In rice (Oryza sativa), adventitious roots emerge at the stem nodes in response to submergence. To facilitate root emergence, epidermal cells above root primordia undergo cell death. Root growth and cell death are controlled by ethylene via reactive oxygen species signaling. In addition, growing roots exert a local force on epidermal cells. The mechanical signal provides spatial resolution to ethylene-induced local cell death.
Abstract
A central question in biology is how spatial information is conveyed to locally establish a developmental program. Rice (Oryza sativa) can survive flash floods by the emergence of adventitious roots from the stem. Epidermal cells that overlie adventitious root primordia undergo cell death to facilitate root emergence. Root growth and epidermal cell death are both controlled by ethylene. This study aimed to identify the signal responsible for the spatial control of cell death. Epidermal cell death correlated with the proximity to root primordia in wild-type and ADVENTITIOUS ROOTLESS1 plants, indicating that the root emits a spatial signal. Ethylene-induced root growth generated a mechanical force of ∼18 millinewtons within 1 h. Force application to epidermal cells above root primordia caused cell death in a dose-dependent manner and was inhibited by 1-methylcyclopropene or diphenylene iodonium, an inhibitor of NADPH oxidase. Exposure of epidermal cells not overlying a root to either force and ethylene or force and the catalase inhibitor aminotriazole induced ectopic cell death. Genetic downregulation of the reactive oxygen species (ROS) scavenger METALLOTHIONEIN2b likewise promoted force-induced ectopic cell death. Hence, reprogramming of epidermal cell fate by the volatile plant hormone ethylene requires two signals: mechanosensing for spatial resolution and ROS for cell death signaling.
INTRODUCTION
For plants, flooding is a frequently encountered environmental stress that results in reduced gas diffusion and, as a consequence, oxygen deprivation in submerged plant parts. In response, plants have evolved several adaptations that help to relieve or to mitigate low oxygen conditions (Bailey-Serres and Voesenek, 2008; Bailey-Serres et al., 2012). One major consequence of reduced oxygen tension is a shutdown of respiratory ATP synthesis, which results in metabolic adaptations. Altered gene expression that accommodates anaerobic metabolism is regulated by transcription factors of subgroup VII of the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) gene family. This subgroup includes SUBMERGECE1A-1 and SNORKEL genes of rice (Oryza sativa) (Xu et al., 2006; Hattori et al., 2009) and RAP2.2, RAP2.12, ERF71/HRE2, and ERF73/HRE1 of Arabidopsis thaliana (Hinz et al., 2010; Licausi et al., 2011). Some ERFs, such as ERF71/HRE2 and ERF73/HRE1, are expressed at elevated levels in hypoxic conditions. RAP2.12 and ERF71/HRE2 proteins are controlled at the level of protein stability (Gibbs et al., 2011; Licausi et al., 2011). At normoxic conditions, RAP2.12 and ERF71/HRE2 are targeted for protein degradation via the N-end rule pathway and for protein stabilization in response to low oxygen.
The gaseous hormone ethylene accumulates rapidly in submerged tissue upon flooding and mediates many adaptations to submergence. These include control of stem growth inhibition during the quiescence response in submergence-resistant rice plants as well as rapid stem growth, which helps deepwater rice to escape flooding (Xu et al., 2006; Hattori et al., 2009). Furthermore, ethylene promotes growth of adventitious roots in rice (Lorbiecke and Sauter, 1999; Steffens et al., 2006), Rumex palustris (Peeters et al., 2002), Vigna radiata (Robbins et al., 1985), and tomato (Solanum lycopersicum) (Phatak et al., 1981). Ethylene insensitivity resulted in the formation of fewer adventitious roots in tobacco (Nicotiana tabacum) (McDonald and Visser, 2003) and in reduced growth of adventitious roots in petunia (Petunia × hybrida) (Clark et al., 1999). Adventitious roots can replace the soil-borne roots when these become dysfunctional because of oxygen shortage as a result of flooding.
Secondary roots develop either de novo within older roots (lateral roots) or shoots (adventitious roots). In rice, adventitious root primordia are formed at the nodes as part of normal development (Bleecker et al., 1986). Adventitious root formation is controlled by GNOM, which encodes a GDP/GTP exchange factor for small G proteins of the ADP-ribosylation factor class (ARF-GEF; Liu et al., 2009). The ARF-GEF GNOM is also involved in lateral root formation, but the transcription factors CROWN ROOTLESS1/ADVENTITIOUS ROOTLESS1 (CRL1/ARL1) and CRL5 specifically control adventitious root formation (Inukai et al., 2005; Liu et al., 2005; Kitomi et al., 2011). CRL5 encodes a member of the AP2/ERF family (Kitomi et al., 2011). CRL1/ARL1 (known as ARL1) was described as an auxin- and ethylene-responsive gene and belongs to the ASYMMETRIC LEAVES2 (AS2)/LATERAL ORGAN BOUNDARIES (LOB) domain (LBD) gene family. Mutants with reduced ARL1 or CRL5 expression have reduced numbers of adventitious roots, and the arl1/crl5 double mutant completely lacks adventitious roots (Liu et al., 2005; Kitomi et al., 2011). To emerge from their native organ, secondary roots must penetrate inner cell layers and the epidermis, which acts as a physical barrier to protect the organ. In Arabidopsis, lateral roots have to penetrate three cell layers, the endodermis, the cortex, and the epidermis (Péret et al., 2009), and genes encoding for cell wall–loosening enzymes, such as expansin and pectate lyase, were shown to be expressed in front of emerging lateral root primordia (Neuteboom et al., 1999; Swarup et al., 2008). Epidermal cells are particularly well attached to each other. In Arabidopsis, epidermal cell separation is facilitated through enhanced xyloglucan endotransglycosylase/hydrolase activity, which was observed at sites of lateral root emergence (Vissenberg et al., 2005; Swarup et al., 2008). Local expression of a cutinase in epidermal cells overlying emerging lateral roots further indicates that the cuticle is weakened before root penetration (Takahashi et al., 2010).
In rice, emergence of adventitious roots is preceded by death of those nodal epidermal cells that overlie adventitious root primordia. It was hypothesized that this cell death response prevents damage to the emerging root tip (Mergemann and Sauter, 2000). Similar to growth of adventitious root primordia, epidermal cell death is controlled by ethylene and is mediated by reactive oxygen species (ROS). Regulation of root growth and epidermal cell death by the same signals provides a means to coordinate adventitious root growth with local weakening of the epidermal cell barrier (Steffens and Sauter, 2005, 2009a). Epidermal cells above root primordia that undergo cell death were shown to have a distinct molecular identity, with a transcriptome that is largely different from that of other nodal epidermal cells (Steffens and Sauter, 2009a). The unique molecular identity and the defined developmental fate of epidermal cells overlying adventitious root primordia raise the question of how and when this specific cell death fate is acquired. Epidermal cell death is strictly confined to sites of adventitious root formation; therefore, we hypothesized that adventitious root primordia emit a signal that determines epidermal cell death identity. We further hypothesized that this signal might be mechanical force. To test these hypotheses, we analyzed the force exerted by growing adventitious roots and the effect that a mechanical force of similar strength has when applied ectopically. These studies revealed that the emerging adventitious root communicates with the epidermis via mechanosignaling. The mechanical force is however not sufficient but in addition requires ethylene to promote cell death. At the cellular level, ethylene-mediated root growth and ethylene-dependent cell death require ROS activity.
