This work examines, in moss and seed plants, the role of cyclic nucleotide gated Ca2+ channels in acquired thermotolerance, finding that thermo-responsive channels in the plasma membrane act as thermosensors that signal for the accumulation of molecular defenses, heat shock proteins in particular, setting up a transient response for thermoprotection.
Abstract
Typically at dawn on a hot summer day, land plants need precise molecular thermometers to sense harmless increments in the ambient temperature to induce a timely heat shock response (HSR) and accumulate protective heat shock proteins in anticipation of harmful temperatures at mid-day. Here, we found that the cyclic nucleotide gated calcium channel (CNGC) CNGCb gene from Physcomitrella patens and its Arabidopsis thaliana ortholog CNGC2, encode a component of cyclic nucleotide gated Ca2+ channels that act as the primary thermosensors of land plant cells. Disruption of CNGCb or CNGC2 produced a hyper-thermosensitive phenotype, giving rise to an HSR and acquired thermotolerance at significantly milder heat-priming treatments than in wild-type plants. In an aequorin-expressing moss, CNGCb loss-of-function caused a hyper-thermoresponsive Ca2+ influx and altered Ca2+ signaling. Patch clamp recordings on moss protoplasts showed the presence of three distinct thermoresponsive Ca2+ channels in wild-type cells. Deletion of CNGCb led to a total absence of one and increased the open probability of the remaining two thermoresponsive Ca2+ channels. Thus, CNGC2 and CNGCb are expected to form heteromeric Ca2+ channels with other related CNGCs. These channels in the plasma membrane respond to increments in the ambient temperature by triggering an optimal HSR, leading to the onset of plant acquired thermotolerance.
INTRODUCTION
Plants are sessile organisms that cannot escape environmental stresses, including heat shock. During a mild rise of the ambient temperature, plants cells need precise sensing devices to anticipate potentially damaging heat conditions. Plants use specific signaling pathways to elaborate molecular defenses against upcoming heat damage in a timely fashion. A harmful heat stress may cause the hyperfluidization and disruption of membranes (Horváth et al., 1998; Sangwan et al., 2002), inactivate proteins by unfolding, misfolding, and aggregation (Sharma et al., 2010), cause metabolic imbalance (Vierling, 1991), and generate reactive oxygen species (Dat et al., 1998; Gong et al., 1998; Volkov et al., 2006). These various types of damage, which are amplified by other environmental stresses (Mittler, 2006), may impair photosynthesis (Hall, 2000) and cause the death of sensitive tissues, in particular of reproductive organs, with severe consequences for plant development, growth, reproduction, and crop yields (Ahuja et al., 2010; Mittler and Blumwald, 2010). To protect labile macromolecules from heat damage and repair or degrade damaged macromolecules, prokaryotes and eukaryotes have developed a specific network of heat-inducible cellular and molecular defenses, generally referred to as the heat shock response (HSR). The HSR is a highly conserved cellular response to a gradual or sharp rise of temperature above the physiological growth range (Wahid et al., 2007; Mittler et al., 2012). During and following the HSR, hundreds of specific genes, up to 4% of the higher plant genome, become predominantly upregulated and hundreds of so-called heat shock proteins (Hsps) accumulate in various cellular compartments (Finka et al., 2011; Mittler et al., 2012). Thus, while being exposed to initially moderate increases in temperature that gradually develop into a more severe heat shock (Larkindale and Vierling, 2008), plants need to send an early signal for the timely accumulation of Hsps and metabolites, while also readjusting their pH and redox potentials and reducing their photosynthetic and transpiration activities (Mittler et al., 2012). Together, these mechanisms establish acquired thermotolerance: a transient ability of plants to survive a limited exposure, typically for a few hours, to otherwise lethal temperatures. The success of plant-acquired thermotolerance thus strictly depends on the effectiveness of a preceding period of so-called heat priming, when the plant still experiences nonlethal temperatures and during which the HSR is optimally induced (Mittler, 2006; Larkindale and Vierling, 2008).
Among the Hsps that accumulate to the largest extent during heat priming are the molecular chaperones that belong to several canonical conserved families in prokaryotes and eukaryotes, such as the small Hsps (sHsps), Hsp100s, Hsp90s, Hsp70s, Hsp60s, and their cochaperones (Finka et al., 2011). Whereas most molecular chaperones can prevent protein aggregation, some can use ATP to convert already misfolded and aggregated proteins into unfolded polypeptides that can recover their native conformation after the stress (Sharma et al., 2010) or become degraded by ATP-regulated proteases (Hinault et al., 2011). The sHsps are of particular interest to the understanding of the molecular basis for plant-acquired thermotolerance because they are among the most strongly expressed Hsps during a heat priming treatment (Mittler et al., 2012) and their accumulation in various cellular compartments correlates with acquired thermotolerance (Dafny-Yelin et al., 2008; Larkindale and Vierling, 2008; Finka et al., 2011). Their mode of action involves both membrane and protein protection: sHsps form large oligomers (Stengel et al., 2010) that during heat stress transiently dissociate into amphiphilic dimers, which upon inserting into lipid bilayers may stabilize hyperfluidized membranes (Tsvetkova et al., 2002). Moreover, the sHsps contain a conserved α-crystalline domain, whose reversible interaction with abnormally exposed hydrophobic polypeptide segments can prevent misfolding and aggregation of heat-labile proteins (Ingolia and Craig, 1982; Jakob et al., 1993; Stengel et al., 2010). After stress, sHsp-bound damaged proteins are thus optimally exposed to the scavenging ATPase chaperones Hsp70 and Hsp101 (possibly also Hsp60 and Hsp90) that can unfold them into natively refoldable species (Buchner et al., 1991; Veinger et al., 1998; Goloubinoff et al., 1999; Mogk et al., 2003; Sharma et al., 2010). In agreement with the in vitro protective functions of sHsps, mutants in various sHsp genes have impaired acquired thermotolerance phenotypes (Kimpel and Key, 1985; Lee et al., 2005; Dafny-Yelin et al., 2008; Luján et al., 2009; Shakeel et al., 2011). Members of the other classes of molecular chaperones may also become strongly upregulated in heat-primed plants, albeit to a lesser extent than the sHsps. As with sHsps, loss-of-function mutants of the disaggregating cytosolic chaperone Hsp101, and of other members of the Hsp70/Hsp40 or Hsp90 chaperone families, have reduced tolerance to thermal stress (Lee and Schöffl, 1996; Queitsch et al., 2000; Yamada et al., 2007; Su and Li, 2008).
Because of the high energetic and metabolic cost of having to maintain high levels of Hsps, all organisms, including plants, have developed mechanisms to tightly repress the expression of hundreds of Hsps during nonstressful temperatures, while maintaining readiness to derepress and overexpress them under heat-priming conditions (Finka et al., 2011; Mittler et al., 2012). The effectiveness of this strategy strongly depends on the capacity of cellular thermosensors to detect moderate subnoxious increments in ambient temperatures to integrate these into an effective HSR signal leading to the timely accumulation of protective Hsps to withstand an upcoming noxious heat shock.
Highly sensitive molecular thermosensors involving both a protein and a plasma membrane component have been identified in the plasma membrane of the moss Physcomitrella patens (Saidi et al., 2009, 2010). Heat causes a transient opening of Ca2+ channels, allowing a specific inward flux of extracellular Ca2+ ions from the apoplast (Wu et al., 2010) possibly via modulation of membrane fluidity (Saidi et al., 2009). Electrophysiology of reversibly heated moss protoplasts established that the primary plant thermosensors are heat-responsive Ca2+ channels in the plasma membrane (Saidi et al., 2009). This was further confirmed by testing the effects of artificial membrane fluidizers (Saidi et al., 2005), Ca2+ chelators, specific Ca2+ channel blockers, and specific Ca2+ ionophores in reporter moss lines constitutively expressing the Ca2+-sensing protein aequorin or conditionally expressing the β-glucuronidase (GUS) reporter enzyme from a heat-inducible promoter (Saidi et al., 2009).
Here, we report that the cyclic nucleotide gated calcium channel (CNGC) CNGCb gene from P. patens mosses and its orthologs CNGC2 and CNGC4 from Arabidopsis thaliana encode an essential component of the thermosensory machinery of land plant cells. Arabidopsis CNGC2 and moss CNGCb are involved in the control of the plant’s HSR and acquired thermotolerance. A targeted CNGCb deletion led to the loss of a thermoresponsive Ca2+ channel in the plasma membrane and produced a hypersensitive temperature-dependent Ca2+ influx into the moss cells and corresponding hyper-thermoresponsive profiles of HSR activation. In addition, both moss CNGCb and Arabidopsis CNGC2 mutants showed a similar phenotype of hyper-thermoresponsive acquired thermotolerance.
