residues that fall into a consensus Asn-Xaa-Ser/Thr sequence motif, we first examined all of the asparagine residues in the extracellular domain of TβRII. Only N-linked consensus sequences located within the extracellular-facing domains are exposed to N-linked glycosylation enzymes in the ER lumen during biosynthesis [20,21,31]. In the extracellular domain of TβRII in human isoforms A and B, mouse isoforms A and B, rat and zebrafish, there were 13, 12, 9, 8, 9 and 17 asparagine residues respectively. However, only 3, 2, 3, 2, 2 and 4 asparagine residues respectively satisfied the exact requirement for N-linked glycosylation (Figure 2A). Interestingly, among them, only two asparagine residues were conserved in mammals and other vertebrates ranging from human to zebrafish.
Figure 2. Two conserved asparagine residues are essential for N-linked glycosylation of TβRII.
(A) N-terminal amino acid sequence alignment of human TβRII isoform A (hTβRII-A), human TβRII isoform B (hTβRII-B), mouse TβRII isoform A (mTβRII-A), mouse TβRII isoform B (mTβRII-B), rat TβRII (rTβRII) and zebrafish TβRII (zTβRII). Asparagine residues are boxed. Asn-Xaa-Ser/Thr motifs are shaded. Asterisks indicate conserved residues. Arrowheads indicate conserved asparagine residues in the Asn-Xaa-Ser/Thr motif. (B and C) Western blot analysis showing the protein expression of TβRII in A549 cells. (B) FLAG-tagged TβRII of WT and three dNG mutants N70Q, N94Q and N70/94Q. (C) WT, N70/94Q, KIF-treated WT and Tun-treated WT TβRII. Note that the single mutants or KIF treatment (10 μg/ml for 24 h) reduced N-linked glycosylation levels of TβRII, whereas the double mutant or Tun treatment (1 μg/ml for 12 h) completely block the N-linked glycosylation. (D) Western blots of Endo H- and PNGase F-treated WT and dNG mutants of TβRII. Note that PNGase F treatment removed all N-glycosylated forms of TβRII, whereas Endo H treatment did not remove heavily glycosylated forms of TβRII.
The two conserved asparagine residues of TβRII mediate N-linked glycosylation of TβRII
To examine whether the two conserved asparagine residues were essential for N-linked glycosylation of TβRII, we generated three dNG mutants of the mouse TβRII isoform B, in which one or both of the asparagine residues was converted into glutamine: N70Q, N94Q and N70/94Q. Compared with WT TβRII, all three dNG mutants of TβRII showed no upper bands over approximately 72 kDa (Figure 2B). The N70/94Q double mutant showed only a single band at approximately 64 kDa, which was the same as that of Tun-treated WT TβRII (Figure 2C), indicating that both Asn70 and Asn94 might be indispensable for N-linked glycosylation of TβRII. In addition, the upper bands of the two single mutants exhibited a similar, albeit lower, pattern to those of WT TβRII, strongly suggesting that each asparagine residue might equally contribute to the N-linked glycosylation of TβRII. To confirm that the upper bands of both WT and the two single mutants were caused by N-linked glycosylation, we used PNGase F to remove all N-glycans from TβRII. Indeed, only one single band at ~64 kDa was detected in WT and in the two single mutants after PNGase F treatment (Figure 2D). Interestingly, the bottom band of WT TβRII was detected at a higher molecular mass (~68 kDa) than those of the two single mutants (~66 kDa), which were larger than that of the double mutant (~64 kDa). The ~68 kDa band of WT TβRII was shown in the KIF-treated WT TβRII proteins, indicating that it represented the high-mannose type of N-glycosylated TβRII (Figure 2C). To study the size differences between the bottom bands of WT and dNG mutants, we used another enzyme, Endo H. Unlike PNGase F, Endo H can cleave only unprocessed core oligosaccharide chains, such as high-mannose N-glycans, but is unable to function on more fully processed oligosaccharides, such as hybrid or complex N-glycans [17,32,33]. Upon Endo H treatment, most of the multi-upper bands remained intact, whereas the bottom bands of WT TβRII as well as the two single mutants appeared at reduced sizes (~64 kDa) that were comparable with those of the double dNG mutant (Figure 2D). These results suggested that TβRII proteins undergoing core N-linked glycosylation at the one or two remaining asparagine residue(s) might be detected as the bottom bands (~68 kDa or ~66 kDa respectively). Moreover, the difference of the size shift between WT (~68 kDa to ~64 kDa) and the two single mutants (~66 kDa to ~64 kDa) suggested that the two conserved asparagine residues contributed equally to the core N-linked glycosylation, adding a molecular mass of ~2 kDa each. Collectively, our results clearly showed the essential role of both Asn70 and Asn94 of TβRII in the whole N-linked glycosylation process, consisting of the core N-linked glycosylation, processed in the ER by high-mannose N-glycans, and the subsequent N-linked glycosylation, processed in the post-ER compartment by hybrid or complex N-glycans. In addition, unlike found in a previous study [34], Asn70 and Asn94 are the only requirement for N-linked glycosylation of TβRII because our double -dNG mutant showed the completely non-glycosylated form.