RESULTS
Development and Emergence of Adventitious Roots in Relation to Epidermal Cell Death
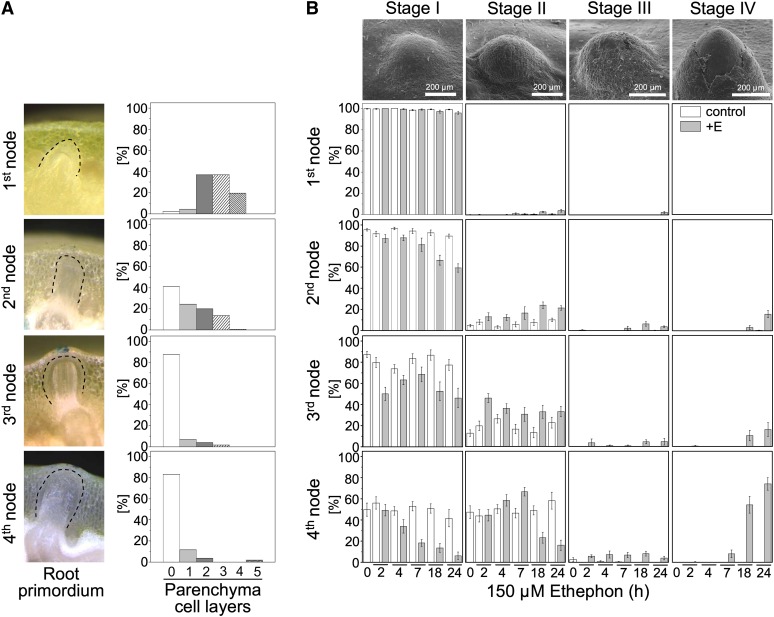
Rice stems develop adventitious root primordia at their nodes as part of normal development (Bleecker et al., 1986; Figure 1A). These primordia emerge when the stem is submerged as a result of flooding whereby growth of adventitious roots is induced by ethylene (see Supplemental Figures 1A and 1B online). 1-Methylcyclopropene (1-MCP), an inhibitor of ethylene perception, effectively suppresses ethylene-induced adventitious root growth (see Supplemental Figure 1A online). Root emergence is preceded by death of those nodal epidermal cells that cover the adventitious root primordium (Mergemann and Sauter, 2000; Figure 1B).
Figure 1.
Development of Adventitious Root Primordia in Relation to Epidermal Cell Death.
(A) Adventitious roots at the first to fourth node and the number of parenchymal cell layers between the root tip and the epidermis at each node in rice cultivar PG56. Data presented are means (n = 22 to 45).
(B) Scanning electron microscopy views of emerging adventitious root primordia at the third node of rice cultivar PG56. At stage I, all cells overlying a primordium are alive; at stage II, single cells are dead; at stage III, a patch of epidermal cells has undergone cell death; and at stage IV, the epidermis has cracked open, and the underlying root primordium is visible. Stem sections were treated with 150 μM of ethephon (E) for up to 24 h, and percentages of the four stages were determined at all four nodes using Evans Blue staining. Data presented are mean ± se (n > 11).
Bars in (B) = 200 μm.
[See online article for color version of this figure.]
Root growth and epidermal cell death in rice require proper timing and spatial coordination to minimize tissue damage and reduce the risk for infection. In the absence of an external stimulus, root primordia grow at a slow rate without emerging while nodes mature (Figure 1A, left column). At the first node, the root tip is separated from the epidermis by two to four parenchymal cell layers (Figure 1A, right column). As nodes get older, the primordia get closer to the epidermis. At the third and fourth nodes, nearly all roots are in immediate contact with the epidermis, which remains intact in the absence of a growth-inducing stimulus.
We next analyzed ethylene-induced epidermal cell death at each node of rice cultivar Pin Gaew 56 (PG56) (Figure 1B). Cell death stages were defined as described elsewhere (Mergemann and Sauter, 2000). Briefly, in stage I, all cells are alive. Single dead cells are observed in stage II. A continuous patch of dead cells defines stage III. At stage IV, the epidermis is cracked open, making a place for an emerging root (Figure 1B, Top). The detailed analysis of stages I to IV revealed that the lag phases of cell death induced by the ethylene-releasing compound ethephon were shorter and that the extent of cell death was greater at older nodes than at younger nodes (Figure 1B, Bottom). The lag phase was inversely related to the number of parenchymal cell layers present between the root tip and the epidermis. Root penetration and lengths of penetrated roots increased as nodes matured (see Supplemental Figure 1B online).
Epidermal Cell Death Is Dependent on the Presence of Adventitious Root Primordia
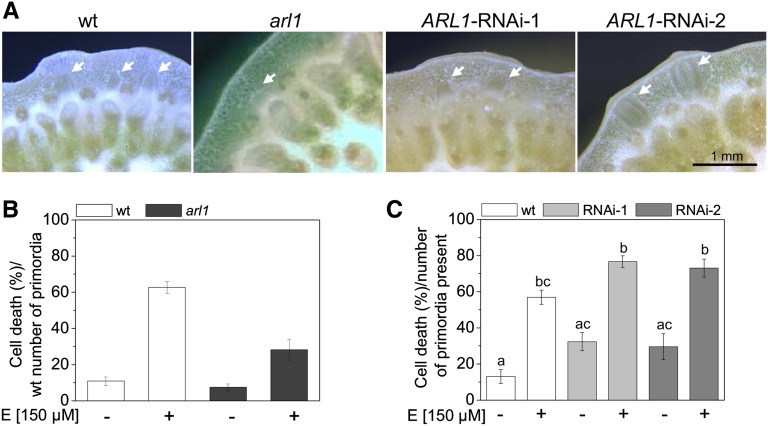
The formation of adventitious roots in rice requires ARL1 (Inukai et al., 2005; Liu et al., 2005). The arl1 deletion mutant and two ARL1-RNA interference (RNAi) lines, RNAi-1 and RNAi-2, from rice cultivar Zhonghua 11 were previously shown to have significantly fewer adventitious roots. The arl1 deletion mutant has an average of four, and the ARL1-RNAi-1 and ARL1-RNAi-2 lines have seven root primordia per node as compared with 13 in the wild-type cultivar (Figure 2A). These knockdown mutants were therefore considered to be useful to assess whether the presence of adventitious roots was required for the induction of epidermal cell death.
Figure 2.
Epidermal Cell Death Is Dependent on the Presence of an Adventitious Root Primordium.
(A) Cross sections from third nodes of rice cultivar Zhonghua 11 wild-type (wt), arl1, and ARL1-RNAi-1 and ARL1-RNAi-2. Knockdown lines have fewer roots than the wild type. Arrows indicate root primordia.
(B) Rice wild-type and arl1 stem sections were treated with 150 μM of ethephon (E) for 48 h, and cell death at the third node was determined based on wild-type numbers of adventitious root primordia. Results are means (±se) from 22 to 28 stem sections analyzed per genotype and treatment. Ethephon-induced cell death rates of the wild type are significantly higher than the control and higher than cell death rates of arl1 at P < 0.001 (ANOVA).
(C) Cell death observed after treatment of wild-type, ARL1-RNAi-1, and ARL1-RNAi-2 stem sections with 150 μM of ethephon for 48 h were calculated as percentages of the number of roots present (ANOVA; P < 0.001). Data presented are means (±se; n = 23 stem sections analyzed per treatment). a, b, and c indicate significantly different values.
A dose–response analysis of ethephon-induced cell death at the third node of wild-type rice cultivar Zhonghua 11 showed that epidermal cell death was induced in a dose-dependent manner by ethephon (see Supplemental Table 1 online) as was shown previously for other rice cultivars (Mergemann and Sauter, 2000; Steffens and Sauter, 2005). Treatment with 15 μM of ethephon resulted in 8.0 and 19.0% cell death after 22 h and 48 h, respectively, as compared with 1.0 and 10.9% in controls. Treatment with 150 μM of ethephon resulted in 36.3 and 56.9% cell death after 22 h and 48 h, respectively. The percentage of cell death induced by 150 μM of ethephon was 20.8% in arl1 as compared with 51.8% in the wild type based on the wild-type root numbers (Figure 2B). Based on the actual number of roots present in the respective genotypes, induction of cell death occurred at similar rates in ARL1-RNAi lines and the wild type (Figure 2C). Epidermal cell death was observed exclusively above root primordia in both genotypes. The mutant analysis supported the idea that ethylene-induced epidermal cell death is dependent on an underlying root primordium.