RESULTS
Bioinformatic Analysis of Moss and Arabidopsis CNGC Genes
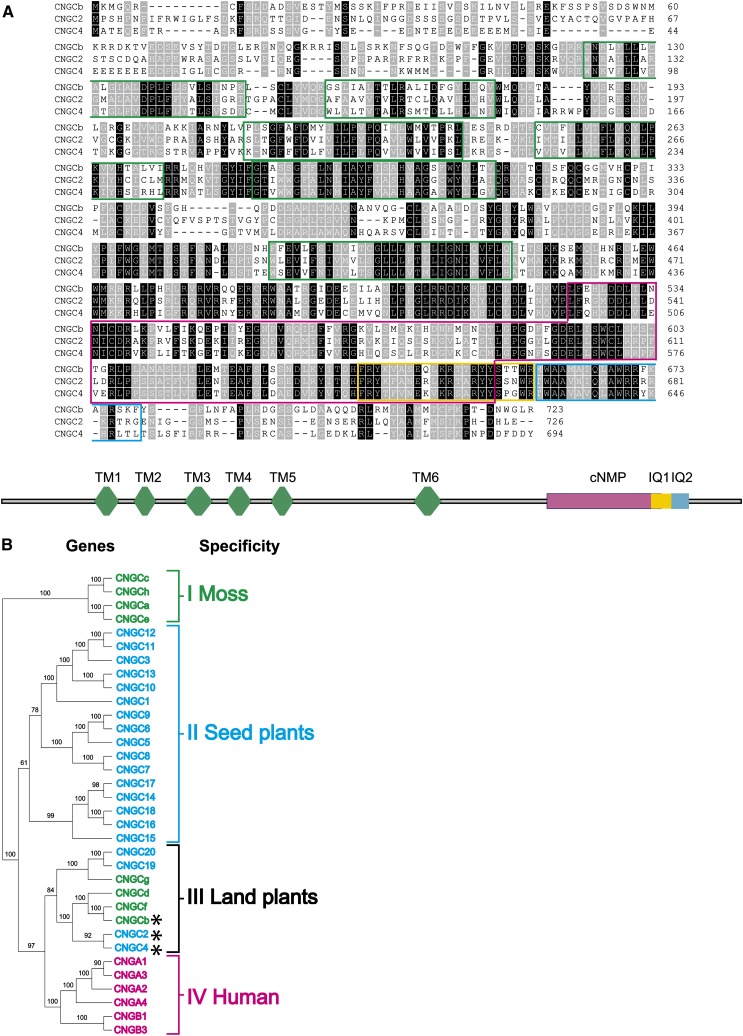
Initial evidence for the involvement of CNGCs in thermosensory functions in nematodes (Mori, 1999; Prahlad et al., 2008) centered our bioinformatic search for putative plant thermosensors on the 20 CNGCs from the seed plant Arabidopsis and the nine CNGCs (named CNGCa-i) from the moss P. patens (Wheeler and Brownlee, 2008; see Supplemental Figure 1 online). Similar to animal CNGCs, a majority of land plant CNGC genes encode polypeptides of ∼700 residues, with six predicted transmembrane segments, a large cytosolic C-terminal domain containing a cyclic nucleotide binding domain (cNMP) (Clough et al., 2000), and a highly conserved atypical calmodulin binding domain, IQ1 (Arazi et al., 2000), flanked by a second canonical calmodulin domain, IQ2 (Figure 1A).
Figure 1.
Phylogenetic and Structural Organization of Moss and Arabidopsis CNGCs.
(A) Alignment of the deduced amino acid sequences of moss CNGCb with Arabidopsis CNGC2 and CNGC4. Black background denotes identical residues and gray background similar residues. The six predicted transmembrane helices are delimited by green boxes, the cyclic nucleotide binding domain by a red box, the canonical calmodulin binding domains (IQ1) by a yellow box, and the second noncanonical calmodulin binding domain (IQ2) by a blue box.
(B) Plant and human amino acid sequences of CNGCs (see Supplemental Data Set 1 online) are compared in an unrooted neighbor-joining tree (see Methods). The tree shows four main gene clusters that are significantly different: cluster I (green), containing only moss-specific CNGCs; cluster II (blue), containing only plant specific CNGCs; cluster III (black), containing both moss and seed plant CNGCs and is therefore inferred to be land plant specific; and cluster IV (magenta), containing only human CNGCs. The CNGCb, CNGC2, and CNGC4 that presumably encode thermosensory Ca2+channels in land plants are marked with asterisks.
Applying a neighbor-joining tree algorithm (see Methods) to the predicted amino acid sequences of the eight full-length moss CNGCs, the 20 Arabidopsis, and the six human CNGCs generated a tree with four significantly distinct monophyletic clusters: the first (I), containing only moss polypeptide sequences; the second (II), containing only seed plant (Arabidopsis) sequences; the third (III), containing a mixture of four Arabidopsis sequences, including CNGC2, CNGC4, CNGC19, and CNGC20 and four moss sequences (CNGCb, CNGCd, CNGCf, and CNGCg); and the fourth, as expected containing only human sequences (Figure 1B). Whereas the first gene cluster was hypothesized to carry only moss-specific CNGC functions, the second seed plant-specific functions and the fourth only human-specific functions, the third monophyletic cluster was of particular interest because it contained both moss and Arabidopsis CNGC sequences, implying that it could carry some primordial functions that were already effective in the ancestor of land plants (mosses and seed plants). Indeed, to colonize land, aquatic plant ancestors would have needed to develop the ability to sense and react to temperature variations, which were sharper and more extreme in a gaseous than in an aqueous environment. We therefore hypothesized that one or several of the cluster III CNGCs may carry thermosensory functions.
Disruption of Moss CNGCb and Arabidopsis CNGC2 Impairs Plant Growth and Heat Sensing
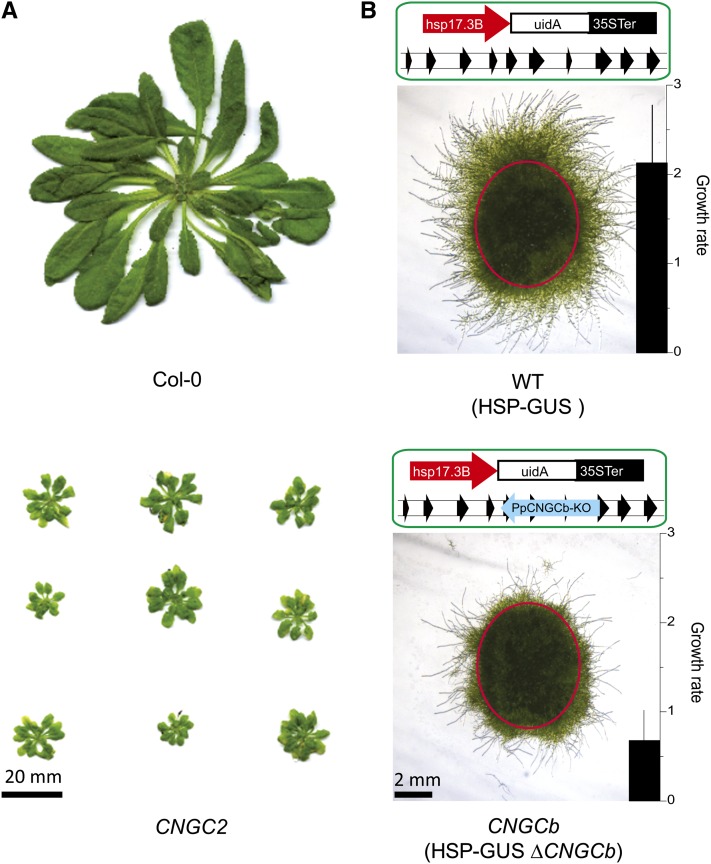
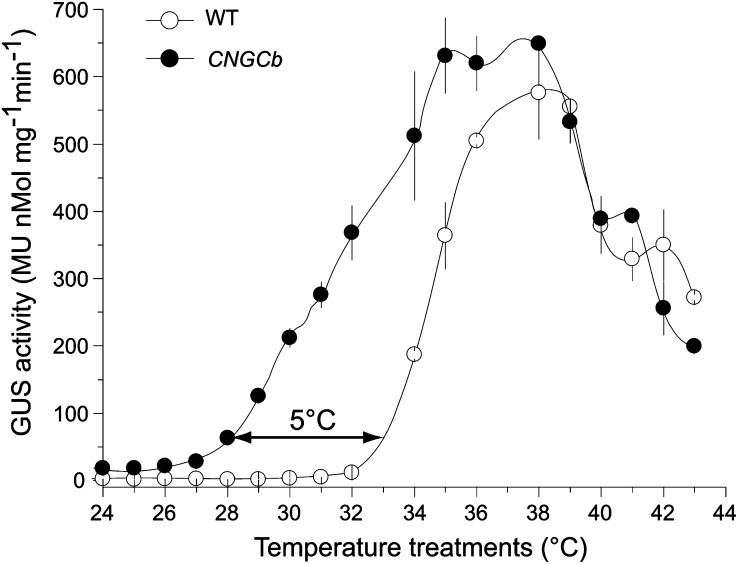
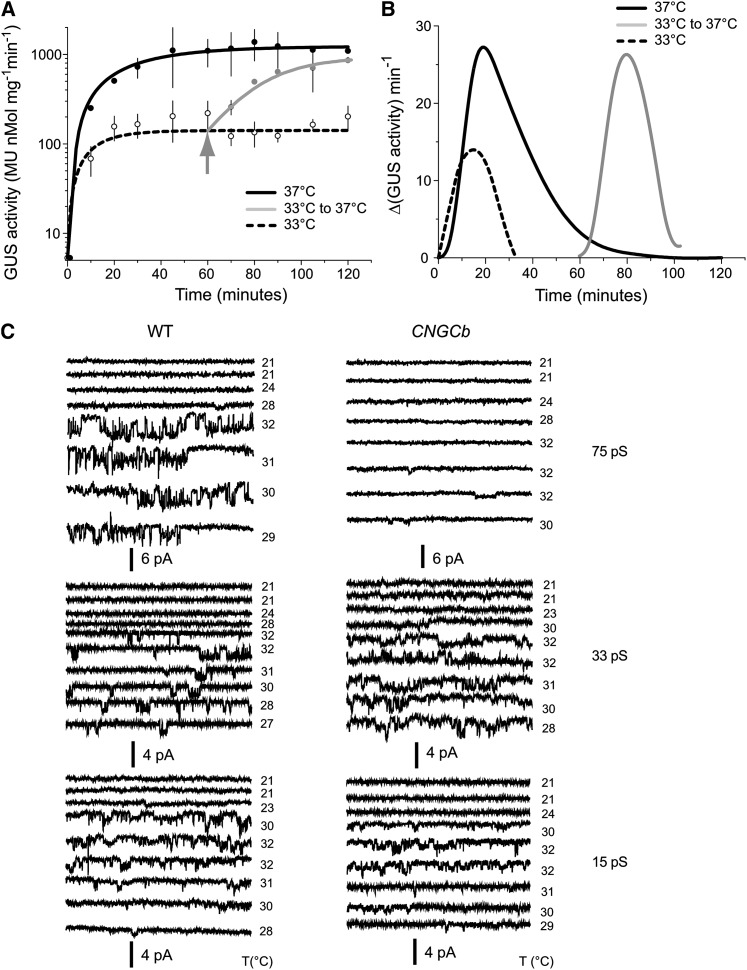
To investigate the role of plant CNGCs in heat sensing, we analyzed Arabidopsis and moss mutants disrupted in the CNGC2 and CNGCb genes, respectively. Using targeted mutagenesis (Schaefer and Zrÿd, 1997), we disrupted the CNGCb gene in a HSP-GUS moss background (Saidi et al., 2005) (see Supplemental Figure 2B online). The HSP-GUS line was specifically designed to monitor temperature-dependent expression of the GUS reporter enzyme from a recombinant heat-inducible promoter, GmHsp17.3B (Saidi et al., 2005). Similar to the phenotype of growth retardation that was described earlier for the Arabidopsis CNGC2 (Clough et al., 2000; Figure 2A), the moss protonemal tissues of the CNGCb mutant grew ∼2.5 times slower than the wild type, without other detectable cellular, morphological, or developmental defects (Figure 2B). We then compared the ability of the CNGCb mutant to respond to a 60-min heat treatment at various increasing temperatures, from 24°C up to 44°C, by quantifying enzymatic activity of the accumulated GUS reporter protein (Figure 3A). As long as the heat treatments were below 32°C, virtually no GUS expression was found in the wild-type HSP-GUS tissues. Above 32°C, when the heat treatments attained 33, 34, and 36°C, the corresponding GUS levels were 10, 50, and 90%, respectively, of the maximum level, which was observed at 38°C (Figure 3A). Remarkably, in the CNGCb mutant, the same GUS expression thresholds were reached at much lower temperatures, at 28, 31, and 34.5°C, respectively, representing a sensitivity downshift in the range of Hsp-inducing temperatures of 5°C. The same hyper-thermosensitive phenotype was observed in four different individually selected stable mutants isolated from the same moss transformation (see Supplemental Figure 2A online). Although reacting very differently at physiological temperatures, both mutant and wild-type strains showed the maximal GUS expression at 38°C. Both the wild type and mutant were similarly vulnerable to excessive heat, as reflected by the same half inhibition of GUS production observed at 42°C.
