Abstract
The pattern recognition receptor FLAGELLIN SENSING2 (FLS2) renders plant cells responsive to subnanomolar concentrations of flg22, the active epitope of bacterial flagellin. We recently observed that a preparation of the peptide IDL1, a signal known to regulate abscission processes via the receptor kinases HAESA and HAESA-like2, apparently triggered Arabidopsis thaliana cells in an FLS2-dependent manner. However, closer investigation revealed that this activity was due to contamination by a flg22-type peptide, and newly synthesized IDL1 peptide was completely inactive in FLS2 signaling. This raised alert over contamination events occurring in the process of synthesis or handling of peptides. Two recent reports have suggested that FLS2 has further specificities for structurally unrelated peptides derived from CLV3 and from Ax21. We thus scrutinized these peptides for activity in Arabidopsis cells as well. While responding to <1 nM flg22, Arabidopsis cells proved blind even to 100 μM concentrations of CLV3p and axYs22. Our results confirm the exquisite sensitivity and selectivity of FLS2 for flg22. They also show that inadvertent contaminations with flg22-type peptides do occur and can be detected even in trace amounts by FLS2.
During the last years, the pattern recognition receptor FLAGELLIN SENSING2 (FLS2) and its cognate microbe-associated molecular pattern (MAMP), the peptide flg22 (Felix et al., 1999), have widely been used to study plant innate immunity (Boller and Felix, 2009). Typically, in FLS2-expressing Arabidopsis thaliana cells, flg22 stimulates rapid changes of ion fluxes, including extracellular alkalinization and an induction of defense-related genes, such as FRK1, at threshold concentrations of 10 to 100 pM, while fls2 mutants lacking the receptor kinase FLS2 are completely unresponsive to flg22 (Boller and Felix, 2009). These findings demonstrate that FLS2 