Adventitious Root Growth Depends on Ethylene and ROS Signaling
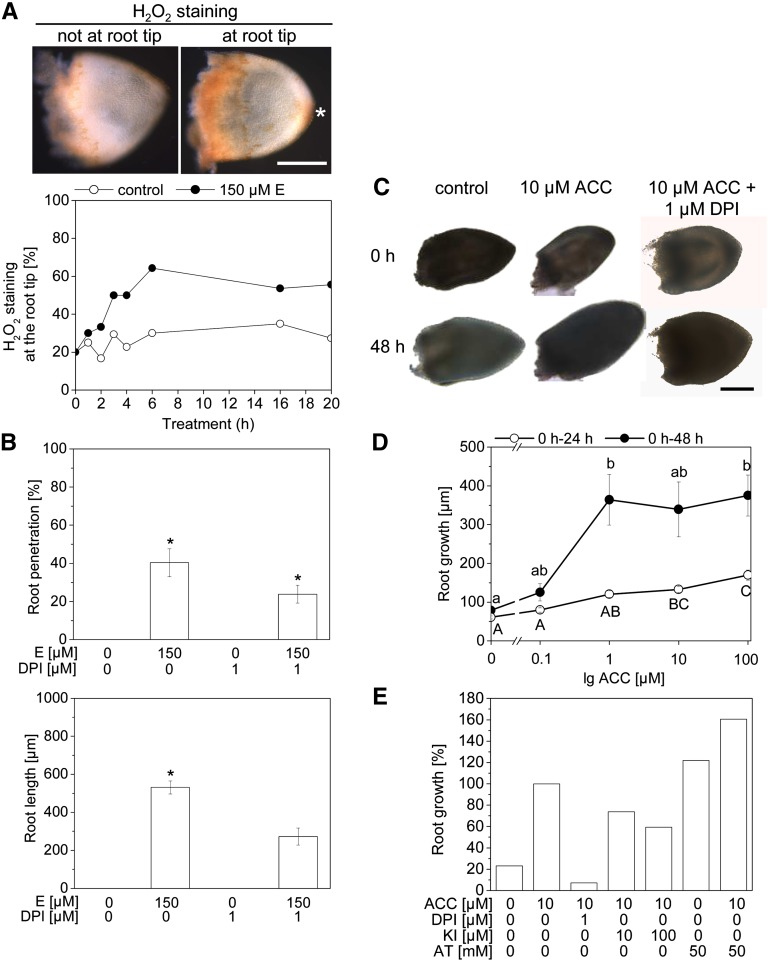
To understand how ethylene-induced adventitious root growth is mediated at the cellular level, we analyzed a possible role of ROS in this response. Hydrogen peroxide (H2O2) was visualized with 3,3′-diaminobenzidine (DAB) in isolated adventitious roots of the third node of rice cultivar PG56 (Figure 3A, Top). Staining was observed at the root base, which was wounded as a result of root isolation, and at the root tip. Ethylene-treated adventitious root primordia displayed enhanced H2O2 production at the tip in comparison with untreated controls after 4 to 20 h (Figure 3A, Bottom). We next analyzed whether ethylene-induced growth of root primordia was dependent on ROS homeostasis and/or production (Figures 3B, 3C, and 3E). Endogenous ROS levels were lowered through inhibition of NADPH oxidase with 1 μM of diphenylene iodonium (DPI). DPI partially inhibited ethylene-induced primordia growth when applied to rice stems (Figure 3B) and fully inhibited growth of isolated root primordia (Figures 3C and 3E). Growth of isolated root primordia was promoted by 1-aminocyclopropane-1-carboxylate (ACC), the natural precursor of ethylene, at concentrations of 1, 10, and 100 μM (Figures 3C and 3D). Potassium iodide (KI), an H2O2 scavenger, partially inhibited primordia growth (Figure 3E). Vice versa, the catalase inhibitor 3-amino-1,2,4-triazole (AT), which promotes ROS accumulation, promoted growth in the absence of ethylene and enhanced ethylene-induced root growth (Figure 3E). Genetic downregulation of METALLOTHIONEIN2b (MT2b), which encodes a ROS scavenger, did not alter ethylene-induced root growth (see Supplemental Figure 2A online), even though MT2b::Tos17 plants showed enhanced accumulation of O2− and H2O2 at the root tip (see Supplemental Figure 2C online).
Figure 3.
Ethylene-Induced Adventitious Root Growth Is Dependent on ROS.
(A) Stem sections of rice cultivar PG56 were treated with or without 150 μM of ethephon (E) for up to 20 h. Isolated adventitious roots were stained with DAB to visualize H2O2. Adventitious root with H2O2 staining after 6 h with 150 μM of ethephon (Top). Asterisk indicates stained root tip. Percentage of H2O2 staining at the root tip after treatment with or without ethephon for the times indicated (Bottom). Data are means (n = 10 to 20).
(B) Stem sections were treated with or without 150 μM of ethephon for 48 h with or without 1 μM of DPI. The number of penetrated roots and root lengths were measured. Data are means ± se (n = 24 to 27). Asterisks indicate significantly different values (ANOVA; P < 0.001).
(C) Isolated adventitious root primordia were treated with 10 μM of ACC with or without 1 μM of DPI for 48 h.
(D) Isolated adventitious root primordia were treated with up to 100 μM of ACC, and the increase in length of each primordium was determined after 24 and 48 h. Increases in root lengths are means ± se (n = 20 to 41). Different letters indicate significantly different values (ANOVA; P < 0.001).
(E) Isolated adventitious roots were treated for 48 h with 10 μM of ACC, 1 μM of DPI, 10 or 100 μM of KI, or 50 mM of AT at the combinations indicated. ACC-induced root growth was set as 100, and all other values were calculated as a percentage of that. Data are means (n > 30).
Bars in (A) and (C) = 200 μm.
In summary, inhibitor studies indicated that ethylene-induced adventitious root growth is mediated by ROS whereby changes in ROS activity resulting from downregulation of MT2b were not favorable for growth promotion. Ethylene is a volatile hormone that accumulates in all flooded plant cells, and ROS mediate both epidermal cell death and root growth; therefore, we pursued the question of how spatial information is exchanged between root and epidermis to ensure that only those cells overlying a root tip are induced to undergo cell death.