Figure 2.
Arabidopsis CNGC2 and Moss CNGCb Mutants Have Growth Defects.
(A) Images of 8-week-old Arabidopsis wild-type Col-0 (top) and the mutant CNGC2 plants (bottom) grown in soil at 20°C in short days.
(B) Scheme of the genetic background and the corresponding pictures of the morphological appearance of whole moss colony inoculates (delimited by a red circle) 6 d after their replating on minimal medium. The wild type like HSP-GUS strain is denoted as wild type (WT; top) and the HSP-GUS ΔCNGCb as CNGCb (bottom). Net relative growth rates and sd from three independent measurements beyond the red circle are presented as black bar graphs on the bottom right corners of the images.
Figure 3.
Deletion of CNGCb Causes a Hyper-Thermosensitive Moss Phenotype.
Induced accumulation of the reporter GUS enzyme from the recombinant reporter promoter GmHsp17.3 (Saidi et al., 2005, 2007, 2009) in the control wild-type (WT) moss (open symbols) and the CNGCb mutant (closed symbols), following 1 h exposure at the indicated temperatures, and a 16-h recovery at 22°C. The double arrow shows that 10% of the maximal GUS accumulation is obtained at 28°C in the mutant compared with 33°C in the wild-type moss. Means and sd are from three independent experiments. MU, 4-methylumbelliferone glucuronide.
CNGCb Regulates the Heat-Induced Ca2+ Influx into the Cytoplasm
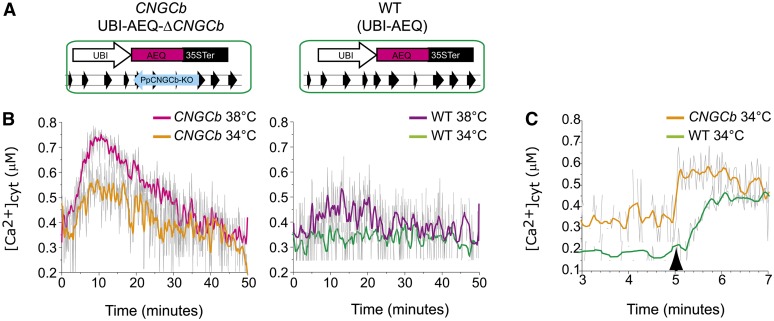
The moss plant thermosensors behave as Ca2+ channels undergoing heat-induced transient openings, which in turn mediate a transient Ca2+ influx (Saidi et al., 2009). To assess the effect of the CNGCb loss of function on the dynamics of cytosolic Ca2+ during the various phases of a mild temperature upshift, we introduced an UBI-AEQ cassette expressing the Ca2+ sensor aequorin into the HSP-GUS ΔCNGCb background, (Figure 4A). As we have previously demonstrated in the control (Saidi et al., 2009), an abrupt rise from 24 to 36°C triggers (in wild-type UBI-AEQ) a maximal accumulation of cytosolic Ca2+ within the first 10 to 15 min, which thereafter subsides to near baseline levels despite the ongoing heat shock (Figure 4B). The decrease of cytosolic Ca2+ during this refractory phase can be attributed to the closure of heat-responsive channels in combination with ongoing Ca2+ extrusion from the cytoplasm by active transporters. When an abrupt, continuous heat treatment at 34 or 38°C was applied to 24°C-grown CNGCb mutants, a stronger Ca2+ influx, reaching up to 3.5-fold higher cytoplasmic concentrations of Ca2+, was observed during the first 15 min at both inducing temperatures. Even during the first 30 min of the continuous exposure to 34 or 38°C, the cytoplasmic Ca2+ levels remain higher in CNGCb cells, which contained on average 1.5-fold more Ca2+ ions than wild-type cells (Figures 4B and 4C; see Supplemental Figure 3 online).
Figure 4.
CNGCb Deletion Causes a Hyper-Thermoresponsive Ca2+ Influx and Elevated Cytoplasmic Ca2+.
(A) Scheme of the genetic background of the stable reporter moss strains UBI-AEQ ΔCNGCb (CNGCb) and UBI-AEQ (wild type [WT]).
(B) Moss protonemata grown at 25°C were preincubated with coelenterazine and then exposed to 34 or 38°C. The Ca2+-dependent luminescence of aequorin was recorded every 6 s for a period of 50 min in living tissues of CNGCb (left) and wild-type (right) strains. cyt, cytoplasmic.
(C) The same 25°C-grown moss protonemata were preincubated with 14 mM EGTA and coelenterazine followed by exposure to the mild intermediate temperature of 34°C for 5 min and then supplemented with an excess of external Ca2+ (5 mM CaCl2 final concentration; black arrow). Ca2+-dependent luminescence was recorded every 1.5 s for a period of 10 min (here are shown only 3 to 7 min) in the CNGCb (orange line) or the wild-type (green line) strain. Raw unsmoothed luminescence data are in gray, and the resulting partially smoothed curves are colored.
To ascertain that the plasma membrane Ca2+ conductance in the CNGCb mutant was higher than in the wild type, we further exposed 24°C-grown tissues to 34°C in the presence of the calcium chelator EGTA. When, after 5 min during which the sample was allowed to fully reach 34°C, an excess Ca2+ (5 mM) was supplemented to cause a sudden availability of external Ca2+, and this led to a sharp rise in the cytosolic Ca2+ in the mutant that was at least 20 times faster than in the wild-type cells (Figure 4C). This confirms that at this threshold inducing temperature, a large number of Ca2+ channels in the plasma membrane of the CNGCb mutant are open, whereas this is not the case for the wild type.