has an exquisite sensitivity as a flagellin receptor and that FLS2 is the only receptor for the flg22 ligand in Arabidopsis. Our previous results also indicated an exquisite selectivity of FLS2 with regard to its ligand. For example, the flg22 peptide of Agrobacterium tumefaciens (flg22A.tum.) is completely inactive as a ligand of FLS2 or as a stimulus for FLS2-dependent responses and therefore has often been used as a negative control in assays for flg22-induced responses (Felix et al., 1999; Asai et al., 2002).
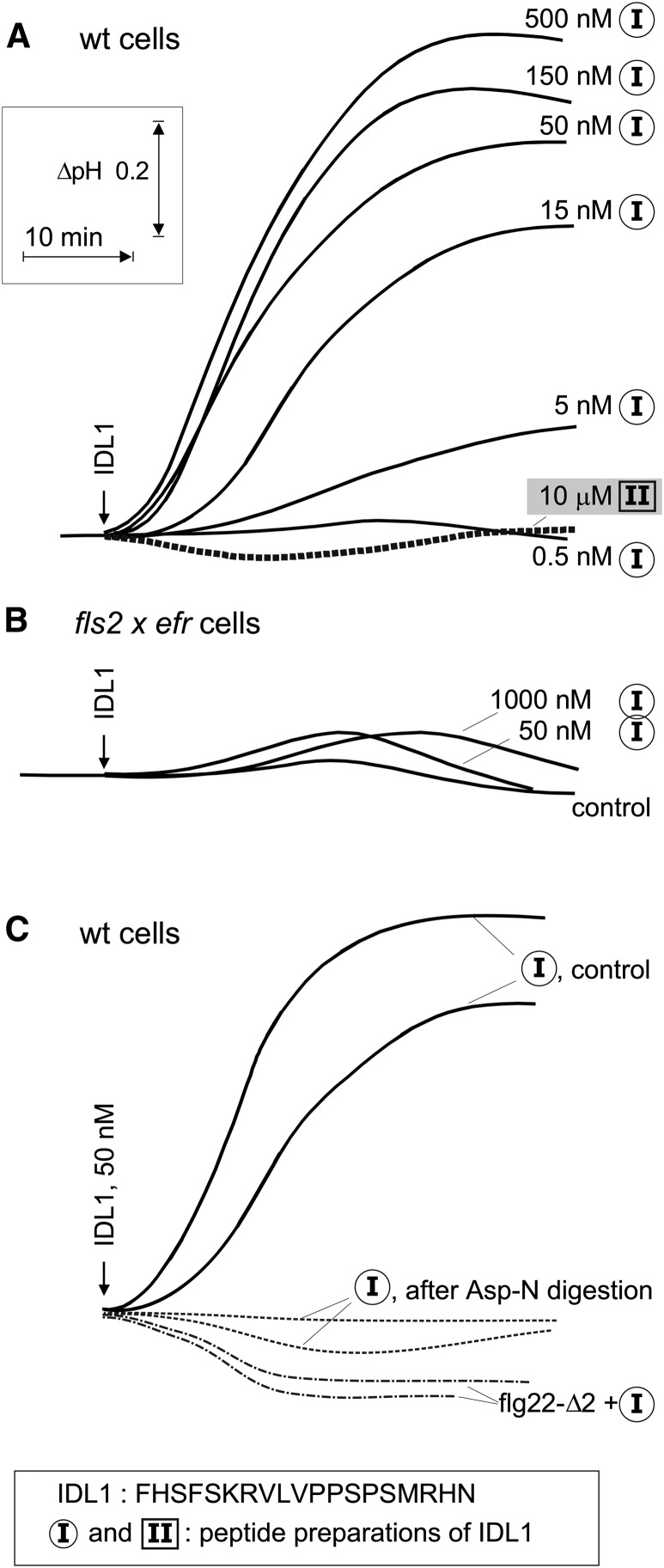
Recently, we observed that a synthetic preparation of IDL1, an endogenous peptide signal involved in abscission processes (Stenvik et al., 2008), showed considerable activity as an inducer of MAMP responses and stimulated extracellular alkalinization in Arabidopsis cells at a threshold level of <5 nM (Figure 1A). More surprisingly, when examined in a cell culture of the fls2 efr double mutant, no significant medium alkalinization was detectable after treatment with the IDL1 preparation (Figure 1B). To check if FLS2 was involved in the response to the IDL1 preparation, we made use of the inhibitor flg22-Δ2, which acts as a specific antagonist of flg22 in Arabidopsis (Bauer et al., 2001). Indeed, presence of 30 μM flg22-Δ2 completely abolished the response to 50 nM IDL1 (Figure 1C).
Figure 1.
Effects of IDL1 Peptides on Extracellular pH in Suspension-Cultured Arabidopsis Cells.
(A) Alkalinization in response to different doses of two independent preparations of the IDL1 peptide (preparations I and II). wt, wild-type.
(B) Alkalinization response in cells from the fls2 efr double mutant.
(C) Alkalinization response in wild-type cells to preparation I of IDL1 alone, to preparation I in combination with the flg22 antagonist flg22-Δ2 (30 μM), or to preparation I after digestion (overnight, 37°C) with endoproteinase AspN, as indicated.