Ethylene Signaling Results in Generation of a Mechanical Force
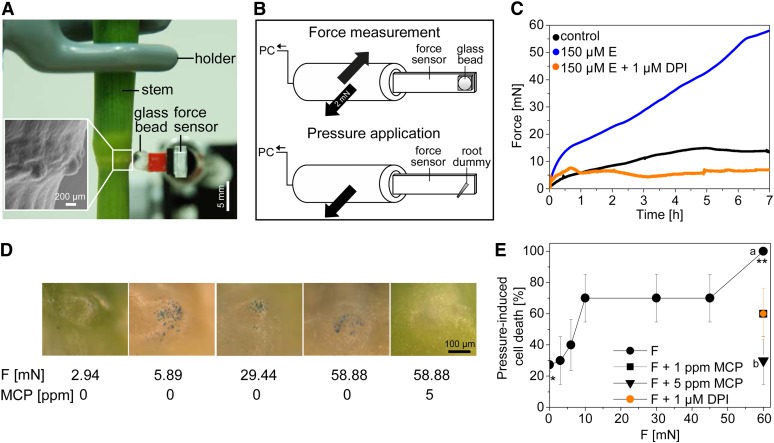
Based on the proximity of the emerging root and the epidermal cells, we hypothesized that growing root primordia emit a positional signal that determines epidermal cell death. We further hypothesized that the signal might be mechanical. To test whether roots exert a mechanical force, we placed a force sensor on the epidermis above a root primordium (Figures 4A and 4B). In rice cultivar PG56, application of 150 μM of ethephon, an optimal concentration for induction of root growth and cell death, generated a force of ∼15 millinewtons (mN) within 30 min, which increased to ∼59 mN within 7 h. In control roots, force values remained lower throughout the 7-h period and did not exceed 15 mN (Figure 4C). Cell death was previously shown to be induced within 1.5 h of ethephon treatment, and roots penetrated the epidermis after 7 to 10 h at the third node (see Supplemental Figure 1B online; Steffens and Sauter, 2005). No force was induced by 150 μM of ethephon when a force sensor was placed above nodal epidermis cells not covering an adventitious root (see Supplemental Figure 3 online). These data support the idea that a mechanical signal is generated by the growing adventitious root rather than ethylene-induced turgor pressure in the epidermis.
Figure 4.
Ethylene Promotes Root Growth via ROS Signaling and Consequently Generates a Mechanical Force.
(A) A stem section encompassing the third node was fixed in place, and a glass bead attached to a force sensor was placed on top of a root primordium of rice cultivar PG56. The insert shows a scanning electron micrograph of a node with bulging epidermis-covered primordia.
(B) Schematic presentation of a force sensor used for force measurements and of a root dummy used for pressure application. PC, computer.
(C) Forces generated above root primordia that were treated with or without 150 μM of ethephon (E) or with 150 μM of ethephon after pretreatment with 1 μM of DPI. Data are means (n = 3 to 6).
(D) Evans Blue staining of the epidermis overlying root primordia after 2 h of force (F) application with or without pretreatment with 5 ppm of 1-MCP as indicated.
(E) Dose-dependent cell death in response to 2 h of pressure application with a root dummy. The applied force corresponds to the maximal force measured in (C). *,** P < 0.001 (t test). Pretreatment with 1 or 5 ppm of 1-MCP was for 2 h. a,b P < 0.001 (t test). Pretreatment with 1 μM of DPI was for 3 h. Data are means (± se; n = 10 each data point).
To analyze whether the ethylene-generated force depended on ROS production, stems were pretreated with 1 μM of DPI before ethylene treatment to inhibit NADPH oxidase. In the presence of DPI, ethylene-dependent force was abolished (Figure 4C). Thus, ethylene promotes root growth via ROS, and this growth is responsible for the generation of a mechanical force that is exerted on epidermal cells.
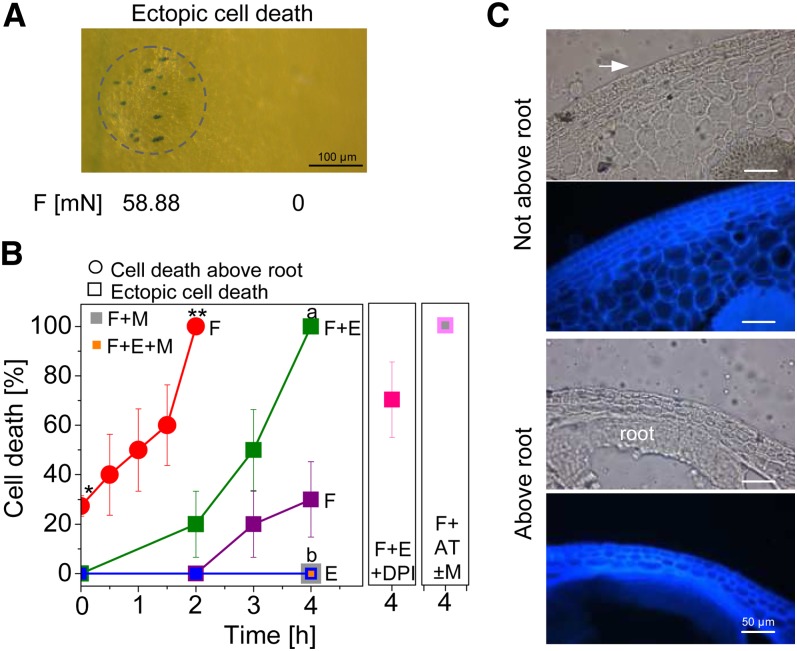
We next designed a root dummy with the diameter corresponding to the average size of a root tip at the third node (Figures 4A and 4B) to apply a force to the epidermis above root primordia from the outside. The forces applied were from 2.94 mN (or 0.97 MPa average pressure) to 58.88 mN (or 2.64 MPa average pressure), encompassing the range of forces generated by growth-induced root primordia (Figure 4C; see Supplemental Figure 4 online). Cell death was observed in 70% of epidermal patches after application of 8.82 mN for 2 h. With 58.88 mN, 100% cell death induction was observed after 2 h. A time-course experiment indicated that cell death increased with time and was fully induced after force application of 58.88 mN for 2 h (Figure 5B, red line).
Figure 5.
Induction of Ectopic Epidermal Cell Death by Mechanical Stimulation Is Dependent on Ethylene and Mediated through ROS.
(A) Death of epidermal cells that are not localized above a root is induced by force (F) in rice cultivar PG56. Cell death is strictly confined to the site of force application (indicated by a dashed circle).
(B) Application of 58.88 mN of force significantly (*,** P < 0.001; t test) promotes death of epidermal cells above root primordia within 2 h. Epidermal cells that are not localized above a root do not undergo cell death in response to 150 μM of ethephon (E, blue line). Application of 58.88 mN of force promotes 30% ectopic cell death after 4 h (magenta line). Cell death is fully inhibited by 5 ppm of 1-MCP (M; a,b P < 0.001; t test) (4 h, gray square). Joint application of force and ethephon fully promotes death of epidermal cells that do not cover a root (F+E, green line). Ectopic cell death is fully inhibited by 5 ppm of 1-MCP (a,b P < 0.001) (4 h, orange square) and is reduced to 70% by 1 μM of DPI (4 h, magenta square). Force in the presence of 50 mM of AT fully promoted ectopic cell death independent of ethylene perception as analyzed in the absence of 1-MCP (4 h, light magenta square) and in the presence of 5 ppm of 1-MCP (4 h, gray square). Values indicate means ± se (n = 10 for all data points except n = 3 for force + 50 mM of AT + 5 ppm of 1-MCP).
(C) Cross sections of epidermal cells overlying or not overlying a root primordium. Light micrograph (Top); autofluorescence (Bottom) highlighting cell walls (λex= 360 to 370 nm, emission was detected with a BP420 nm filter).
Bar in (A) = 100 μm; bars in (C) = 50 μm.
To find out whether ethylene signaling was required for the mechanically induced cell death response, stems were pretreated with 1-MCP to block ethylene perception (Figure 4E). Epidermal cell death induced by a force of 58.88 mN was partially inhibited with 1 ppm of 1-MCP; complete inhibition of inducible cell death was achieved with 5 ppm of 1-MCP, indicating that the force signal was mediated through the ethylene signaling pathway and that epidermal cell death is a hormonally controlled physiological response rather than the result of sheer physical destruction. To understand whether ROS production is a prerequisite for pressure-induced cell death, we used the NADPH oxidase inhibitor DPI. Pretreatment with DPI partially inhibited pressure-induced cell death, suggesting a role for ROS in mechanostimulated signal transduction (Figure 4E).