dNG single mutants of TβRII, but not the double mutant, can be localized on the cell surface
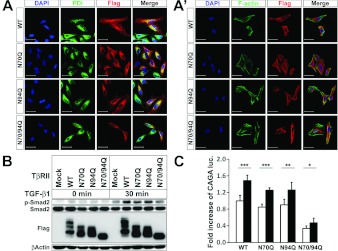
We next examined the subcellular localization of the WT and dNG mutants of TβRII. Similar to WT TβRII, N70Q and N94Q TβRII were mainly localized on the cell surface. This observation suggested that single N-linked glycosylated TβRII at Asn70 or Asn94 was capable of localizing on the cell surface (Figures 3A and 3A'). In contrast, N70/94Q TβRII proteins were predominantly accumulated in the perinuclear region, merging with PDI, an ER marker (Figure 3A). Since this pattern was strongly reminiscent of Tun-treated TβRII localization, we again assayed TGF-β signalling activity in A549 cells. Cells were transiently transfected with WT or the dNG mutants of TβRII and stimulated with TGF-β1. Upon TGF-β1 treatment, the phosphorylation level of Smad2 in the WT TβRII-transfected cells was higher than that of the mock-transfected control cells, but was comparable with that of each of the single-mutant-transfected cell lines (Figure 3B). This result implied that the single N-linked glycosylated TβRII at Asn70 or Asn94 could bind to TGF-β1 and transduce the signal. Importantly, the phospho-Smad2 level in the N70/94Q double mutant was much lower than that of the N70Q and N94Q single mutants, and was similar to that of the mock-transfected control cells (Figure 3B). The data indicated that non-glycosylated TβRII proteins, which were not able to be transported to the cell surface to bind to TGF-β1, were defective in signalling to downstream transducers. Additionally, we performed a (CAGA)12-luciferase reporter assay with the same context and obtained the same result. Consistent with the increased phosphorylation level of Smad2, the (CAGA)12-luciferase gene was significantly induced in the N70Q and N94Q as well as WT TβRII upon TGF-β1 stimulation (Figure 3C). The N70/94Q TβRII also exhibited (CAGA)12-luciferase gene induction with statistical significance, albeit much lower than those of WT and single-mutant TβRII, which probably reflected the endogenous TβRII effect (Figure 3C).
Figure 3. Defective N-linked glycosylation hinders cell surface transportation of TβRII and reduces Smad2 phosphorylation and (CAGA)12-luciferase transcriptional activity.
(A and A') Fluorescence micrograph showing the localization of transiently transfected FLAG-tagged WT, N70Q, N94Q and N70/94Q TβRII in HeLa cells. Cells were stained with an anti-FLAG antibody (red), PDI (green) (A) and phalloidin (F-actin; green) (A'). Two single mutants, N70Q and N94Q TβRII, seem to be transported to the cell surface, whereas N70/94Q does not. Scale bars, 50 μm. (B) FLAG-tagged WT, N70Q, N94Q and N70/94Q TβRII were transiently transfected into A549 cells. At 28 h after transfection, cells were treated with or without TGF-β1 (5 ng/ml for 30 min). Cell extracts were immunoblotted with anti-FLAG, anti-phospho-Smad2 or anti-Smad2 antibody. β-Actin was detected as a loading control. (C) WT, N70Q, N94Q and N70/94Q TβRII were co-transfected with (CAGA)12-luciferase and β-galatosidase into A549 cells. At 12 h after transfection, TGF-β1 (5 ng/ml) was added to the medium for 16 h. Cells were then collected for luciferase (luc.) and β-galatosidase assays. Results represent luciferase activity related to β-galactosidase activity and are means±S.E.M. for experiments in triplicate. Note that the two single dNG mutants of TβRII (N70Q and N94Q) are comparable with WT TβRII in inducing Smad2 phosphorylation and (CAGA)12-luciferase transcription. ***P<0.001; **P<0.01; *P<0.05.