Based on strong genetic evidence, IDL1 is thought to act as a regulator of abscission processes via the receptor kinases HAESA and HAESA-like 2 (Stenvik et al., 2008), but why should FLS2 be involved? The IDL1 preparation was ∼100-fold less effective than authentic flg22 preparations, as indicated by the EC50 values of 0.1 nM for flg22 (Bauer et al., 2001) and 10 nM for IDL1 (Figure 1A), respectively. We hypothesized that the IDL1 preparation might be contaminated by a peptide of the flg22 type. In contrast with IDL1, which has no acidic amino acid residues, flg22 contains two Asp residues that are important for its biological activity on FLS2 (Felix et al., 1999). We used this difference for selective digestion by the endoproteinase AspN, which cuts peptides N-terminal of Asp residues. Indeed, no activity was left in IDL1 after digestion (Figure 1C). This strongly indicated that the activity was not associated with the IDL1 peptide itself but rather with a flg22-type of contamination. Repurification of IDL1 by C18 reverse-phase chromatography could not separate the IDL1 peptide from the flg22 type of activity. Apart from a dominating signal for IDL1, mass spectrometry analysis of this fraction also revealed faint mass signatures characteristic for flg22 and its spontaneous derivative containing pyroglutamate at its N terminus (see Supplemental Figure 1 online). Together, these results clearly pinpointed a contamination as the source of the flg22-like activity in IDL1.
Where did this contamination occur? We observed the same activity with a second, unopened tube from the same batch of the synthetic IDL1 peptide, indicating that a putative flg22 contamination had occurred prior to arrival in our lab, most likely in the company providing the peptide. We therefore resynthesized a new batch of IDL1 and found that it did not cause alkalinization in Arabidopsis wild-type cells even at a concentration of 10 μM (preparation II, Figure 1A). A further, independent, batch of an IDL1-derived peptide with a C-terminal extension by two amino acid residues similarly failed to induce alkalinization in the Arabidopsis wild-type cells (data not shown). These results made us acutely aware of a potential contamination problem when working with peptides unrelated to flg22. Indeed, two recent reports have suggested that FLS2 perceives two additional, unrelated, peptidic signals derived from either CLV3 (Lee et al., 2011) or Ax21 (Danna et al., 2011), respectively. What if these unexpected results were due to inadvertent contamination by flg22 as well?