Mechanical Force Promotes Ectopic Epidermal Cell Death in an Ethylene- and ROS-Dependent Manner
Cell death induced by pressure was restricted to the epidermal area to which force was applied (Figure 4D), indicating that mechanical and chemical signaling must be perceived in the same cells to activate the cell death pathway. To test whether these two signals were in fact sufficient to generally promote cell death, we next applied these stimuli to epidermal cells that do not normally die (Figure 5). Treatment with ethylene did not induce death of nodal epidermal cells not overlying a root. Application of 58.88 mN of force caused 20 and 30% cell death after 3 h and 4 h, respectively (Figures 5A and 5B). Simultaneous application of 58.88 mN of force and 150 μM of ethephon caused cell death with a 20% probability after 2 h and with a 100% probability after 4 h (Figure 5B), supporting the view that both signals act in a synergistic manner. As was observed for epidermal cells overlying root primordia, ectopic cell death was restricted to the area of force application (Figure 5A). Cell death induced by force alone or by force in the presence of ethylene was fully inhibited with 5 ppm of 1-MCP, indicating dependence on ethylene signaling, and was partially inhibited by 1 μM of DPI, indicating at least partial dependence on ROS production (Figure 5B). To further test the hypothesis that ROS act as a signal, the catalase inhibitor AT was applied. Application of 50 mM of AT significantly increased epidermal cell death above adventitious roots from 27.4% (±2.3%) in controls to 38.4% (±3.7%) after 4 h (t test, P > 0.05) but not ectopically. Application of 50 mM of AT and a local force of 58.88 mN for 4 h caused ectopic cell death with a 100% probability (Figure 5B). Force-induced cell death in the presence of AT and 5 ppm of 1-MCP also occurred with a 100% probability, indicating that ROS act downstream of ethylene (Figure 5B). In wild-type rice cultivar Nipponbare, treatment with 150 μM of ethephon induced a force of ∼40 mN after 3 h (see Supplemental Figure 2B online). The MT2b::Tos17 mutant in the Nipponbare background displayed significantly enhanced ectopic cell death after application of 40 mN of force for 1 h as compared with the wild type (see Supplemental Figure 2D online). MT2b is downregulated in epidermal cells by ethylene (Steffens and Sauter, 2009a). These results indicate that downregulation of MT2b in epidermal cells alters ROS homeostasis to promote cell death, whereas growth induction of root primordia by ROS is not mediated via MT2b (see Supplemental Figures 2A, 2B, and 2D online). Taken together, data provided show that physical stimulation in concert with ethylene signaling is sufficient to promote epidermal cell death through altering ROS homeostasis and/or signaling, whereby spatial specificity is provided by the mechanical signal component (Figures 4D and 5A).
DISCUSSION
Epidermal Cell Death Correlates with Proximity to Adventitious Root Primordia
In rice, adventitious roots are formed early during node formation and grow at a slow rate as the node matures (Bleecker et al., 1986). Although two to five parenchymal cell layers separate root and epidermis at the youngest node, close to 90% of all root primordia are in direct contact with the epidermis at the third youngest node. Root emergence is induced by ethylene, which rapidly accumulates in submerged plants. Before root emergence, the overlying epidermal cells undergo cell death to make way for the penetrating root. At older nodes, single dead cells were observed above 30 and 50% of the root primordia at the third and fourth node, respectively, even when plants were neither submerged nor treated with ethylene, indicating that proximity to the root over time may trigger cell death. However, this cell death response did not spread but was confined to single cells. Ethylene promoted cell death at all nodes. Ethylene-induced cell death was inversely related to the number of parenchymal cell layers present between the root tip and the epidermis. Highest rates of epidermal cell death were observed at older nodes where roots are in immediate contact with the epidermis. Furthermore, reduction in the number of adventitious roots in arl1 resulted in overall reduced cell death rates whereby epidermal cell death was strictly confined to sites of underlying roots. When based on the number of root primordia present in each respective genotype, cell death was not significantly different between wild-type and ARL1-RNAi lines. The correlative evidence thus suggested that the presence of a root may directly be required for cell death signaling. Such communication may occur through chemical or mechanical signaling.
Emergence of Secondary Roots Facilitated by Local Cell Death Requires Spatial Resolution
Auxin controls secondary root formation. In Arabidopsis, auxin was shown to control emergence of lateral roots through the auxin influx carrier LIKE AUX1 3 (LAX3; Bhalerao et al., 2002; Swarup et al., 2008). LAX3 promotes accumulation of auxin in cortical and epidermal cells overlying emerging lateral root primordia. Furthermore, the expression of cell wall–remodelling enzymes in these cells was shown to be LAX3-dependent (Swarup et al., 2008). In rice, lateral root development requires the degradation of IAA11 and IAA13, two Aux/IAA proteins (Kitomi et al., 2012; Zhu et al., 2012), but changes in cortical or epidermal cells were not described to be induced by emerging lateral roots.
In rice, epidermal cell death occurs before adventitious root emergence. Both processes depend on ethylene, are promoted by gibberellin, and are inhibited by abscisic acid (Lorbiecke and Sauter, 1999; Mergemann and Sauter, 2000; Steffens et al., 2006). Epidermal cell death above adventitious roots in rice is mediated by RGA1/D1, which encodes the sole Gα subunit of heterotrimeric G proteins (Steffens and Sauter, 2009b). d1 mutants displayed reduced epidermal cell death induction by ethylene or H2O2, indicating that G protein signaling is essential for epidermal cell death. Data provided here show that the adventitious root provides information to the epidermis by means of mechanical signaling to locally induce cell death in the epidermis.
The plant epidermis was long hypothesized to have mechanosensing and transducing ability. Indirect experimental evidence and computer modeling from the shoot apical meristem (SAM) of Arabidopsis indicated that epidermal cell division was altered in response to local mechanical stress (Hamant et al., 2008). There are additional hints that mechanical forces led to reprogramming of cells during development. Mechanical signaling led to reorientation of microtubules in the SAM of Arabidopsis (Hamant et al., 2008) and was involved in primordium formation in the SAM of tomato (Kierzkowski et al., 2012). Different elastic properties in slow- and fast-growing areas of the apex resulted in differential growth and organogenesis within the SAM. Local cell wall loosening by expansin or relaxation of the cell wall promoted bulging and normal organ development in the periphery of the meristem of tomato and tobacco plants, indicating that development depends on mechanical signaling (Fleming et al., 1997; Pien et al., 2001).
Adventitious Root Growth Is Mediated by ROS
The balance between ROS production and scavenging is indispensable for cell death and growth regulation. In growing Arabidopsis roots, O2− and H2O2 were shown to accumulate in distinct zones regulated by the bHLH transcription factor UPBEAT1 (UPB1), which represses specific peroxidase genes (Dunand et al., 2007; Tsukagoshi et al., 2010). We showed that ethylene-induced adventitious root growth is mediated by ROS. Inhibition of O2− production through NADPH oxidase, the major source for ROS, inhibited ethylene-induced growth of adventitious roots. ROS detoxification is achieved by enzymes, such as ascorbate peroxidase, catalase, and superoxide dismutase (Jwa et al., 2006), or nonenzymatically through ascorbate, glutathione, and MT2b, a member of the metallothionein family. Genetic downregulation of MT2b resulted in increased H2O2 levels in suspension-cultured rice cells (Wong et al., 2004). Elevation of internal ROS levels through inhibition of catalase by AT promoted adventitious root growth, whereas reduction of internal ROS levels by the H2O2 scavenger KI reduced ethylene-induced growth, pointing to ROS as key mediators of ethylene-induced adventitious root growth in rice. Reduction of internal H2O2 level by genetic downregulation of MT2b had no effect on ethylene-induced adventitious root growth, possibly indicating that ROS homeostasis has to be altered in a defined way for root growth to be induced.