The HSR in Moss Is Regulated by Several Heat Sensors with Different Activation Thresholds
When the wild-type HSP-GUS reporter strain was exposed to a mild temperature upshift of 25°C→33°C, a 100-fold increase in cytoplasmic GUS was observed following 20 to 30 min of the heat treatment (Figure 5A). As best illustrated by the derivative of the GUS accumulation curve, the net progression of the heat shock signal reached a maximum at 20 min, which thereafter decayed within 10 min to zero despite the ongoing mild temperature treatment (Figure 5B). This is the attenuation of the heat shock signal, resulting from the spontaneous closure of the thermoactivated Ca2+ channels (Saidi et al., 2009). Noticeably, when 60 min after the start of the first mild temperature upshift to 33°C, the temperature was further raised to 37°C, the moss tissues readily responded by producing a fully developed heat shock signal, reaching an approximate net 1000-fold GUS accumulation, similar in intensity to the maximal GUS response produced by a unique temperature upshift from 25 to 37°C (Figure 5A). This strongly suggests the presence of at least two distinct populations of plant thermosensors: one that readily responds to 33°C and another that only becomes activated at higher temperatures. Thus, the plant thermosensitive Ca2+ channels distribute into several subpopulations with different thresholds of thermal activation.
Figure 5.
Mosses Have Multiple Thermosensors with Different Activation Thresholds.
(A) GUS levels in tissues of wild-type HSP-GUS moss reporter line grown at 25°C, exposed either continuously for 2 h at 33°C (dashed line) or at 37°C (black line) or discontinuously, first for 1 h at 33°C then for 1 h at 37°C (gray line). Recovery was done at 25°C for 16 h. Means and sd are from three independent repeats. MU, 4-methylumbelliferone glucuronide.
(B) The time-dependent evolution of the net HSR from (A), expressed as the time-dependent averaged differences of the rates of GUS accumulation per minute.
(C) Changes in membrane conductance reflecting the activity of Ca2+-permeable channels in moss protoplast attached patches, during a mild temperature upshift from 21°C up to 32°C and back to 29°C. In wild-type (WT) protoplasts (left-hand panels), three distinct temperature-induced conductances are observed with 75-pS (top), 33-pS (middle), and 15-pS (bottom) unitary conductances. In the CNGCb mutant (right-hand panels), the 75-pS conductance was not recorded. By contrast, the 33-pS and possibly also the 15-pS conductance in the mutant protoplasts acquired a higher Po at comparable temperatures. Each trace was recorded at −80 mV and has a duration of 0.5 s, and temperatures are denoted on the right-hand side.
Electrophysiology Shows the Presence of Three Different Thermoresponsive Ca2+ Channels
Patch clamp analysis of moss protoplasts at room temperature typically shows a complete lack of ion channel activity (Saidi et al., 2009). However, an increase in bath temperature from 21°C to temperatures >30°C leads to induction of channel activity in a proportion (10 to 15%) of cells. Three different types of ion channel were recorded that showed inward current and mean unitary conductances with sd of 15 (±2.4), 33 (±3.9), and 75 (±4.4) pS, respectively (Figure 5C, left-hand panels; see Supplemental Figure 4 online). The different conductances occurred with frequencies of around 0.24, 0.22, and 0.15 (n = 55). Ba2+ is frequently used as an analog for Ca2+ (Clay, 1991). Although in theory the recorded inward currents could be carried by an outward flux of anions such as Cl−, measurement of the membrane potential and cellular Cl− content make this extremely unlikely (see Supplemental Figure 4 online). The aforementioned in vivo aequorin records (Figure 4) independently confirmed the presence of an inward Ca2+ flux during heat shock at 34°C and above; therefore, we refer to the observed conductances as Ca2+ permeable channels. In contrast with the three conductances in wild-type cells, the mutant protoplasts only showed activity of the 15- and 33-pS channels in response to the same temperature upshifts (Figure 5C, right-hand panels), with frequencies of 0.12 and 0.14 (n = 44).
Since loss of CNGCb caused a stronger influx of Ca2+ in this genotype, we assessed whether altered gating properties of the 15- and 33-pS channels could underlie this phenomenon. We therefore measured the open probability (Po) during a 50-s window after the application of temperature rises in the patch clamp chamber. The mean Po of the 15- and 33-pS channels in the wild-type protoplasts was 0.089 (±0.06), while the corresponding value in the mutant was nearly twice large at 0.171 (±0.12). The latter difference was only weakly significant (P < 0.1); clearly, the main effect of the deletion of CNGCb was that it eliminated the presence of active 75-pS channels.3
Moss CNGCb and Arabidopsis CNGC2 and CNGC4 Mutants Develop Futile Acquired Thermotolerance
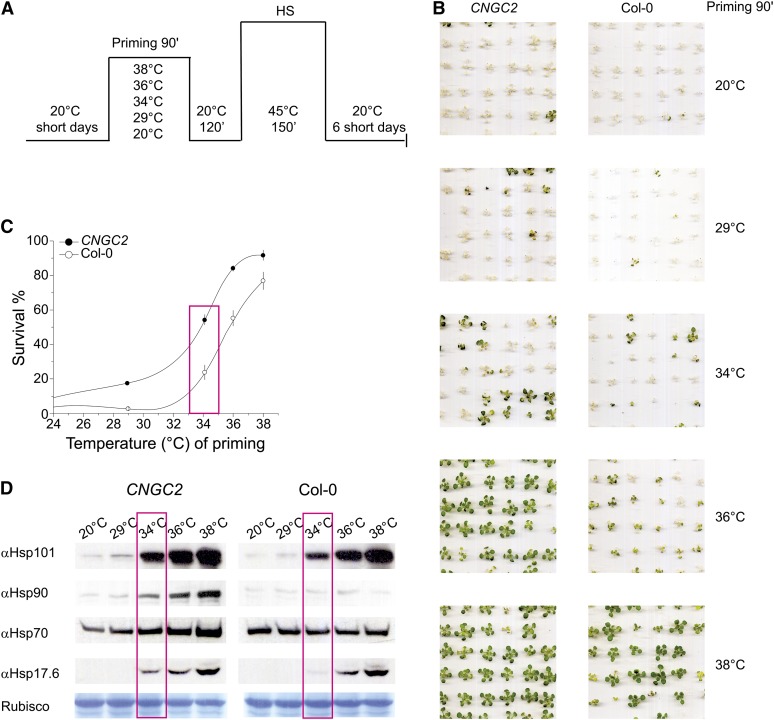
We next addressed how the hyper-thermosensitive phenotype of the mutants affects their priming profile for acquired thermotolerance. As expected from the general upregulation of the plant HSR at lower inducing temperatures, the moss CNGCb mutant developed acquired thermotolerance at lower priming temperatures. Hence, priming pretreatment at 34°C led to a significant thermotolerance in the CNGCb mutant compared with the wild type (see Supplemental Figure 5A online). This was confirmed with Arabidopsis seedlings lacking functional CNGC2, which developed acquired thermotolerance in response to much lower priming temperatures than the Columbia-0 (Col-0) seedlings: 20% of surviving CNGC2 mutants were observed following a priming treatment at 29°C (i.e., 4.5°C lower than necessary to provide 20% survival in the Col-0 seedlings). Similarly, following priming at 35°C, 70% of the CNGC2 mutant seedlings survived compared with only 37% for Col-0 seedlings (Figures 6A to 6C). Immunoblots showed that following 90-min incubation at the various priming temperatures and 2-h recovery, the CNGC2 mutant seedlings accumulated more Hsp101, Hsp90, and Hsp17.6 than Col-0 seedlings (Figure 6D). The same apparent gain of acquired thermotolerance at lower priming temperatures was also observed with Arabidopsis CNGC4 mutant seedlings (i.e., in the closest relative of the CNGC2 gene) (see Supplemental Figure 5B online). These data strongly indicate that the gene products of moss CNGCb and of Arabidopsis CNGC2 and CNGC4 are pivotal components of the land plant thermosensory machinery controlling the HSR and acquired thermotolerance.
Figure 6.
Arabidopsis CNGC2 Mutant Seedlings Develop Futile Acquired Thermotolerance.
(A) Scheme showing the duration and temperatures of treatments leading to Arabidopsis seedling acquired thermotolerance: 15-d-old Col-0 seedlings or 19-d-old seedlings lacking CNGC2 at the same developmental stage as Col-0 were primed during 90 min at the indicated temperatures, then incubated back for 120 min at 20°C, then for 150 min at 45°C, then back for 6 d at 20°C. HS, heat shock.
(B) Pictures of plants taken 6 d after the noxious heat shock at 45°C. The priming temperature is indicated on the right.
(C) Scored percentage of survival and sd of Col-0 (open circles) and CNGC2 seedlings (closed circles) treated as in (B).
(D) Immunoblots showing the relative expression levels of Hsp101, Hsp90, Hsp70, and Hsp17.6 in Arabidopsis CNGC2 mutant seedlings and Col-0 seedlings following 90-min priming at the indicated temperatures and 120 min at 20°C. Boxes highlight the priming temperature of 34°C, at which Hsp levels are higher in the CNGC2 mutant, and correlates with their improved survival to noxious heat shock (60% for CNGC2 compared with 25% for Col-0). Below, Coomassie blue staining of the 55-kD Rubisco large subunit in the same samples, showing equal protein loading.
[See online article for color version of this figure.]