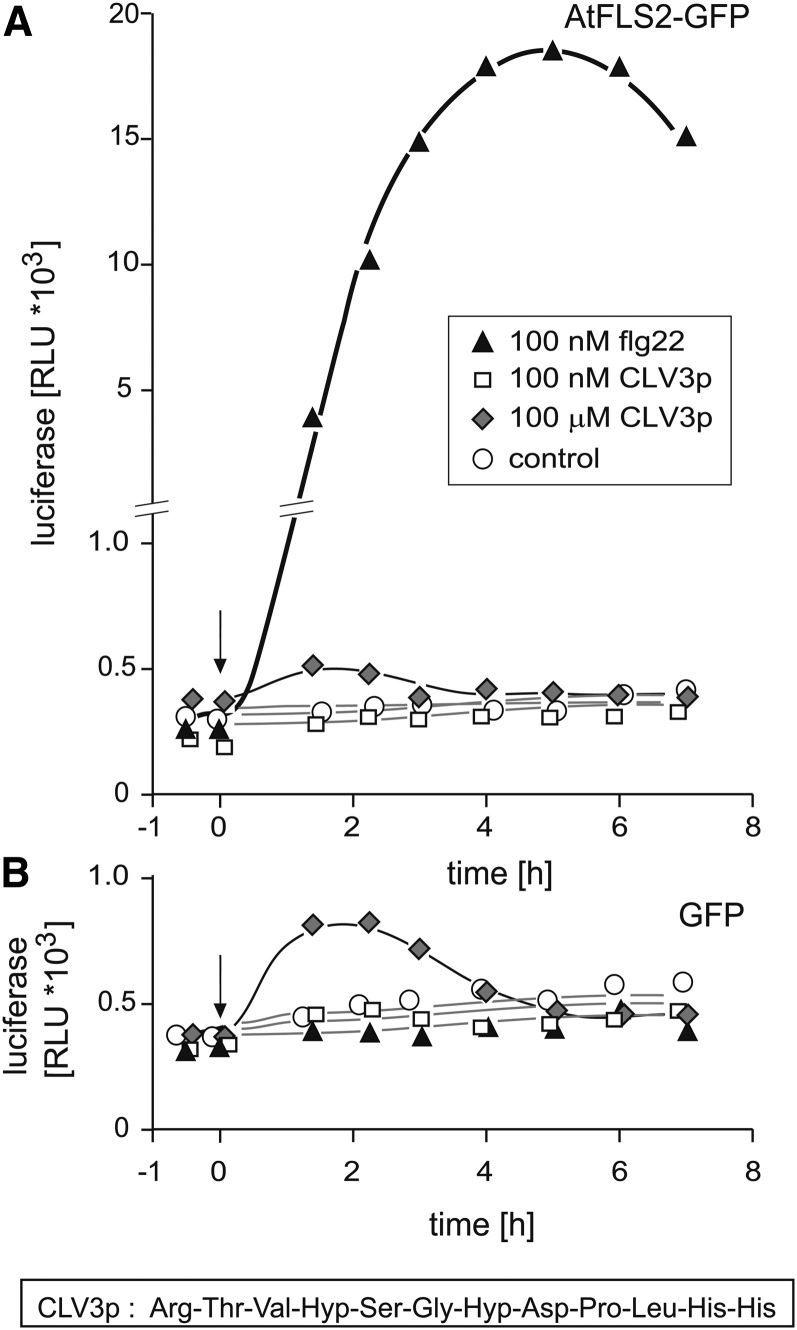
In a recent study (Mueller et al., 2012), we compared the FLS2 orthologs from Arabidopsis and tomato (Solanum lycopersicum) and their chimeras, making use of protoplasts from fls2 mutant plants transformed simultaneously with constructs encoding one of the FLS2 orthologs and a pFRK1:luciferase reporter, an assay system originally introduced by Asai et al. (2002). Protoplasts with both versions of FLS2 exhibited exquisite sensitivity to picomolar concentrations of flg22. However, they failed to respond to the hydroxylated CLV3 peptides CLV3-ΔAra3-ΔH (12 amino acids) and CLV3-ΔAra3 (13 amino acids) (described in Ohyama et al., 2009), termed CLV3p and CLV3p-H in our article (see Figure 1 in Mueller et al., 2012). Indeed, even when applied at a concentration of 100 μM, the 12–amino acid CLV3p caused no significant response in protoplasts expressing FLS2 from Arabidopsis (Figure 2A). A marginal transient increase in luminescence occurred in the first 2 h of the experiment, but this effect was also seen in the absence of FLS2 (Figure 3B), demonstrating that it had nothing to do with FLS2-dependent activation of the reporter gene. Our preparation of the CLV3p peptide exhibited the expected strong inhibitory effect in root growth assays with wild-type Arabidopsis and with fls2 mutants but not with the mutant clv2-1 (see Supplemental Figure 2 online).
Figure 2.
The CLV3p Peptide (Arg-Thr-Val-Hyp-Ser-Gly-Hyp-Asp-Pro-Leu-His-His, CLV3-ΔAra3-ΔH in Ohyama et al., 2009) Does Not Induce Expression of the Reporter pFRK1:luciferase via the Receptor FLS2.
Mesophyll protoplasts from efr×fls2 mutants were transformed with pFRK1:luciferase (pFRK1, promoter of the flagellin responsive receptor kinase 1) together with p35S:FLS2-GFP (A) or p35S:GFP (B) and tested for responsiveness to CLV3p and flg22 as indicated. GFP, green fluorescent protein; RLU, relative light units.
Figure 3.