Mechanostimulation and Ethylene Determine Cell Death Fate through ROS Signaling
Our study revealed that a growing adventitious root exerts a strong force of up to 60 mN on epidermal cells. Application of the same force strength resulted in death of epidermal cells overlying an adventitious root and even more so of epidermal cells that do not normally undergo cell death. However, mechanical force did not suffice to promote cell death; rather, ethylene signaling was required in addition, because inhibition of ethylene perception by 1-MCP completely abolished the cell death response. Elevation of endogenous ROS levels was a prerequisite for cell death induction, because inhibition of ROS production partially inhibited the cell death response. Ethylene or ROS alone did not promote ectopic cell death, but applied jointly with force, ethylene or ROS elicited a local cell death response in the epidermis independent of the presence of a root (Figure 6). The degree of the response clearly indicated synergistic effects of mechanostimulation and ethylene/ROS signaling. Ethylene is a gaseous hormone that is likely not localized to single or few cells, because neither transport nor degradation mechanisms are known to exist. Especially during submergence, ethylene likely accumulates throughout the plant, because its diffusion rate in water is 10,000 times lower than in air (Jackson, 1985). A mechanical signal seems like an ideal means to provide spatial resolution to the ubiquitous ethylene signal and is particularly well-suited in this case, where root growth and emergence need to be coordinated with epidermal cell death in a timely manner.
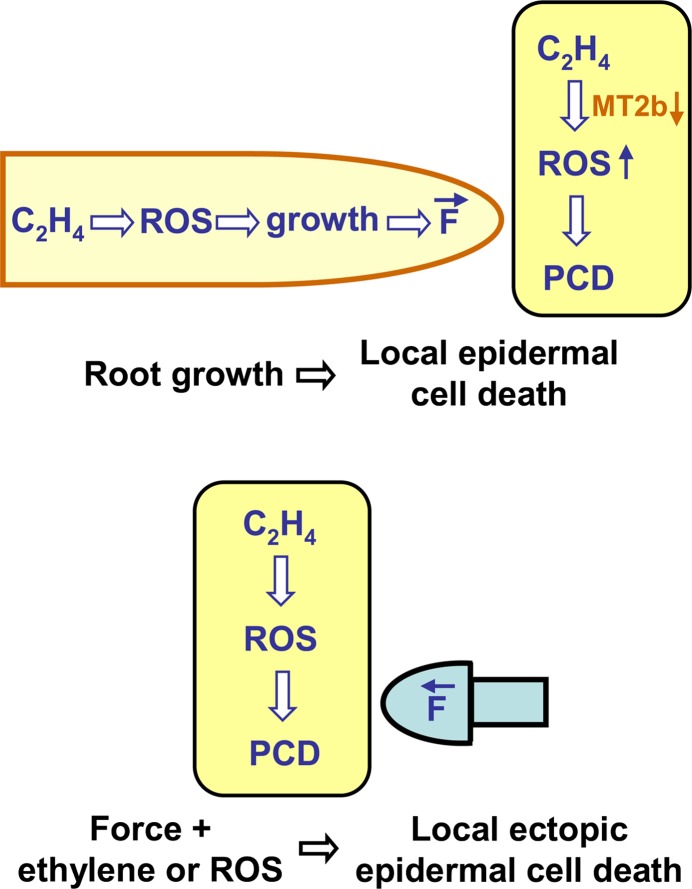
Figure 6.
Model of Epidermal Cell Death Induced by Mechanical, Ethylene, and ROS Signaling.
Ethylene promotes ROS production in the root and in epidermal cells. ROS promote root growth (Top), which generates a local force (F) on overlying epidermal cells. In epidermal cells, ROS accumulate because of reduced scavenging from downregulated MT2b. Mechanical signaling (generated artificially, Bottom) and ROS are required and are sufficient to promote death of epidermal cells whereby the mechanical signal provides the spatial information to locally restrict cell death. PCD, programmed cell death.
[See online article for color version of this figure.]
The longer lag phase observed in ectopically induced cell death as compared with cell death observed in epidermal cells overlying a root may indicate that key factors required for cell death have to be synthesized de novo in epidermal cells that are not localized above a root. One of these factors might be ethylene itself. Ethylene accumulation and release were shown to be induced in plants by mechanical stimuli. A pressure of 1 bar (101 kPa) applied to maize (Zea mays) seedlings stimulated the rate of ethylene synthesis within 1 h (He et al., 1996). Etiolated pea (Pisum sativum) plants produced more ethylene when they grew through a barrier, and were therefore mechanostimulated, compared with unimpeded plants (Goeschl et al., 1966). It is furthermore conceivable that force, ethylene, or ROS signaling in rice alters protein activities required for cell death induction or execution. Epidermal cells that are not overlying a root primordium possess a transcriptome that is distinct from that of epidermal cells overlying root primordia before cell death induction, indicating that the two cell types are primed differently (Steffens and Sauter, 2009a).
Putative candidates that sense mechanostimulation might be mechanosensitive channels. Responses to mechanostimulation were shown to be mediated by an increase in cytosolic Ca2+ and apoplastic ROS, and in pH changes in the cytoplasm and apoplast (Chehab et al., 2009). In Escherichia coli, the mechanosensitive ion channel MECHANOSENSITIVE CHANNEL OF SMALL CONDUCTANCE (MscS) mediates protection from osmotic shock (Haswell et al., 2008). Arabidopsis possesses 10 and rice possesses six MSL proteins related to MscS (MscS-LIKE), of which the plasma membrane–localized MSL9 and MSL10 homologs were shown to be stretch-activated ion-permissive channel proteins (Haswell et al., 2008). Furthermore, the Arabidopsis plasma membrane protein Ca2+-PERMEABLE MECHANOSENSITVE CHANNEL1 (MCA1) mediates Ca2+ uptake. Arabidopsis mca1-null roots cannot sense or respond to agar hardness, indicating that MCA1 is a mechanosensitive Ca2+ channel (Nakagawa et al., 2007). In rice, Ca2+ influx and ROS production induced by hypoosmotic stress requires MCA1, a plasma membrane–localized, Ca2+-permeable mechanosensitive channel (Kurusu et al., 2012). Overall, however, functional analysis of ion-permeable channels in mechanosignaling is largely missing for plants, and essentially nothing is known about other molecular components of signal transduction of local pressure in plants. The identification of cell death as an inducible cellular response to mechanostimulation may aide future studies of the molecular mechanisms that govern mechanical signaling.
Conclusion
A central question in developmental biology is how spatial information is conveyed to locally establish a developmental program. The study presented here shows that mechanostimulation is combined with chemical signaling to achieve just that during submergence-related adventitious root growth. Our results demonstrate that epidermal cell death is under dual control by ethylene, which is present ubiquitously in submerged tissues, and a mechanical signal that provides spatial information (Figure 6). Ethylene-induced growth and epidermal cell death are mediated by ROS signaling. The requirement for more than one signal provides high safety standards for the initiation of irreversible cell death. It prevents unwarranted damage to the protective epidermal cell layer by either mechanical impedance or ethylene and/or ROS accumulation and thus limits vulnerability to pathogen infection.
