Maize (Zea mays) is one of the most important cereal grains, and considerable effort has been focused on improving this source of human nutrition (Gibbon and Larkins, 2005). Most of the weight of maize kernels is the endosperm, and the protein composition of the endosperm is an important determiner of kernel texture and nutritional value. Zeins make up over half of endosperm total protein, and alterations in zein content lead to changes in nutritional quality and the appearance of the maize kernel.
Numerous classical opaque mutants, named for the maize kernel phenotype, have been isolated and often result from decreased zein synthesis in the endosperm. Some have abnormal protein bodies, which are critical for proper zein accumulation in the kernel. Also, inhibition of zein gene expression by RNA interference phenocopies the opaque phenotype (Wu and Messing, 2010). However, in the classic maize mutant opaque1 (O1;), the amount and amino acid composition of zein and nonzein proteins is similar to the wild type, suggesting no defect of zein synthesis in this mutant.
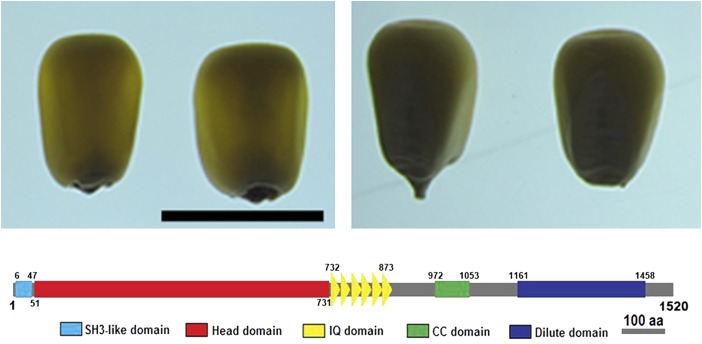
Using map-based positional cloning, Wang et al. (pages 3447–3462) show that O1 encodes a myosin XI motor protein that shares many structural features with known myosins (see figure). These include an N-terminal SH3-like domain (unknown function), a myosin head motor domain (actin binding, ATP hydrolysis, and force generation), a neck domain with a series of six IQ motifs (binding sites for myosin light chains and calmodulin), a coiled-coil domain (homodimerization), and a C-terminal DIL domain (anchoring to cargo via attachments to organelle-specific receptors).
Wild type (top left) and O1 (top right) maize kernels. O1 encodes a myosin XI motor protein (bottom) containing domains for actin binding and ATP hydrolysis (red), myosin light chain binding (yellow), homodimerization (green), and cargo anchoring (blue). Bar = 1 cm. aa, amino acids. (Adapted and reprinted from Wang et al. [2012], Figures 1 [top] and 3 [bottom].)
Consistent with the role of myosin XI proteins in endomembrane system function, the authors show that the O1 mutant has abnormal, dilated endoplasmic reticulum (ER) and small, misshapen protein bodies. In O1 kernels, protein bodies were roughly 60% the size of wild-type protein bodies, with a compensating increase in their overall number. Therefore, total endosperm protein and amounts of zein and nonzein proteins were identical for the wild type and O1, and SDS-PAGE analysis showed no obvious differences in zein protein composition.
O1, which is highly expressed in kernels, was shown to be most abundant in the endomembrane fraction of kernels. Further analysis showed the O1 was associated preferentially with the ER membrane. Because cargo attachment is mediated by myosin tails, the authors used a dominant-negative strategy to analyze the function of O1 in vivo by coexpressing an ER marker protein and the O1 tail domain fused to enhanced yellow fluorescent protein. In Nicotiana benthamiana, they showed that overexpression of the O1 tail domain significantly inhibited ER motility.
Although myosin has a role in formation of ER tubules in yeast and plants, the authors found no evidence of interaction either with the maize homologs of proteins known to be involved in ER tubule formation or with other maize protein body proteins. However, subsequent analysis showed that the O1 C-terminal tail domain interacted with an HSP70 interacting protein (HIP), suggesting that the HIP acts as an adaptor between O1 and the ER. Based on the above evidence and the observation that myosin XI is the major protein responsible for ER streaming (Ueda et al., 2010), the authors propose a model for the role of O1 in ER motility and protein body formation in maize kernels.
References
- Gibbon B.C., Larkins B.A. (2005). Molecular genetic approaches to developing quality protein maize. Trends Genet. 21: 227–233 [DOI] [PubMed] [Google Scholar]
- Ueda H., Yokota E., Kutsuna N., Shimada T., Tamura K., Shimmen T., Hasezawa S., Dolja V.V., Hara-Nishimura I. (2010). Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc. Natl. Acad. Sci. USA 107: 6894–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wang F., Wang G., Wang F., Zhang X., Zhong M., Zhang J., Lin D., Tang Y., Xu Z., Song R. (2012). Opaque1 encodes a myosin XI motor protein that is required for endoplasmic reticulum motility and protein body formation in maize endosperm. Plant Cell 24: 3447–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Messing J. (2010). RNA interference-mediated change in protein body morphology and seed opacity through loss of different zein proteins. Plant Physiol. 153: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]