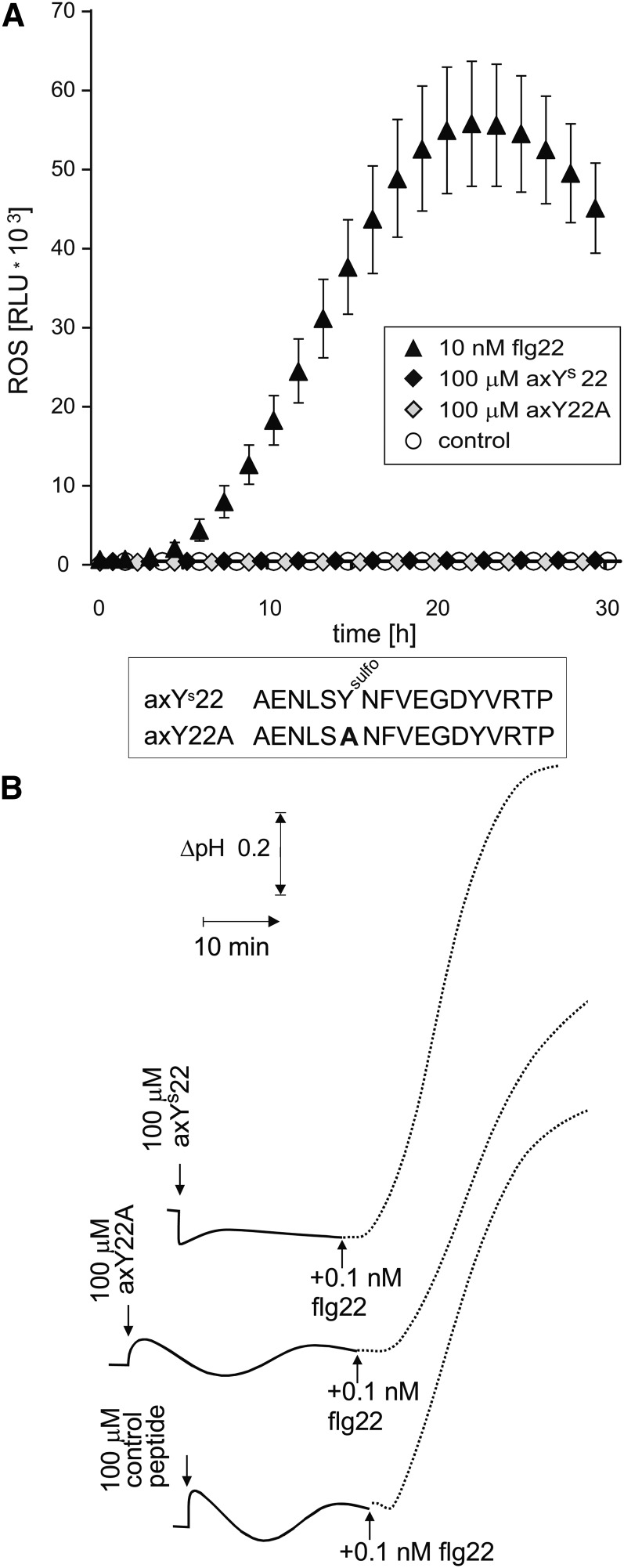
Ax21-Derived Peptides axYs22 and axY22A Show No Activity as Inducers of Oxidative Burst and Medium Alkalinization in Arabidopsis.
(A) Oxidative burst in leaf pieces of Arabidopsis treated with axYs22, axY22A, or flg22 as indicated. Reactive oxygen species (ROS) were determined by light emission (relative light units [RLU] of the luminometer) in a luminol-based assay. Values and error bars represent mean ± se of n = 6 replicates. (Error bars in all samples not treated with flg22 were smaller than 100 relative light units.)
(B) Extracellular alkalinization in cell cultures of Arabidopsis treated with axYs22, axY22A, or a control peptide (SASRSRIQDADFAAETANLSRSQILQQAGTA) in combination with flg22, as indicated.