Defective N-linked glycosylation blocks cell surface transport of TβRII and subsequently reduces TGF-β signalling
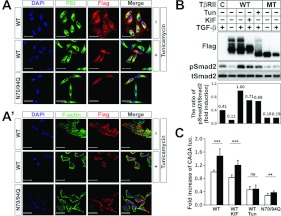
To further confirm that defective N-glycosylation caused transport incompetence of TβRII to the cell surface, we compared the subcellular localization of the Tun-treated WT TβRII with the N70/94Q TβRII. By using fluorescence confocal microscopy, we observed that the localization pattern of N70/94Q TβRII was analogous to that of the Tun-treated WT TβRII (Figure 4A and 4A'). Importantly, they showed predominant accumulation in the perinuclear region by merging with PDI (Figure 4A). Comparable subcellular localization patterns of N70/94Q and Tun-treated WT TβRII led to the examination of the activity of N70/94Q TβRII in TGF-β signalling. We investigated the degrees of TGF-β sensitivity in the N70/94Q TβRII (non-glycosylated form)-, the KIF-treated WT TβRII (high-mannose type)- or the Tun-treated WT TβRII (deglycosylated form)-transfected A549 cells. As expected, Smad2 phosphorylation in the WT TβRII-transfected cells was approximately 2.5-fold higher than those in the empty-vector-transfected control cells upon TGF-β1 treatment (Figure 4B). Importantly, the phospho-Smad2 level in the N70/94Q double mutant was not only markedly lower than that of the WT TβRII-transfected cells (only 19% induction), but also lower than that of the KIF-treated WT TβRII-transfected cells (~3.7-fold decrease) (Figure 4B). The Tun-treated WT TβRII also showed a decreased level of phospho-Smad2, although this reduction was not as dramatic as that shown in Figure 1(E), implying the incomplete inhibition of Tun (Figure 4B). Likewise, the (CAGA)12-luciferase reporter assay showed that the Tun-treated TβRII did not increase the reporter gene induction (Figure 4C). The different induction ratios between the phospho-Smad2 levels and (CAGA)12-luciferase reporter gene levels could be explained by the incomplete Tun effect to deglycosylate TβRII and the existence of endogenous TβRII. Collectively, these results indicated that completely non-glycosylated TβRII proteins, which seemed unable to be transported to the cell surface to bind to TGF-β1, might be totally defective in the TGF-β signal transduction.
Figure 4. Deglycosylated TβRII blocks TGF-β signalling and cell surface transport of TβRII.
(A and A') Fluorescence micrographs showing the localization of transiently transfected FLAG-tagged WT and N70/94Q TβRII in HeLa cells. Cells were stained with an anti-FLAG antibody (red), PDI (green) (A) or phalloidin (F-actin; green) (A'). Tun treatment was at 1 μg/ml for 12 h. Note that WT TβRII proteins are co-stained with phalloidin at the cell surface. However, Tun-treated WT and N70/94Q TβRIIs accumulate in the perinuclear region, almost merging with PDI. Scale bars, 50 μm. (B) WT and N70/94Q double mutant (MT) TβRII were transiently transfected into A549 cells. At 8 h of transfection, cells transfected with WT TβRII were treated with KIF (10 μg/ml for 24 h) and Tun (1 μg/ml for 12 h), followed by TGF-β1 treatment (5 ng/ml for 30 min). Cell extracts were immunoblotted with anti-FLAG, anti-phospho-Smad2 and anti-Smad2. Band intensities representing phospho-Smad2 and Smad2 expression levels were converted by densitometry using ImageJ software into the ratio of phospho-Smad2 to Smad2. Note that Smad2 phosphorylation is dramatically reduced in the N70/94Q TβRII-transfected cells. (C) WT and N70/94Q TβRII were co-transfected with (CAGA)12-luciferase and β-galatosidase into A549 cells. At 8 h after transfection, cells transfected with WT TβRII were treated with KIF and Tun, followed by TGF-β1 treatment (5 ng/ml for 16 h). Cells were then collected for luciferase (luc.) and β-galatosidase assays. Results represent luciferase activity related to β-galactosidase activity and are means±S.E.M. for experiments in triplicate. Note that the double dNG mutant (N70/94Q) significantly decreased (CAGA)12-luciferase transcriptional activity. ***P<0.001; **P<0.01; ns, not significant.