DISCUSSION
Acquired Thermotolerance as an Inducible Mechanism against Damage from Noxious Heat
During the last three decades, the average global temperature has been increasing by ∼0.6°C (Hansen et al., 2006), causing more frequent and acute heat waves (Schär et al., 2004) with devastating effects on biodiversity (Thomas et al., 2004), ecosystem productivity (Ciais et al., 2005), and crop yields (Lobell et al., 2011). In extreme terrestrial climates, the daily rise of the sun may cause a gradual increase in the ambient temperature of up to 40°C in <12 h. Heat stress may affect photosynthesis, particularly the labile components of photosystem II (Berry and Bjorkman, 1980) and ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco) (Haldimann and Feller, 2004). Heat inactivation of photosystem II causes an imbalance of the electron flow and the accumulation of reactive oxygen species (ROS) in the thylakoids, damaging lipids and proteins (Koussevitzky et al., 2008). The imbalance of tissue gas concentrations favors the catabolic oxygenase activity of Rubisco, in turn increasing photoinhibition and ROS production (Allakhverdiev et al., 2008).
Whereas land plants use constitutively expressed long-term strategies of basal heat resistance to withstand the gradual warming of the climate, they also need conditionally expressed short-term strategies of acquired thermotolerance at the cellular and molecular levels to withstand more acute and frequent heat waves. Acquired thermotolerance is a transient phenotype resulting from the successful prior induction of various molecular defenses, whose output is survival to an otherwise deadly heat shock episode (Mittler, 2006; Larkindale and Vierling, 2008). It is therefore essential that plant cells can sense small increments of ambient temperatures and integrate these changes into an effective HSR. This proceeds in the form of the timely accumulation of protective metabolites and heat shock proteins (Finka et al., 2011), many of which are molecular chaperones that efficiently protect thermolabile macromolecules, in particular components of the photosynthetic apparatus (Heckathorn et al., 1998; Salvucci, 2008), and rehabilitate cell proteostasis in general (Finka et al., 2010).
An Ancestral CNGC Subclade Codes for Land Plant Thermosensors
Using biological assays with reporter moss strains, electrophysiology, and biochemical and pharmacological approaches, we have previously shown that the primary thermosensors of the moss P. patens are specific thermoresponsive Ca2+ channels in the plasma membrane (Saidi et al., 2009). Temperature-induced fluidity increments in the plasma membrane caused a transient channel opening and an influx of external Ca2+ into the cytoplasm, which was followed within minutes by channel closing despite the ongoing heat-inducing conditions, allowing Ca2+ pumps to extrude excess Ca2+ from the cytoplasm (Saidi et al., 2009). Yet, the precise identity of the genes encoding for the plant thermosensors remained unclear. To address the nature of the primary temperature perception events leading to land plant HSR and acquired thermotolerance (Saidi et al., 2011), we used two complementing model land plants, the moss P. patens and the seed plant Arabidopsis, and focused on CNGCs because of their ability to mediate various cellular responses to biotic and abiotic stresses by way of controlled influxes of extracellular Ca2+ into the cytoplasm (Mäser et al., 2001). Of particular interest were the Arabidopsis CNGC2 and CNGC4 genes because mutant phenotypes have been previously described: during pathogen attack, both have defective programmed cell death (PCD), while maintaining effective gene-for-gene resistance, and they were accordingly called defense no death1 (dnd1) and dnd2 (Clough et al., 2000; Jurkowski et al., 2004). Moreover, both dnd1 and dnd2 mutants showed a general hypersensitivity to Ca2+ in the medium, impaired growth, defective reproduction, and defective seed development (Balagué et al., 2003; Jurkowski et al., 2004; Chaiwongsar et al., 2009).
Whereas in the same monophyletic clade their most closely related genes, CNGC19 and CNGC20, may be involved in salt stress sensing and tolerance (Kugler et al., 2009), other more distantly related Arabidopsis CNGCs may still carry theromosensory-related roles in plant development, and mutants may display phenotypes related to ion homeostasis, uptake, transport, and tolerance to heavy metal poisoning (Kaplan et al., 2007). Our bioinformatic analysis identified a monophyletic gene cluster comprising the three moss genes CNGCb, CNGCd, and CNGCf, alongside Arabidopsis CNGC2 and CNGC4, as possible ancestral thermosensory CNGCs since the initial colonization of land by the common ancestor of mosses and seed plants. The thermosensory function was confirmed by targeted mutagenesis of CNGCb and by analyzing acquired thermotolerance in the dnd1 and dnd2 mutants: CNGCb, CNGC2, and CNGC4 were found to be major regulatory components of the land plant thermosensory machinery controlling the HSR and acquired thermotolerance. Strengthening this finding, CNGC6 was recently found to be involved in tissue-specific thermosensing, Hsp expression, and Arabidopsis thermotolerance (Gao et al., 2012). This is not unexpected given that phylogeny places CNGC6 in a seed plant–specific clade, which stemmed from the ancestral thermosensory clade. Therefore, in addition to CNGC2 and 4, other seed plant–specific CNGCs may contribute to the fine-tuning of the plant thermosensory machinery in different tissues and organs. A Genevestigator analysis of the 20 Arabidopsis CNGCs mRNA levels revealed that many seed-plant specific genes are expressed at very low levels in all plant organs, whereas CNGC2 is by far the most massively expressed in all tissues, yet with large variations between organs and developmental stages (see Supplemental Figure 6 online). It is tempting to speculate that by controlling CNGC2 expression levels, different tissues can alter the heterooligomeric composition of thermosensory CNGC channels, implying that the same inducing temperature could produce different types and intensities of HSRs in the various organs of the same plant.
The Thermosensory Role of CNGCs in Animals and Plants
In the worm Caenorhabditis elegans, there is a nonautonomous neuroendocrine HSR pathway that depends on the thermosensory neuron AFD that detects temperature through cyclic guanosine monophosphate–gated channels containing TAX-4/TAX-2 subunits, which are regulated by transmembrane guanylate cyclases (Prahlad et al., 2008; Ramot et al., 2008). In vertebrates, six members of the CNGC family, CNGA1-A4, CNGB1, and CNGB3 (Bradley et al., 2001), are involved in light perception (Hardie and Raghu, 2001), but they are also significantly expressed in nonsensory organs that may indicate a potential role in thermosensing (Kraus-Friedmann, 2000). In addition to the few CNGC genes, algae, yeast, and animals have genes encoding for transient receptor potential ion channels (TRPs) (Wheeler and Brownlee, 2008) that structurally resemble CNGCs. They also form tetrameric channels composed of subunits containing each six transmembrane spans and a C-terminal calmodulin binding domain. At the N-terminal side, the TRPs possess ankyrin repeat domains. However, TRPs do not have cyclic nucleotide binding domains (Nilius and Owsianik, 2011). A main characteristic of TRPV1-4 is their transient activation in response to heat, allowing transient Ca2+ entry in the cytosol (Venkatachalam and Montell, 2007). Interestingly, a splice variant of TRPV1 causes a remarkable hyper-thermoresponsive phenotype in the thermosensory organ of vampire bats, which, as in the case of the ΔCNGCb moss mutant, responded to significantly lower temperatures (ΔT = 6°C) than tissues expressing the nonspliced variant in the same animal (Gracheva et al., 2011). However, whereas TRPV1 clearly initiates and mediates an effective heat signaling mechanism between neuronal cells, it is not known whether the TRPV1-mediated cytosolic Ca2+ transient also triggers an autonomous HSR in the form of Hsp synthesis and accumulation in individual cells.
Thus, it would appear that when colonizing land, plants relinquished their TRP genes in favor of new functionally diverse CNGC genes, possibly to meet the new environmental challenges of a terrestrial life style, with more extreme and frequent variations of environmental cues. The combination of various CNGC isoforms to encode specific types of Ca2+ channels could help assigning different Ca2+ signatures to disparate stimuli that all generate increases in cytoplasmic Ca2+, including heat shock (Larkindale and Knight, 2002), oxidative stress (Evans et al., 2005), freezing (Knight et al., 1991, 1996), salt and drought stress (Knight et al., 1997; Ranf et al., 2008), excess light (Shacklock et al., 1992), and biological stress (Knight et al., 1991; Ehrhardt et al., 1996; Kosuta et al., 2008).
The Organization of Plant Thermosensitive CNGC Channels
We found that the moss CNGCb mutant was highly deregulated in temperature sensing, which demonstrates that CNGCb is a central component of the plant thermosensory machinery. Yet, the CNGCb mutant was apparently not a simple loss-of-thermosensing mutant, but it rather displayed a strong hyper-thermoresponsive phenotype, with an optimal HSR at significantly lower temperatures than the wild type. The aequorin-expressing CNGCb mutant confirmed that the hyper-thermoresponsive phenotype was directly associated with a systematic higher background of cytoplasmic Ca2+ in the CNGCb mutant cells and about a twofold heat-induced transient increase of cytoplasmic Ca2+ during the first 15 min of a sustained heat treatment compared with wild-type cells (Figures 4A and 4B). Abnormal constitutive high levels of Ca2+ ions could be cytotoxic by interfering with other Ca2+-dependent signaling pathways, such as the plant response to pathogens, and generate the defense-no-death phenotype of the Arabidopsis dnd1 and dnd2 mutants (Chaiwongsar et al., 2009) and growth defects evocative of Ca2+ poisoning and accelerated senescence (Ali et al., 2007)
Sequential mild and then strong heat shock treatments of the HSP-GUS moss reporter strain indicated the presence of multiple thermosensors (Figure 5), each with a different threshold of thermal activation. Electrophysiology confirmed that in response to various mild temperature increments, wild-type plasma membranes showed activation of at least three distinct ion channels, with 75, 33, and 15 pS conductance, respectively. The 75-pS channel was not observed in the mutated CNGCb background, confirming that it is indeed a loss-of-function mutant.