In previous work by Lee et al. (2009), the sulfated peptide axYs22, derived from the protein Ax21 of the pathogenic bacterium Xanthomonas oryzae, but not its variant form axY22A, in which the sulfotyrosine was replaced by an Ala, have been reported to cause a resistance response in rice (Oryza sativa) expressing the receptor kinase XA21. Recently, preparations of both of these two peptides have been reported to stimulate immune responses in Arabidopsis when applied at concentrations of 1 to 100 μM (Danna et al., 2011; Figures 1 to 3). Surprisingly, this activation was dependent on the presence of a functional FLS2 receptor, suggesting that these peptides are acting as ligands for FLS2 as well. We obtained fresh preparations of both axYs22 and axY22A. In our hands, both peptides were completely inactive at concentrations up to 100 μM in oxidative burst and alkalinization assays (Figure 3). The cells used for the alkalinization assays strongly responded to 100 pM of authentic flg22, indicating that the Ax21-related peptides were at least a million times less efficient to induce alkalinization via FLS2 (Figure 3B).
The peptide flg22-Δ2 functions as a competitive antagonist that specifically inhibits flg22-induced responses in Arabidopsis (Bauer et al., 2001). Since the FLS2-dependent responses to CLV3p and the Ax21 peptides were reported to be inhibited by excess flg22-Δ2 (Danna et al., 2011; Lee et al., 2011), we also checked whether CLV3p or the Ax21 peptides might interfere with the binding of flg22 to FLS2 (Figure 4). The receptor FLS2 binds carrier-free 125I-Tyr-flg22 with a high affinity, and this binding can be specifically competed by 10 μM unlabeled flg22 but neither by 30 μM CLV3p nor by 30 μM axYs22 (Figure 4). Thus, we cannot confirm that CLV3p or axYs22 can directly interact and activate FLS2. While inadvertent contamination is a possible explanation, we cannot finally explain the obvious discrepancies to the results in the Lee et al. (2011) and Danna et al. (2011) reports. Also, since our analysis focused on direct and immediate effects on FLS2, we cannot comment on effects that high concentrations of peptides like CLV3p or axYs22 might exert on prolonged treatment. For example, induction of plant resistance is a highly complex process that develops over days and involves two living systems. Rather than on the host cells, peptides applied might act on the pathogenic bacteria and influence their synthesis of flagellin or their assembly/disassembly of flagellin subunits into flagellar structures. At least for the ax21 peptides, described as a quorum sensing type of signals for bacteria, this is an option to be considered.
Figure 4.
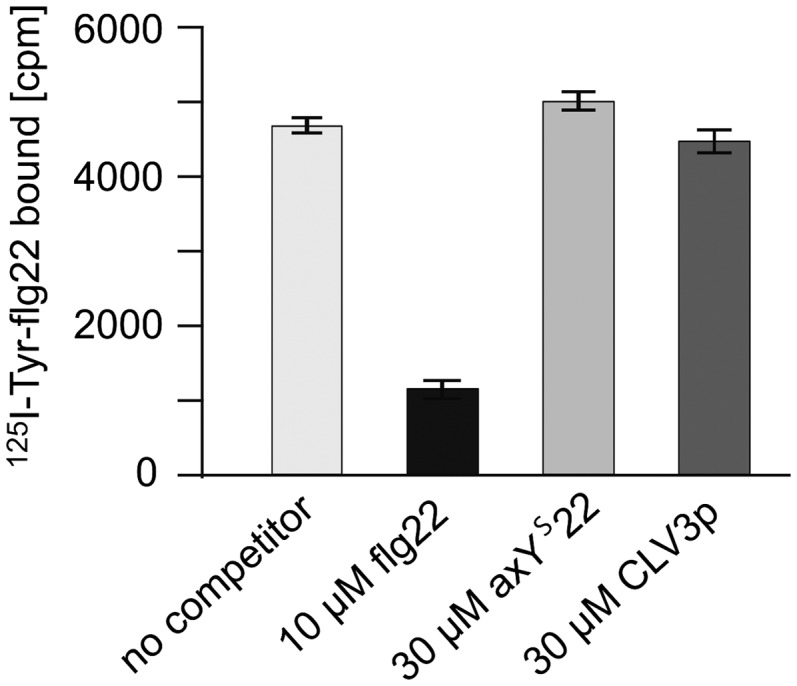
The Peptides axYs22 and CLV3p Do Not Compete for Binding of flg22 to FLS2.