METHODS
Plant Materials and Growth Conditions
Seeds of wild-type, arl1, ARL1-RNAi-1, and ARL1-RNAi-2 lines of rice (Oryza sativa) subsp japonica cv Zhonghua 11 were obtained from Ping Wu (Zhejiang University, Zijingang Campus, Hangzhou, China). Wild-type and MT2b::Tos17 lines of rice subsp japonica cv Nipponbare were obtained from Akio Miyao (Genome Research Centre, Ibaraki, Japan). Seeds of deepwater rice subsp indica cv PG56 were originally obtained from the International Rice Research Institute (Los Banos, Philippines). Plants were grown for 12 to 18 weeks (Sauter, 1997); stem sections of 20 cm in length were excised (Steffens and Sauter, 2005). For data shown in Figure 1B, stem sections included the first and second, the second and third, or the third and fourth nodes. For all other measurements, stems including the second and third nodes were used. Stems were incubated at 27°C in continuous light with 150 μmol of photons m−2 s−1. The percentage of penetrated roots was based on the number of adventitious root primordia present. Average length of adventitious roots was calculated from roots that had emerged. Ethephon (2-chloroethanephosphoric acid; Sigma-Aldrich) was applied in an aqueous solution at 150 μM; ethylene (Air Liquide) was applied at 2.5 ppm; 1-MCP (AgroFresh) was applied before ethephon or ethylene treatments at the concentration and for the duration indicated; 1 μM of DPI (Sigma-Aldrich) was applied 3 h before treatments.
Measurement of Growth and H2O2 Staining of Isolated Adventitious Roots
Single adventitious roots were isolated from the third node of rice cv PG56 plants and of rice cv Nipponbare wild-type and MT2b::Tos17 plants. To obtain adventitious roots, 50-mm–long stem sections were cut 20 mm below the third node. Segments were surface-sterilized for 2 min with 2% (v/v) sodium hypochlorite and washed three times with sterile water. Single adventitious roots were isolated and laid on plates containing 0.5× Murashige and Skoog medium (Murashige and Skoog, 1962) with 1.5% (w/v) Suc and 0.4% (w/v) Gelrite. The media were supplemented with 1 or 10 μM of ACC, 1 μM of DPI, 10 or 100 μM of KI (H2O2 scavenger), or 50 mM of AT (catalase inhibitor). Plates were left in the dark at 27°C. Adventitious roots were photographed after 0, 24, and 48 h using a microscope (Olympus) with a charge-coupled device camera. Lengths of roots were analyzed with the program ImageJ, and the increase in root lengths between 0 and 24 h and between 0 and 48 h was calculated.
H2O2 and O2− Staining of Single Adventitious Roots
Stem sections of rice cv PG56 and of rice cv Nipponbare wild-type and MT2b::Tos17 were prepared and treated for the times indicated with 150 μM of ethephon or without ethephon as a control. Adventitious roots were isolated as described previously. To detect H2O2 and O2− in situ, single adventitious roots were placed for 10 min in DAB (Enhanced Liquid Substrate System; Sigma-Aldrich) or nitroblue tetrazolium (1 mM of nitroblue tetrazolium, 10 mM of potassium buffer, pH 6.1) solution, respectively. The staining reaction was stopped with sterile water. Staining was observed with a microscope, and pictures of single adventitious roots were taken with a charge-coupled device camera. H2O2 and O2− staining of root tips was scored in comparison with unstained root tips and calculated as percentage of all roots analyzed per time point.
Evans Blue Staining and Microscopy Analyses
Dead cells were stained with Evans Blue as described previously (Mergemann and Sauter, 2000). Cell death was specified according to the extent of cell death, with stage I indicating no dead cells, stage II indicating single dead cells, stage III indicating a continuous patch of dead cells, and stage IV indicating cracked epidermis and possibly emerged roots, or overall cell death was calculated as stages II to IV taken together divided by the number of root primordia present and was expressed as a percentage. In force experiments with one epidermal patch targeted at a time, the number of cell death events was divided by the number of experiments performed and was expressed as a percentage. Nodal tissue was prepared for light microscopy and scanning electron microscopy as described previously (Steffens and Sauter, 2009a). Scanning electron micrograph pictures were taken with a DSM 940 (Zeiss; Microscopy Center, University of Kiel).
Force Measurements and Pressure Application
The force exerted by a root primordium was measured with a force transducer (10-g capacity; World Precision Instruments), which was installed on a motorized DC3314R micromanipulator with an MS314 controller (World Precision Instruments). The force signal from the sensor was amplified and digitalized using an MP100WSW data acquisition system (Biopac Systems). A stem section was fixed with a holder and was covered with moist filter paper to prevent drying. A glass bead of 3 mm in diameter was glued with cyanoacrylate glue (5925 elastomer; Kisling Deutschland GmbH) to the force sensor. The bead was brought into direct contact with the epidermis above an adventitious root primordium, and a load of ∼2 mN was applied. Force-time curves were recorded using AcqKnowledge 3.7.0 software (Biopac Systems). To study pressure-induced epidermal cell death, a root dummy with a tip diameter of 200 μm was mounted onto the force sensor. This diameter corresponded to the average diameter of adventitious root primordia as determined from scanning electron micrograph pictures. Load forces between 2.94 mN and 58.88 mN were applied as indicated to the epidermis with the root dummy, with the force sensor signal being monitored throughout the application period. Induction of cell death was subsequently determined with Evans Blue staining. When force was applied for less than 2 h (58.88 mN), stem sections remained in the holder until cell death was determined after 2-h total incubation period (e.g., stems treated for 1 h were left untreated for another hour before cell death rates were determined).
Statistical Analysis
Statistical analyses were performed with Minitab. Percentage of cell death was transformed with arcsine√(x/100) to obtain normal distributed data. Comparison of means was analyzed for statistical significance with an analysis of variance (ANOVA) (Tukey test) or a two-sample t test. Normal distribution and constant variance of data were verified before statistical analysis, and the P value was set to P < 0.001 if one of both conditions was not achieved.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Ethylene Promotion of Adventitious Root Growth Displays a Developmental Gradient.
Supplemental Figure 2. Repression of MT2b Enhances Pressure-Induced Cell Death.
Supplemental Figure 3. Force Generation Is Dependent on the Presence of a Root.
Supplemental Figure 4. Calculation of the Pressure at Each Force Applied.
Supplemental Table 1. Dose–Response Curve of Ethephon-Induced Epidermal Cell Death in Rice Cultivar Zhonghua 11.
Supplementary Material
Acknowledgments
We thank Ping Wu (Zhejiang University, Zijingang Campus, Hangzhou, China) for providing wild-type and arl1, ARL1-RNAi-1, and ARL1-RNAi-2 lines of rice cultivar Zhonghua 11 and Akio Miyao (Genome Research Centre, Ibaraki, Japan) for supplying the MT2b::Tos17 insertion line NE7013. This article was supported by the Deutsche Forschungsgemeinschaft.
AUTHOR CONTRIBUTIONS
B.S., A.K., S.N.G., and M.S. designed the research; B.S. and A.K. performed research; A.K. and S.N.G. contributed new analytical tools; B.S. analyzed data; B.S. and M.S. wrote the article.