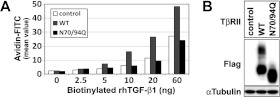
Furthermore, we were interested in clarifying whether the total absence of the N-linked glycosylation affected the number of TβRII proteins expressed on the cell surface. To address this issue, we performed a ligand-binding assay by using the WT and the N70/94Q double mutant version of TβRII. The ability of TGF-β1 to bind to WT or N70/94Q TβRII was evaluated with various concentrations of biotinylated TGF-β1 (ranging from 2.5 to 60 ng) in HEK-293T cells (Figure 5). Since the HEK-293T cell line had endogenous TβRII, TGF-β1 binding was also increased dose-dependently in control HEK-293T cells (Figure 5A). Notably, WT TβRII-transfected cells showed an almost 2-fold increase in ligand binding (Figure 5A). However, the ligand binding in the N70/94Q-transfected HEK-293T cells was comparable with that of the mock-transfected control cells (Figure 5A). The data showing the lack of increased binding efficiency in the N70/94Q-transfected HEK-293T cells might be explained by the inability of N70/94Q TβRII to travel to the cell surface and thereby add to the pool of active TβRII molecules. The alternative explanation was that TGF-β1 could bind to endogenous TβRII, but not to the N70/94Q version, even if N70/94Q TβRII was expressed on the cell surface. However, according to our microscopic data, the latter possibility became unlikely because N70/94Q was observed mostly in the perinuclear region, mainly in the ER, but not on the cell surface (Figure 4A).
Figure 5. Defective N-linked glycosylation of TβRII suppresses ligand-binding affinity.
(A) Representative flow cytometric analysis of receptor density for recombinant human TGF-β1 (rhTGF-β1) at the cell surface. Various amounts of biotinylated TGF-β1 (2.5–60 ng) were added to 105 HEK-293T cells that were transiently transfected with empty vector, WT or N70/94Q TβRII. The numbers of biotinylated TGF-β1-bound TβRII were quantified using rhTGF-β1. (B) Control Western blot of WT and N70/94Q TβRII transiently transfected into HEK-293T cells used in the ligand-binding assay (A).
DISCUSSION
In the present study, we provide evidence suggesting that TGF-β sensitivity can be regulated by different maturation levels of N-linked glycosylation of TβRII in different cells. Since the same WT TβRII construct was transfected into various cell lines, the different levels of N-linked glycosylation of transfected WT TβRII implied that the cellular machinery controlling N-linked glycosylation process in the ER and Golgi might function differently in each cell line (Figures 1A and 1B). Although the functional differences in the regulation of N-linked glycosylation in various cell lines has not been precisely elucidated yet, it is evident from this study that the more fully processed N-glycosylation (the complex type; WT in the case of TβRII) rather than less fully processed N-glycosylation (the high-mannose type; KIF-treated TβRII) or non-glycosylation (the N70/94Q dNG mutant or Tun-treated TβRII in the present study) would render TβRII more sensitive to its ligand binding on the cell surface. Various factors besides the N-linked glycosylation of TβRII are engaged in TGF-β1 responsiveness in cellular systems. Thus in order to investigate the sole effect of N-linked glycosylation on TGF-β1 responsiveness, it was important to examine TGF-β sensitivity by applying different N-linked glycosylated levels in one single cell system. In this regard, A549 cell line was an excellent model because it exhibited moderate levels of N-linked glycosylation of TβRII and responded well to TGF-β1, compared with other cell lines. In A549 cells, we clearly demonstrate that inhibiting or blocking the N-linked glycosylation of TβRII can regulate TGF-β sensitivity. In addition, the same phenomena are observed in other cell lines, such as the MKN1 human gastric cancer cell line and the HEK-293 cell line (Supplementary Figure S1). Moreover, we demonstrate that the TβRII proteins undergo complicated N-linked glycosylation processes towards a complex type via a high-mannose type of N-glycan. We also show that the high-mannose type of TβRII proteins can be localized on the cell surface and interact with TGF-β1 to transduce the signal, although its efficiency to activate Smad2 phosphorylation is slightly lower than that of the complex type of TβRII (Figures 1 and 4).