Vertebrate CNGCs typically form heterotetramers (Flynn et al., 2001; Zheng and Zagotta, 2004), but CNGC subunit composition is unknown in plants. The electrophysiology data, in combination with cytosolic calcium measurements, suggest a model in which plant CNGCs may also assemble into heterooligomeric Ca2+ channels, with the 75-pS channel activity critically depending on the presence of CNGCb subunits. The disregulated Ca2+ influx observed in the CNGCb mutant implies that this subunit also impacts on other Ca2+ channels. This may result from the imperfect replacement of CNGCb subunits in the 33- and 15-pS channels by orthologous CNGCs, for example, CNGCd and CNGCf, causing hyper-thermosensitivity. Future biochemical and electrophysiological studies of single and multiple mutants in other related CNGCs will be necessary to answer this question.
One question raised by our findings concerns the apparent juxtaposition between Ca2+ as a general secondary messenger and the high specificity of the heat shock signal. Previously, we showed that heat stress does not activate the specific osmosensitive LEA promoter and, conversely, that mannitol stress does not activate the specific thermosensitive GmHsp17.3B promoter (Saidi et al., 2009). Yet, in both cases, signal transduction strictly depends on the transient entry of external Ca2+ through Ca2+ channels in the plasma membrane (Saidi et al., 2009). Stimulus specificity of the Ca2+ signal can be introduced in many ways, including amplitude and frequency modulation (Dodd et al., 2010), and for the heat signal, this could include a localized steep rise in Ca2+ to form a transient signaling complex relying on the binding of a specific calmodulin. This scenario is supported by the central role of CAM3 in controlling the plant HSR in Arabidopsis (Liu et al., 2005; Liu et al., 2007). Further downstream steps in the heat shock signal cascade would than rely on the activation of specific Ca2+-dependent protein kinases and phosphatases by CAM3 (Liu et al., 2007, 2008; Zhang et al., 2009), Hsp90-mediated activation of rotamases (Meiri et al., 2010), and, finally, the activation of the heat shock transcription factors (Li et al., 2004; Liu et al., 2005; von Koskull-Döring et al., 2007; Chan-Schaminet et al., 2009; Li et al., 2010; Liu et al., 2011), leading to the HSR and the onset of acquired thermotolerance (Mittler et al., 2012). The identification of the central components of the primary thermosensors is a first step to understand how land plants may survive deadly episodes of heat waves in a context of global warming. Not only the HS signaling pathway is to be further deciphered (Mittler et al., 2012), but also how the components of the HSR, the Hsps, and the metabolites in particular collaborate in a network of complementing molecular mechanisms, coalescing into acquired thermotolerance.
Another important question raised by our findings is how other environmental cues may integrate into this thermosensory CNGC-mediated HSR pathway. Hsps sometimes accumulate in various plant tissues under isothermal conditions (Saidi et al., 2005; Finka et al., 2011), for example, in response to phytohormones, such as abscisic acid (Snyman and Cronjé, 2008), pollutants (Saidi et al., 2007), or when exposed to combinations of mild heat and dehydration stresses (Rizhsky et al., 2004). It is tempting to speculate that the thermosensory CNGC2 and CNGC4, either on their own or in combination with more distantly related CNGCs, such as CNGC6 (Gao et al., 2012), form heteromeric Ca2+ channels that can trigger Hsp expression at constant temperature and, as such, mediate plant resistances to various environmental stresses in addition to thermotolerance.
Acquired Thermotolerance and Apoptosis
Our data agree with previous observations (Larkindale and Vierling, 2008) that nonprimed plants that are directly treated with a noxious heat shock show no apparent damage in their photosynthesis efficiency when measured immediately after the noxious stress. Rather, the nonprimed plants needed six full days to die, whereas the heat-primed plants all survived. This suggests that nonprimed plants may have slowly died as a consequence of the activation of a heat-induced PCD and not because of direct heat damage per se. Whereas apoptosis, meaning literally “dropping off the leaves” in Greek, could provide plants with a reasonable means to evade excessive heat, it is not clear what would be the evolutionary advantage of total apoptosis for a nonprimed plant. One possible explanation would be that the very slow, 6-d-long process leading to plant death may be caused by heat-induced ROS-mediated DNA damage (Vacca et al., 2004), which would be effectively prevented by the timely heat priming–induced accumulation of catalase and ascorbate peroxidase (Vanderauwera et al., 2011; Mittler et al., 2012). In addition to enzymatic ROS scavengers, other mechanisms leading to plant-acquired thermotolerance may depend on the accumulation of Hsp chaperones during and following the heat-priming treatment. The downshift in priming temperatures needed to induce acquired thermotolerance in the dnd1 and dnd2 mutants, correlated with an excessive expression of Hsp chaperones at noninducing temperatures. This is in agreement with previous observations that the constitutive overexpression of Hsp17.7 in carrot (Daucus carota; Malik et al., 1999) or of Hsp25.3-P (Härndahl et al., 1999) and Hsp101 in Arabidopsis (Queitsch et al., 2000) provide some degree of acquired thermotolerance without heat priming. In animals too, the activities of Hsp chaperones (e.g., Hsp70 and sHsps), can block PCD by inhibiting caspase activation and IκB activity (Beere, 2004; Weiss et al., 2007). It is not unlikely that as in animal cells, the upregulation of specific Hsp chaperones in plant cells in response to heat priming, hormones, or drugs (Saidi et al., 2007; Finka et al., 2011), could inhibit the signaling for heat-induced PCD. Overall, such a mechanism would increase chances of plant survival from noxious heat stress. Thus, in agreement with the initial observations that the Arabidopsis dnd1 and dnd2 mutants are PCD deficient in their response to a biotic stress (Clough et al., 2000; Mäser et al., 2001; Balagué et al., 2003), we find here that the same mutants are PCD deficient in their response to noxious heat stress. It should be noted that gaining acquired thermotolerance at suboptimal priming temperatures is not necessarily beneficial; in the field, such mutant plants would frequently trigger a futile costly accumulation of Hsps as unnecessary defenses against subnoxious and nondamaging temperatures.
Our findings show that land plants can feel mild temperature increments via specific CNGC-based thermosensors in the plasma membrane. This initial signal leads to the development of a network of Hsp-based defenses, which in turn sets up transient acquired thermotolerance against upcoming noxious heat stresses. Given the anticipated global climate change, these results will be key to the future design of crops with increased resilience to heat variations.
METHODS
Chemicals, GUS, and Aequorin Experimentations
The coelenterazine hcp, EGTA, and 4-methylumbelliferone glucuronide were purchased from Sigma-Aldrich. GUS-specific activities, electrophysiological experiments, and the concentration of cytosolic calcium using reconstituted aequorin system were as described previously (Saidi et al., 2009).
Bioinformatic Analysis
The published CNGC genomic sequences were aligned against available transcripts using the BLAST program (http://www.cosmoss.org). Obtained coding sequences were translated and aligned, and the relationship between Physcomitrella patens CNGC amino acid sequences to each other and to known CNGC sequences from Arabidopsis thaliana and humans was explored using protein sequence comparison algorithms (http://www.clcbio.com). An unrooted neighbor-joining tree of the plant and human amino acid sequences of CNGCs was generated using CLC Sequence Viewer 6. The resulting sequence alignment was submitted to bootstrap analysis using 100 replicates, and the tree was visualized and edited by Treegraph 2 (Stöver and Müller, 2010) (see Supplemental Figure 2 online). With the exception of CNGCf and CNGCi with predicted lengths of 533 and 466 residues, respectively, the remaining predicted moss CNGC sequences share similar lengths of ∼700 amino acid residues, similar to the length of 19 Arabidopsis CNGCs. Transmembrane segments were predicted and double correlated by SPLIT4.0 (http://split.pmfst.hr/split/4/; Juretić et al., 2002) and TMHMM (http://www.cbs.dtu.dk/services/TMHMM/) membrane protein secondary structure prediction servers, whereas cyclic nucleotide binding domains and IQ1 binding domains in plant CNGC genes were assessed using the SMART database (Letunic et al., 2012).
Plasmids and Plant Material
pCNGCb-KO Vector
The 5′ targeting fragment of pCNGCb was amplified using ApaIF (5′-GGGCCCCTATCTATTAACCCCAAGCTCTC-3′) and XhoIR (5′-CTCGAGGACCATCCACAACATTATCTGTA-3′), and 3′ targeting fragment was amplified by SpeIF (5′-ACTAGTGACAACAAGAACGCTGCCGATGGGCA-3′) and SacIIR (5′-CCGCGGTTCGAAGAGTTCGCGAGGTCTTA-3′) using Taq polymerase (Promega). Resulting products were first subcloned in pGEM-T-easy vector (Promega) and digested with ApaI-Xho or SpeI-SacII and subcloned in two steps in the corresponding sites of pBSMDII (Finka et al., 2008) to generate the pCNGCb-KO vector. To minimize the possibility of superintegration of CNGCb-KO cassette into HSP-GUS locus, ∼10 μg of CNGCb-KO transgene was amplified by PCR employing standard T3 and T7 primers followed by enzymatic digestion with ApaI and SacII.
pBUAzeo Vector
The EcoRI fragment of p35S-loxP-Zeo that carries a 35S:Zeocine cassette was inserted into the EcoRI-digested pCNGCb-KO backbone, and resulting plasmid was cut with HindIII and filled in prior insertion of blunt Ubi-Aeq cassette that was digested with Asp718-NotI to generate pBUAzeo.