Glossary
- ROS
reactive oxygen species
- 1-MCP
1-methylcyclopropene
- mN
millinewton
- H2O2
hydrogen peroxide
- DAB
3,3′-diaminobenzidine
- DPI
diphenylene iodonium
- ACC
1-aminocyclopropane-1-carboxylate
- KI
potassium iodide
- AT
3-amino-1,2,4-triazole
- SAM
shoot apical meristem
- ANOVA
analysis of variance
- PG56
Pin Gaew 56
References
- Bailey-Serres J., Fukao T., Gibbs D.J., Holdsworth M.J., Lee S.C., Licausi F., Perata P., Voesenek L.A.C.J., van Dongen J.T. (2012). Making sense of low oxygen sensing. Trends Plant Sci. 17: 129–138 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J., Voesenek L.A.C.J. (2008). Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 59: 313–339 [DOI] [PubMed] [Google Scholar]
- Bhalerao R.P., Eklöf J., Ljung K., Marchant A., Bennett M., Sandberg G. (2002). Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Bleecker A.B., Schuette J.L., Kende H. (1986). Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta 169: 490–497 [DOI] [PubMed] [Google Scholar]
- Chehab E.W., Eich E., Braam J. (2009). Thigmomorphogenesis: A complex plant response to mechano-stimulation. J. Exp. Bot. 60: 43–56 [DOI] [PubMed] [Google Scholar]
- Clark D.G., Gubrium E.K., Barrett J.E., Nell T.A., Klee H.J. (1999). Root formation in ethylene-insensitive plants. Plant Physiol. 121: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunand C., Crèvecoeur M., Penel C. (2007). Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 174: 332–341 [DOI] [PubMed] [Google Scholar]
- Fleming A.J., McQueen-Mason S., Mandel T., Kuhlemeier C. (1997). Induction of leaf primordia by the cell wall protein expansin. Science 276: 1415–1418 [Google Scholar]
- Gibbs D.J., Lee S.C., Isa N.M., Gramuglia S., Fukao T., Bassel G.W., Correia C.S., Corbineau F., Theodoulou F.L., Bailey-Serres J., Holdsworth M.J. (2011). Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl J.D., Rappaport L., Pratt H.K. (1966). Ethylene as a factor regulating the growth of pea epicotyls subjected to physical stress. Plant Physiol. 41: 877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamant O., Heisler M.G., Jönsson H., Krupinski P., Uyttewaal M., Bokov P., Corson F., Sahlin P., Boudaoud A., Meyerowitz E.M., Couder Y., Traas J. (2008). Developmental patterning by mechanical signals in Arabidopsis. Science 322: 1650–1655 [DOI] [PubMed] [Google Scholar]
- Haswell E.S., Peyronnet R., Barbier-Brygoo H., Meyerowitz E.M., Frachisse J.M. (2008). Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 18: 730–734 [DOI] [PubMed] [Google Scholar]
- Hattori Y., et al. (2009). The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460: 1026–1030 [DOI] [PubMed] [Google Scholar]
- He C.J., Finlayson S.A., Drew M.C., Jordan W.R., Morgan P.W. (1996). Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol. 112: 1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M., Wilson I.W., Yang J., Buerstenbinder K., Llewellyn D., Dennis E.S., Sauter M., Dolferus R. (2010). Arabidopsis RAP2.2: An ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 153: 757–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai Y., Sakamoto T., Ueguchi-Tanaka M., Shibata Y., Gomi K., Umemura I., Hasegawa Y., Ashikari M., Kitano H., Matsuoka M. (2005). Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17: 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.B. (1985). Ethylene and responses of plants to soil waterlogging and submergence. Annu. Rev. Plant Physiol. 36: 145–174 [Google Scholar]
- Jwa N.S., Agrawal G.K., Tamogami S., Yonekura M., Han O., Iwahashi H., Rakwal R. (2006). Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem. 44: 261–273 [DOI] [PubMed] [Google Scholar]
- Kierzkowski D., Nakayama N., Routier-Kierzkowska A.L., Weber A., Bayer E., Schorderet M., Reinhardt D., Kuhlemeier C., Smith R.S. (2012). Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science 335: 1096–1099 [DOI] [PubMed] [Google Scholar]
- Kitomi Y., Inahashi H., Takehisa H., Sato Y., Inukai Y. (2012). OsIAA13-mediated auxin signaling is involved in lateral root initiation in rice. Plant Sci. 190: 116–122 [DOI] [PubMed] [Google Scholar]
- Kitomi Y., Ito H., Hobo T., Aya K., Kitano H., Inukai Y. (2011). The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 67: 472–484 [DOI] [PubMed] [Google Scholar]
- Kurusu T., et al. (2012). Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol. 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licausi F., Kosmacz M., Weits D.A., Giuntoli B., Giorgi F.M., Voesenek L.A., Perata P., van Dongen J.T. (2011). Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422 [DOI] [PubMed] [Google Scholar]
- Liu S., Wang J., Wang L., Wang X., Xue Y., Wu P., Shou H. (2009). Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res. 19: 1110–1119 [DOI] [PubMed] [Google Scholar]
- Liu H., Wang S., Yu X., Yu J., He X., Zhang S., Shou H., Wu P. (2005). ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 43: 47–56 [DOI] [PubMed] [Google Scholar]
- Lorbiecke R., Sauter M. (1999). Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 119: 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald M.P., Visser E.J.W. (2003). A study of the interaction between auxin and ethylene in wild type and transgenic ethylene-insensitive tobacco during adventitious root formation induced by stagnant root zone conditions. Plant Biol. 5: 550–556 [Google Scholar]
- Mergemann H., Sauter M. (2000). Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 124: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 15: 473–497 [Google Scholar]
- Nakagawa Y., et al. (2007). Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 104: 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuteboom L.W., Ng J.M.Y., Kuyper M., Clijdesdale O.R., Hooykaas P.J.J., van der Zaal B.J. (1999). Isolation and characterization of cDNA clones corresponding with mRNAs that accumulate during auxin-induced lateral root formation. Plant Mol. Biol. 39: 273–287 [DOI] [PubMed] [Google Scholar]
- Peeters A.J., Cox M.C., Benschop J.J., Vreeburg R.A., Bou J., Voesenek L.A.C.J. (2002). Submergence research using Rumex palustris as a model; looking back and going forward. J. Exp. Bot. 53: 391–398 [DOI] [PubMed] [Google Scholar]
- Péret B., Larrieu A., Bennett M.J. (2009). Lateral root emergence: A difficult birth. J. Exp. Bot. 60: 3637–3643 [DOI] [PubMed] [Google Scholar]
- Phatak S.C., Jaworski C.A., Liptay A. (1981). Flowering and adventitious root growth of tomato cultivars as influenced by ethephon. HortScience 16: 181–182 [Google Scholar]
- Pien S., Wyrzykowska J., McQueen-Mason S., Smart C., Fleming A. (2001). Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc. Natl. Acad. Sci. USA 98: 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J.A., Reid M.S., Paul J.L., Rost T.L. (1985). The effect of ethylene on adventitious root formation in mung bean (Vigna radiata) cuttings. J. Plant Growth Regul. 4: 147–157 [Google Scholar]
- Sauter M. (1997). Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant J. 11: 181–190 [DOI] [PubMed] [Google Scholar]
- Steffens B., Sauter M. (2005). Epidermal cell death in rice is regulated by ethylene, gibberellin, and abscisic acid. Plant Physiol. 139: 713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B., Sauter M. (2009a). Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 21: 184–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B., Sauter M. (2009b). Heterotrimeric G protein signaling is required for epidermal cell death in rice. Plant Physiol. 151: 732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B., Wang J., Sauter M. (2006). Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223: 604–612 [DOI] [PubMed] [Google Scholar]
- Swarup K., et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Shimada T., Kondo M., Tamai A., Mori M., Nishimura M., Hara-Nishimura I. (2010). Ectopic expression of an esterase, which is a candidate for the unidentified plant cutinase, causes cuticular defects in Arabidopsis thaliana. Plant Cell Physiol. 51: 123–131 [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P.N. (2010). Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Vissenberg K., Fry S.C., Pauly M., Höfte H., Verbelen J.P. (2005). XTH acts at the microfibril-matrix interface during cell elongation. J. Exp. Bot. 56: 673–683 [DOI] [PubMed] [Google Scholar]
- Wong H.L., Sakamoto T., Kawasaki T., Umemura K., Shimamoto K. (2004). Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 135: 1447–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Xu X., Fukao T., Canlas P., Maghirang-Rodriguez R., Heuer S., Ismail A.M., Bailey-Serres J., Ronald P.C., Mackill D.J. (2006). Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Zhu Z.X., Liu Y., Liu S.J., Mao C.Z., Wu Y.R., Wu P. (2012). A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol. Plant 5: 154–161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.