Although the glycosylation of TβRII has been previously described, in the present study we clearly demonstrate for the first time that N-linked glycosylation occurs only on the two asparagine residues in the extracellular domain of TβRII and plays crucial roles in its cell surface transportation and TGF-β sensitivity. Interestingly, the two asparagine residues, Asn70 and Asn94, are conserved in human, mouse, rat and even zebrafish, implying that N-linked glycosylation may be a common event as a post-translational modification of TβRII in vertebrates (Figure 2A). Importantly, each asparagine residue seems to be an equal contributor to the N-linked glycosylation of TβRII, because they show the same expression pattern (by Western blot analysis) and almost the same subcellular localization (Figures 3A and 3A'). Moreover, these two residues are the only residues on which TβRII is N-glycosylated, as is evident not only by the molecular mass of N70/94Q (~64 kDa) matching the estimated size of TβRII protein without any modifications, but also by the result that the removal of all N-glycans from WT or N70Q (or N94Q) TβRII recapitulates the size of the unmodified protein (Figures 2B–2D).
We have revealed the role of N-linked glycosylation in the expression of TβRII proteins on the surface membrane. Interestingly, the single glycosylation on Asn70 or Asn94 enables TβRII to be expressed on the surface membrane and activates TGF-β signalling through the phosphorylation of downstream transducers (Figure 3). In contrast, none of the non-glycosylated TβRII seems to be expressed on the cell surface, but accumulates mainly in the ER (Figures 3A and 4A). Therefore our data show that the N-linked glycosylation on both asparagine residues is required for efficient transportation of TβRII protein from the ER to the cell surface. However, it remains unknown whether N-glycans act as a signal for cell surface transport of TβRII proteins, or whether they alter the conformation of TβRII proteins. The change in the protein conformation may somehow lead to cell surface transport [35]. Although we cannot rule out the latter possibility, it may be a less likely explanation, because a different protein, rGH (rat growth hormone), which is normally a non-glycosylated secretory protein, can be transported to the cell surface when it is forced to be N-glycosylated by mutagenesis generating a glycosylation consensus sequence (Asn-Xaa-Ser/Thr) [35]. This result implies that forced N-linked glycosylation without any other changes can be enough to serve as a signal for protein transport to the cell surface. It may support our speculation that the transport of TβRII to the cell surface is due to N-linked glycosylation rather than protein conformational change.
Although the importance of N-linked glycosylation for the cell surface transport of TβRII is elucidated in the present study, it remains to be clarified whether all of the TβRII molecules expressed on the cell surface are N-glycosylated by complex, hybrid or high-mannose N-glycans under normal physiological conditions. It has been reported that non-glycosylated TβRII proteins are also expressed on the cell surface to bind to TGF-β1 [17]. However, this seems inconsistent with our observations that WT TβRII proteins are mainly localized in the ER in the Tun-treated HeLa cells, as well as that the double dNG mutant TβRII protein, N70/94Q, is mostly localized in the ER and unable to bind to TGF-β1 in the extracellular space. Despite the fact that the previous report is based solely on a binding assay of radio-iodinated TGF-β1 in Mv1Lu cells time-dependently treated with Tun [17], the sensitivity of the assay may have detected molecules that are non-glycosylated, but still on the cell surface, suggesting that it is possible that only a very small minority of non-glycosylated TβRII proteins can be expressed on the cell surface, albeit undetectable by indirect immunofluorescence and the TGF-β1-binding assay of the present study.