Binding of 125I-Tyr-flg22 to wild-type Arabidopsis seedlings in the absence of competitor, in the presence of 10 μM unlabeled flg22, or in the presence of 30 μM unlabeled axYs22 or CLV3p. Bars and error bars represent radioactivity (counts per min [cpm]) bound to plant material as means and sd of n = 3 replicates.
Our results confirm the exquisite sensitivity and selectivity of FLS2 for its cognate ligand, flg22. They also show that extreme care must be taken when attempting to assess the effect of peptides on responses that can also be elicited by flg22. Based on our experience, peptide preparations ordered from different commercial suppliers may occasionally be contaminated by flg22-related activity. We observed a contamination corresponding to ∼1% of flg22 equivalents in one of the IDL1 preparations (Figure 1). However, we would like to emphasize that in a peptide preparation applied at 100 μM a contamination by flg22 of only ∼0.0001% (∼1 ppm) can activate FLS2-dependent responses. Using HPLC and mass spectrometry analysis as common checks for purity, suppliers guarantee that a certain percentage, maximally 99%, of the preparation corresponds to the peptide ordered. However, as exemplified for the contaminated IDL1 preparation (see Supplemental Figure 1 online), contaminations at or below 1% can easily go unnoticed. Also, normally, the molecular characteristics of a potential contaminant are not known, so a flg22-type of activity could be present as a partial degradation product or in the form of an unknown flg22 derivative.
We cannot estimate a frequency for cross-contaminations in peptide preparations, but it seems to occur surprisingly often. Over the years, we ordered >100 peptides from various commercial suppliers and had at least two further incidents with flg22-type contaminations. In one of these cases, we ordered, and obtained, flg22 and three structurally unrelated peptides. We found residual flg22-type activity in two out of the three preparations of these unrelated peptides, indicating contamination in the course of commercial peptide synthesis (in this case, by a supplier different from the provider of IDL1) or during handling of these peptides in our lab.
There are reasons why contaminations by flg22 might pose a particular risk. First, flg22 has a tendency to stick to surfaces and we recommended the use of 0.1 M NaCl and 1 mg/mL BSA to prevent loss of the peptide during serial dilutions (Felix et al., 1999). In turn, flg22 adhering to tubings, columns, or glassware might provide a source of contamination for peptides getting handled subsequently. Second, we noticed that lyophilized flg22 can easily pick up electrostatic charge and is prone to float around with the slightest streams of air. This could be a particular problem also for preparations handled by robots of peptide manufacturers. Third, due to a considerable demand by an increasing number of labs, flg22-related peptides have been ordered from various peptide suppliers numerous times and, picking up this peptide as inadvertent contamination has become a considerable problem.
In conclusion, our study complements and extends the commentary by Segonzac et al. (2012) by demonstrating that the receptor FLS2 has an extraordinarily high affinity and selectivity to its ligand, flg22, and that it is completely blind to the peptides IDL1, CLV3p, axYs22, and axY22A even in our most sensitive bioassays. Our results and arguments do not apodictically exclude that a receptor like FLS2 could have a second, physiologically relevant, ligand. Also, there may be chemical structures that inadvertently act as surrogates or mimetics of the true ligand flg22. However, in view of the high selectivity of the FLS2 for its genuine ligand flg22, we think the probability of alien interactors is rather low. By contrast, contaminations with flg22-related molecules can and do occur.