The moss transgenic lines were grown on moss solid medium and transferred when stated to liquid minimal medium as previously described (Saidi et al., 2009). HSP-GUS and UBI-AEQ lines were previously described (Saidi et al., 2007, 2009), respectively. To generate stable transgenic moss CNGCb lines in a HSP-GUS background, a polyethylene glycol–mediated transformation of moss protoplasts was performed using PCR-amplified CNGCb-KO cassette followed by G418 antibiotic selection (Schaefer and Zrÿd, 1997). Several stable lines showing similar levels of GUS activities were isolated, and one mutant line was arbitrarily chosen and used in experiments. The aequorin-expressing CNGCb mutant was created by introduction of pBUAzeo into the previously generated CNGCb mutant line, and zeocine-resistant stable line having aequorin activities were selected and used for the experiments.
For all assays, Arabidopsis seeds were first surface sterilized and planted on Murashige and Skoog (MS) medium plates (MS basal salt mixture) containing 0.43% MS (w/v) and 1% plant agar (Duchefa) and kept at 4°C for 48 h. Plants were then grown in lighted growth chambers at 20°C in short-day conditions (10 h light/14 h dark). Light intensity was ∼100 μmol (photon) m−2 s−1. For Arabidopsis thermotolerance experiments and immunoblots, we used CNGC2 (dnd1) and CNGC4 (dnd2) mutants obtained from the Nottingham Arabidopsis Stock Centre.
Choice of Temperatures
The extreme sensitivity of electrophysiology to mild temperature–induced Ca2+ channel opening in the plasma membrane of moss protoplasts dictated our choice of 32°C as the uppermost temperature to compare mutant and wild-type tissues. By contrast, the poor sensitivity of the online in vivo aequorin measurements dictated our choice to test 34°C as the lowest and 38°C as the uppermost temperature to compare mutant and wild-type tissues.
The 34°C was used in the aequorin experiments as an intermediate threshold, where HSP-GUS was induced only 33% in the wild type and ∼66% in the CNGCb mutant. The 38°C was used as an effective priming temperature in thermotolerance experiments because it was the upper harmless inducing temperature where HSP-GUS was maximally induced, both in the wild type and CNGCb. The noxious temperatures were determined experimentally for their causing, without a prior heat-priming treatment at least 90% plant death and with a prior heat-priming treatment at least 90% plant survival.
Electrophysiological Experimentation
Moss protoplasts were isolated as described (Johannes et al., 1997). In all patch clamp experiments, the external (bath) and pipette solution contained 20 mM BaCl2 and 5 mM MES/Tris, pH 6, and solutions were adjusted with sorbitol to an osmotic pressure of 430 mosM. Data acquisition occurred through a CED A/D converter at 1 kHz, and data were filtered at 0.4 kHz using a six-pole Bessel filter. Bath temperature was controlled by perfusion of bath medium with different temperatures and continuously recorded using an LN stage temperature controller. Open probabilities were calculated for the 15- and 33-pS conductances at −80 mV membrane potential, across 50-s records starting after the imposition of the temperature raise. Po is defined as Po = (t_open/t_total)/n *100, where n is the number of channels in the membrane patch (Maathuis, 2011). Po data are given as the average ± sd using recordings from 11 wild-type protoplasts and six CNGCb mutant protoplasts.
Plant-Acquired Thermotolerance
Moss protoplasts were isolated, and ∼50,000 protoplasts were exposed at priming temperatures as indicated, recovered for 2 h at 25°C, followed by noxious heat shock at 43°C for half an hour and plated. After 7 d, plates were imaged and confluency percentage indicating survival rate was calculated using the ImageJ software (http://rsbweb.nih.gov/ij/). Means and standard errors are from three independent experiments. Col-0 seeds were grown on half-strength MS agar and placed for 2 d at 4°C in darkness followed by growth for 15 d at 22°C using a short daylight regime. To achieve the same developmental stage as Col-0, dnd1 and dnd2 mutant seedlings were grown for 19 and 18 d, respectively, using a short daylight regime. For acquired thermotolerance treatments, only living and fully developed seedlings were used. Each point was obtained from at least 200 plants. Means and standard errors were from four independent repeats of at least 200 plants per experiment as indicated. Plants were photographed after six short days, and survival rates were analyzed using the ImageJ software.
Immunoblot Analyses
Seedlings were treated at 29, 34, 36, and 38°C for 90 min, kept at room temperature for 120 min, and immediately frozen in liquid nitrogen. Frozen tissue was ground in buffer (100 mM Tris-HCl, pH 8.8, 0.5% SDS, 10% glycerol, and 2% β-mercapthoethanol) and centrifuged at 14,000 rpm for 15 min, 4°C. Protein concentrations were determined using the Bio-Rad protein assay according to the manufacturer’s instructions. Standard methods were used for SDS-PAGE separation of protein samples on 10 to 15% (w/v) polyacrylamide gels. Immunoblot analyses were performed as follows: The nitrocellulose membranes (Bio-Rad) containing the transferred proteins were incubated with a rabbit-derived polyclonal antibody against plant Hsp17.6 (StressMark), Hsp70 (Agrisera), Hsp90 (Agrisera), and Hsp100-CT (StressMark) (1/10,000, v/v). Blots were then incubated with anti-rabbit or anti-mouse horseradish peroxidase–conjugated IgG (Bio-Rad) diluted 1/15,000 (v/v). Immune complexes were visualized using the chemiluminescent Immunstar kit (Bio-Rad) according to the manufacturer’s instructions.
Accession Numbers
GenBank/EMBL accession numbers for Arabidopsis and human protein sequences used in the phylogenetic analysis are as follows: O65717 (CNGC1), O65718 (CNGC2), Q9SKD7 (CNGC3), Q94AS9 (CNGC4), Q8RWS9 (CNGC5), O82226 (CNGC6), Q9S9N5 (CNGC7), Q9FXH6 (CNGC8), Q9M0A4 (CNGC9), Q9LNJ0 (CNGC10), Q9SKD6 (CNGC11), Q8GWD2 (CNGC12), Q9LD40 (CNGC13), Q9SJA4 (CNGC14), Q9SL29 (CNGC15), Q9SU64 (CNGC16), Q8L7Z0 (CNGC17), Q9LEQ3 (CNGC18), Q9LDR2 (CNGC19), Q9LD3 (CNGC20), P29973 (CNGA1), Q16280 (CNGA2), Q162810 (CNGA3), Q8IV77 (CNGA4), Q14028 (CNGB1), and Q9NQW8 (CNGB3). Moss cDNA sequences serving for conceptual translation are denoted in Supplemental Figure 1 online.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Alignment of the CNGC Amino Acid Sequences from Physcomitrella patens.
Supplemental Figure 2. Screen and Molecular Analysis of the ΔCNGCb Moss Mutants.
Supplemental Figure 3. Ca2+ Influx during Heat Treatments in CNGCb Mutant and Wild-Type Moss Tissues.
Supplemental Figure 4. Electrophysiology: I/V Curves for All Three Temperature-Responsive Conductances.
Supplemental Figure 5. The Moss CNGCb and Arabidopsis CNGC4 Mutants Acquire Thermotolerance at Lower Priming Temperatures than the Wild-Type Plants.
Supplemental Figure 6. Arabidopsis CNGC Expression Profiles.
Supplemental Data Set 1. Text File of Alignment Corresponding to the Phylogenetic Analysis in Figure 1B.
Acknowledgments
We thank the Swiss National Science Foundation (Grant NSF-0431327) and the University of Lausanne for financing this project in part, Maude Muriset for technical assistance, and Mitsuyasu Hasebe for providing the p35S-loxP-Zeo vector. We thank Jérome Goudet and Robin Liechti for advice on statistical analysis and Hillel Fromm for early discussions that oriented our research to the CNGC2 and CNGC4 genes.
AUTHOR CONTRIBUTIONS
A.F. designed and performed all the experiments, except electrophysiology and acquired thermotolerance in Arabidopsis, and wrote the article. A.F.H.Q. performed the experiments of acquired thermotolerance in Arabidopsis. Y.S. contributed to moss tissue preparation and discussions. F.J.M.M. designed and performed the electrophysiology experiments and contributed to writing the article. P.G. designed and supervised all the experiments except electrophysiology and wrote the article.