Paradoxically, TGF-β1 can function not only as a tumour suppressor, but also as a tumour promoter, even though the mechanisms determining how TGF-β1 switches between two modes of action have not yet been identified [36]. For instance, a process called EMT (epithelial to mesenchymal transition), an important step in tumour progression, is potently induced by TGF-β1. In this regard, the present study might open a possibility that inhibiting N-linked glycosylation can be applied to a novel therapeutic method to slow down or prevent cancer progression by decreasing TGF-β sensitivity of cancer cells.
Several unanswered questions remain, including: how does N-linked glycosylation affect the TβRI/TβRII complex formation? Are there any cases of intrinsic mutations at the sites for N-linked glycosylation of TβRII in cancer or disease systems possessing altered TGF-β signalling? To answer these questions, further investigation is needed, and the present study provides a stepping stone to those future explorations. Moreover, the present study paves the way for further investigation of the importance of the N-linked glycosylation process as a regulatory mechanism controlling the cellular responsiveness of cancer cells to TGF-β1.
Online data
AUTHOR CONTRIBUTION
Young-Woong Kim conceived, designed and performed the research, analysed data and wrote the paper; Jinah Park performed the research, analysed data and helped to write the paper; Hyun-Ju Lee performed parts of the research and analysed data; So-Young Lee analysed data; Seong-Jin Kim conceived the research, collected data and helped to write the paper.
ACKNOWLEDGEMENTS
We thank Dr Ethan A. Kohn for discussion and critical comments; Eun Jin Bae, Brisca Lee, Hae Kyung Ahn and Young Eun Yoo for technical support; and Bora Park, Kathy Park and Yunshin Jung for careful reading of the paper prior to submission.
FUNDING
This work was supported in part by the Bio Technology R&D Program of the National Research Foundation of Korea [grant number 20090081756 (to S.-J.K.)]; and by the World Class University (WCU) programme, National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology [grant number R32-10215].
References
- 1.Shi Y., Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 2.Matsuura I., Denissova N. G., Wang G., He D., Long J., Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 3.Li A. G., Wang D., Feng X. H., Wang X. J. Latent TGFβ1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004;23:1770–1781. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill C. S. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19:36–46. doi: 10.1038/cr.2008.325. [DOI] [PubMed] [Google Scholar]
- 5.Massague J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 6.Filmus J., Kerbel R. S. Development of resistance mechanisms to the growth-inhibitory effects of transforming growth factor-β during tumor progression. Curr. Opin. Oncol. 1993;5:123–129. [PubMed] [Google Scholar]
- 7.Massague J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner S. N., Wagner C., Briedigkeit L., Goos M. Homozygous deletion of the p16INK4a and the p15INK4b tumour suppressor genes in a subset of human sporadic cutaneous malignant melanoma. Br. J. Dermatol. 1998;138:13–21. doi: 10.1046/j.1365-2133.1998.02020.x. [DOI] [PubMed] [Google Scholar]
- 9.Jaffee E. M., Hruban R. H., Canto M., Kern S. E. Focus on pancreas cancer. Cancer Cell. 2002;2:25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 10.Sjoblom T., Jones S., Wood L. D., Parsons D. W., Lin J., Barber T. D., Mandelker D., Leary R. J., Ptak J., Silliman N., et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 11. Reference deleted.
- 12. Reference deleted.