How can contamination of bioactive peptides be recognized and avoided? The first, probably most important, point is a sharpened awareness about in-lab and supplier-dependent sources of contamination. These risks are often ignored, in particular when working with synthetic, purified peptides. A purity of >95 or >99%, as guaranteed by suppliers, is of limited value with respect to highly active contaminants detectable even at the ppm level. Purification offered by suppliers certainly helps to remove chemicals used in the synthesis process and to get rid of many incomplete variants of the peptide ordered. However, at least theoretically, contaminated equipment used during the purification might contribute to the problem rather than to its solution. Dose–response relationships for the peptides under scrutiny are important to consider physiological relevance in general and to compare activities with published data in particular. Thereby, the higher the dose of a peptide applied, the higher the risk to pick up even spurious contaminants. Furthermore, analysis of bioactive peptides should not depend on a single peptide preparation alone. Peptide variants are crucial to elucidate the specificity of an interaction process. The use of several, independently synthesized and handled peptide preparations should help to reliably detect sporadic contamination events and to distinguish contaminants from true ligands. Finally, at least for the particular problem of contamination by flg22, we can offer testing peptide preparations using the sensitive bioassays established in our labs. As long as we have the hands and capacity to handle such requests, we certainly would like to contribute with such a service to detect pirate peptides.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. MALDI-TOF Analysis of IDL1 (Preparation I) after Repurification on C18 Reverse-Phase Column.
Supplemental Figure 2. Effect of CLV3p and flg22 on the Growth of Arabidopsis Seedlings.
Supplementary Material
Acknowledgments
This work was supported by the Swiss National Science Foundation (Grant 127563 to T.B. and Grant 138255 to D.C.) and by financial support from the German Research Foundation to K.M. and A.K.J. as part of the programs SFB446, SFP766, and ERA-Net plant genomics “RLKRLPs.” We thank Reidunn Aalen and Melinka Butenko (Oslo University) for advice with the IDL1 ligands and Martina Bluhm (University of Tuebingen) for help with peptide analysis.
AUTHOR CONTRIBUTIONS
T.B. and G.F. designed the research and wrote the article. H.K. provided and analyzed peptides. K.M., D.C., M.A., and A.K.J. tested peptides for biological activity.
References
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.L., Gómez-Gomez L., Boller T., Ausubel F.M., Sheen J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bauer Z., Gómez-Gómez L., Boller T., Felix G. (2001). Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. J. Biol. Chem. 276: 45669–45676 [DOI] [PubMed] [Google Scholar]
- Boller T., Felix G. (2009). A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Danna C.H., Millet Y.A., Koller T., Han S.W., Bent A.F., Ronald P.C., Ausubel F.M. (2011). The Arabidopsis flagellin receptor FLS2 mediates the perception of Xanthomonas Ax21 secreted peptides. Proc. Natl. Acad. Sci. USA 108: 9286–9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Duran J.D., Volko S., Boller T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Lee H., Chah O.-K., Sheen J. (2011). Stem-cell-triggered immunity through CLV3p-FLS2 signalling. Nature 473: 376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.W., Han S.W., Sririyanum M., Park C.J., Seo Y.S., Ronald P.C. (2009). A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 326: 850–853 [DOI] [PubMed] [Google Scholar]
- Mueller K., Bittel P., Chinchilla D., Jehle A.K., Albert M., Boller T., Felix G. (2012). Chimeric FLS2 receptors reveal the basis for differential flagellin perception in Arabidopsis and tomato. Plant Cell 24: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Shinohara H., Ogawa-Ohnishi M., Matsubayashi Y. (2009). A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat. Chem. Biol. 5: 578–580 [DOI] [PubMed] [Google Scholar]
- Segonzac C., Nimchuk Z.L., Beck M., Tarr P.T., Robatzek S., Meyerowitz E., Zipfel C. (August 24, 2012). The shoot apical meristem regulatory peptide CLV3 does not activate innate immunity. Plant Cell 24: 3186–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvik G.E., Tandstad N.M., Guo Y., Shi C.L., Kristiansen W., Holmgren A., Clark S.E., Aalen R.B., Butenko M.A. (2008). The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in Arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 20: 1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.