- 13.Wrighton K. H., Lin X., Feng X. H. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izzi L., Attisano L. Ubiquitin-dependent regulation of TGFβ signaling in cancer. Neoplasia. 2006;8:677–688. doi: 10.1593/neo.06472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang J. S., Saunier E. F., Akhurst R. J., Derynck R. The type I TGF-β receptor is covalently modified and regulated by sumoylation. Nat. Cell Biol. 2008;10:654–664. doi: 10.1038/ncb1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., et al. Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells R. G., Yankelev H., Lin H. Y., Lodish H. F. Biosynthesis of the type I and type II TGF-β receptors. Implications for complex formation. J. Biol. Chem. 1997;272:11444–11451. doi: 10.1074/jbc.272.17.11444. [DOI] [PubMed] [Google Scholar]
- 18.Turco A., Scarpa S., Coppa A., Baccheschi G., Palumbo C., Leonetti C., Zupi G., Colletta G. Increased TGFβ type II receptor expression suppresses the malignant phenotype and induces differentiation of human neuroblastoma cells. Exp. Cell Res. 2000;255:77–85. doi: 10.1006/excr.1999.4750. [DOI] [PubMed] [Google Scholar]
- 19.Haltiwanger R. S., Lowe J. B. Role of glycosylation in development. Annu. Rev. Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 20.Ohtsubo K., Marth J. D. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Levy O., De la Vieja A., Ginter C. S., Riedel C., Dai G., Carrasco N. N-linked glycosylation of the thyroid Na+/I− symporter (NIS). Implications for its secondary structure model. J. Biol. Chem. 1998;273:22657–22663. doi: 10.1074/jbc.273.35.22657. [DOI] [PubMed] [Google Scholar]
- 22.Velan B., Kronman C., Ordentlich A., Flashner Y., Leitner M., Cohen S., Shafferman A. N-glycosylation of human acetylcholinesterase: effects on activity, stability and biosynthesis. Biochem. J. 1993;296:649–656. doi: 10.1042/bj2960649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waetzig G. H., Chalaris A., Rosenstiel P., Suthaus J., Holland C., Karl N., Vallés Uriarte L., Till A., Scheller J., Grötzinger J., et al. N-linked glycosylation is essential for the stability but not the signaling function of the interleukin-6 signal transducer glycoprotein 130. J. Biol. Chem. 2010;285:1781–1789. doi: 10.1074/jbc.M109.075952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeze H. H. Genetic defects in the human glycome. Nat. Rev. Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- 25.Petrecca K., Atanasiu R., Akhavan A., Shrier A. N-linked glycosylation sites determine HERG channel surface membrane expression. J. Physiol. 1999;515:41–48. doi: 10.1111/j.1469-7793.1999.041ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imperiali B., O'Connor S. E. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr. Opin. Chem. Biol. 1999;3:643–649. doi: 10.1016/s1367-5931(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 27.Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol. Biol. Cell. 1994;5:253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Li J., Li C., Xiao W., Yuan D., Wan G., Ma L. Site-directed mutagenesis by combination of homologous recombination and DpnI digestion of the plasmid template in Escherichia coli. Anal. Biochem. 2008;373:389–391. doi: 10.1016/j.ab.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Otto V. I., Schurpf T., Folkers G., Cummings R. D. Sialylated complex-type N-glycans enhance the signaling activity of soluble intercellular adhesion molecule-1 in mouse astrocytes. J. Biol. Chem. 2004;279:35201–35209. doi: 10.1074/jbc.M404947200. [DOI] [PubMed] [Google Scholar]
- 29.Ulug E. T., Bose H. R., Jr Effect of Tun on the development of the cytopathic effect in Sindbis virus-infected avian fibroblasts. Virology. 1985;143:546–557. doi: 10.1016/0042-6822(85)90393-9. [DOI] [PubMed] [Google Scholar]
- 30.Gut A., Kappeler F., Hyka N., Balda M. S., Hauri H. P., Matter K. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 1998;17:1919–1929. doi: 10.1093/emboj/17.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakin-Eshleman S. H., Spitalnik S. L., Kasturi L. The amino acid at the X position of an Asn-X-Ser sequon is an important determinant of N-linked core-glycosylation efficiency. J. Biol. Chem. 1996;271:6363–6366. doi: 10.1074/jbc.271.11.6363. [DOI] [PubMed] [Google Scholar]
- 32.Tobler A. R., Notterpek L., Naef R., Taylor V., Suter U., Shooter E. M. Transport of Trembler-J mutant peripheral myelin protein 22 is blocked in the intermediate compartment and affects the transport of the wild-type protein by direct interaction. J. Neurosci. 1999;19:2027–2036. doi: 10.1523/JNEUROSCI.19-06-02027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 34.Luga V., McLean S., Le Roy C., O'Connor-McCourt M., Wrana J. L., Di Guglielmo G. M. The extracellular domain of the TGFβ type II receptor regulates membrane raft partitioning. Biochem. J. 2009;421:119–131. doi: 10.1042/BJ20081131. [DOI] [PubMed] [Google Scholar]
- 35.Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985;42:489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- 36.Yang L., Moses H. L. Transforming growth factor β: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–9111. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.