Glossary
- HSR
heat shock response
- Hsp
heat shock protein
- HSP
heat shock protein
- sHsp
small heat shock protein
- GUS
β-glucuronidase
- CNGC
cyclic nucleotide gated calcium channel
- Po
open probability
- Col-0
Columbia-0
- Rubisco
ribulose-1,5-bis-phosphate carboxylase/oxygenase
- ROS
reactive oxygen species
- TRP
transient receptor potential ion channel
- PCD
programmed cell death
- MS
Murashige and Skoog
References
- Ahuja I., de Vos R.C.H., Bones A.M., Hall R.D. (2010). Plant molecular stress responses face climate change. Trends Plant Sci. 15: 664–674 [DOI] [PubMed] [Google Scholar]
- Ali R., Ma W., Lemtiri-Chlieh F., Tsaltas D., Leng Q., von Bodman S., Berkowitz G.A. (2007). Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19: 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev S.I., Kreslavski V.D., Klimov V.V., Los D.A., Carpentier R., Mohanty P. (2008). Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 98: 541–550 [DOI] [PubMed] [Google Scholar]
- Arazi T., Kaplan B., Fromm H. (2000). A high-affinity calmodulin-binding site in a tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 42: 591–601 [DOI] [PubMed] [Google Scholar]
- Balagué C., Lin B.Q., Alcon C., Flottes G., Malmström S., Köhler C., Neuhaus G., Pelletier G., Gaymard F., Roby D. (2003). HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15: 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere H.M. (2004). “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117: 2641–2651 [DOI] [PubMed] [Google Scholar]
- Berry J., Bjorkman O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31: 491–543 [Google Scholar]
- Bradley J., Frings S., Yau K.W., Reed R. (2001). Nomenclature for ion channel subunits. Science 294: 2095–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Schmidt M., Fuchs M., Jaenicke R., Rudolph R., Schmid F.X., Kiefhaber T. (1991). GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry 30: 1586–1591 [DOI] [PubMed] [Google Scholar]
- Chaiwongsar S., Strohm A.K., Roe J.R., Godiwalla R.Y., Chan C.W.M. (2009). A cyclic nucleotide-gated channel is necessary for optimum fertility in high-calcium environments. New Phytol. 183: 76–87 [DOI] [PubMed] [Google Scholar]
- Chan-Schaminet K.Y., Baniwal S.K., Bublak D., Nover L., Scharf K.D. (2009). Specific interaction between tomato HsfA1 and HsfA2 creates hetero-oligomeric superactivator complexes for synergistic activation of heat stress gene expression. J. Biol. Chem. 284: 20848–20857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciais P., et al. (2005). Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437: 529–533 [DOI] [PubMed] [Google Scholar]
- Clay J.R. (1991). A paradox concerning ion permeation of the delayed rectifier potassium ion channel in squid giant axons. J. Physiol. 444: 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Fengler K.A., Yu I.C., Lippok B., Smith R.K., Jr, Bent A.F. (2000). The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 97: 9323–9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafny-Yelin M., Tzfira T., Vainstein A., Adam Z. (2008). Non-redundant functions of sHSP-CIs in acquired thermotolerance and their role in early seed development in Arabidopsis. Plant Mol. Biol. 67: 363–373 [DOI] [PubMed] [Google Scholar]
- Dat J.F., Lopez-Delgado H., Foyer C.H., Scott I.M. (1998). Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 116: 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd A.N., Kudla J., Sanders D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Ehrhardt D.W., Wais R., Long S.R. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85: 673–681 [DOI] [PubMed] [Google Scholar]
- Evans N.H., McAinsh M.R., Hetherington A.M., Knight M.R. (2005). ROS perception in Arabidopsis thaliana: The ozone-induced calcium response. Plant J. 41: 615–626 [DOI] [PubMed] [Google Scholar]
- Finka A., Mattoo R.U.H., Goloubinoff P. (2011). Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells. Cell Stress Chaperones 16: 15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finka A., Saidi Y., Goloubinoff P., Neuhaus J.M., Zrÿd J.P., Schaefer D.G. (2008). The knock-out of ARP3a gene affects F-actin cytoskeleton organization altering cellular tip growth, morphology and development in moss Physcomitrella patens. Cell Motil. Cytoskeleton 65: 769–784 [DOI] [PubMed] [Google Scholar]
- Flynn G.E., Johnson J.P.Jr., Zagotta W.N. (2001). Cyclic nucleotide-gated channels: Shedding light on the opening of a channel pore. Nat. Rev. Neurosci. 2: 643–651 [DOI] [PubMed] [Google Scholar]
- Gao F., Han X., Wu J., Zheng S., Shang Z., Sun D., Zhou R., Li B. (2012). A heat-activated calcium-permeable channel - Arabidopsis cyclic nucleotide-gated ion channel 6 - is involved in heat shock responses. Plant J. 70: 1056–1069 [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Mogk A., Zvi A.P., Tomoyasu T., Bukau B. (1999). Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc. Natl. Acad. Sci. USA 96: 13732–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., van der Luit A.H., Knight M.R., Trewavas A.J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116: 429–437 [Google Scholar]
- Gracheva E.O., Cordero-Morales J.F., González-Carcacía J.A., Ingolia N.T., Manno C., Aranguren C.I., Weissman J.S., Julius D. (2011). Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 476: 88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann P., Feller U. (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 27: 1169–1183 [Google Scholar]
- Hall A.E. (2000). Crop Responses to the Environment. (Boca Raton, FL: CRC Press)
- Hansen J., Sato M., Ruedy R., Lo K., Lea D.W., Medina-Elizade M. (2006). Global temperature change. Proc. Natl. Acad. Sci. USA 103: 14288–14293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R.C., Raghu P. (2001). Visual transduction in Drosophila. Nature 413: 186–193 [DOI] [PubMed] [Google Scholar]
- Härndahl U., Hall R.B., Osteryoung K.W., Vierling E., Bornman J.F., Sundby C. (1999). The chloroplast small heat shock protein undergoes oxidation-dependent conformational changes and may protect plants from oxidative stress. Cell Stress Chaperones 4: 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn S.A., Downs C.A., Sharkey T.D., Coleman J.S. (1998). The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 116: 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinault M.P., Farina-Henriquez-Cuendet A., Goloubinoff P. (2011). Molecular chaperones and associated cellular clearance mechanisms against toxic protein conformers in Parkinson’s disease. Neurodegener. Dis. 8: 397–412 [DOI] [PubMed] [Google Scholar]
- Horváth I., Glatz A., Varvasovszki V., Török Z., Páli T., Balogh G., Kovács E., Nádasdi L., Benkö S., Joó F., Vígh L. (1998). Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: Identification of hsp17 as a “fluidity gene”. Proc. Natl. Acad. Sci. USA 95: 3513–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T.D., Craig E.A. (1982). Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc. Natl. Acad. Sci. USA 79: 2360–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U., Gaestel M., Engel K., Buchner J. (1993). Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268: 1517–1520 [PubMed] [Google Scholar]
- Johannes E., Ermolayeva E., Sanders D. (1997). Red light-induced membrane potential transients in the moss Physcomitrella patens: Ion channel interaction in phytochrome signalling. J. Exp. Bot. 48: 599–608 [DOI] [PubMed] [Google Scholar]
- Juretić D., Zoranić L., Zucić D. (2002). Basic charge clusters and predictions of membrane protein topology. J. Chem. Inf. Comput. Sci. 42: 620–632 [DOI] [PubMed] [Google Scholar]
- Jurkowski G.I., Smith R.K., Jr, Yu I.C., Ham J.H., Sharma S.B., Klessig D.F., Fengler K.A., Bent A.F. (2004). Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “defense, no death” phenotype. Mol. Plant Microbe Interact. 17: 511–520 [DOI] [PubMed] [Google Scholar]
- Kaplan B., Sherman T., Fromm H. (2007). Cyclic nucleotide-gated channels in plants. FEBS Lett. 581: 2237–2246 [DOI] [PubMed] [Google Scholar]
- Kimpel J.A., Key J.L. (1985). Presence of heat-shock mRNAs in field grown soybeans. Plant Physiol. 79: 672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H., Trewavas A.J., Knight M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H., Trewavas A.J., Knight M.R. (1997). Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Knight M.R., Campbell A.K., Smith S.M., Trewavas A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352: 524–526 [DOI] [PubMed] [Google Scholar]
- Kosuta S., Hazledine S., Sun J., Miwa H., Morris R.J., Downie J.A., Oldroyd G.E.D. (2008). Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proc. Natl. Acad. Sci. USA 105: 9823–9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S., Suzuki N., Huntington S., Armijo L., Sha W., Cortes D., Shulaev V., Mittler R. (2008). Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 283: 34197–34203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus-Friedmann N. (2000). Cyclic nucleotide-gated channels in non-sensory organs. Cell Calcium 27: 127–138 [DOI] [PubMed] [Google Scholar]
- Kugler A., Köhler B., Palme K., Wolff P., Dietrich P. (2009). Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol. 9: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J., Knight M.R. (2002). Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J., Vierling E. (2008). Core genome responses involved in acclimation to high temperature. Plant Physiol. 146: 748–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Schöffl F. (1996). An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol. Gen. Genet. 252: 11–19 [DOI] [PubMed] [Google Scholar]
- Lee U., Wie C., Escobar M., Williams B., Hong S.-W., Vierling E. (2005). Genetic analysis reveals domain interactions of Arabidopsis Hsp100/ClpB and cooperation with the small heat shock protein chaperone system. Plant Cell 17: 559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P. (2012). SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 40: D302–D305. [DOI] [PMC free article] [PubMed]
- Li B., Liu H.T., Sun D.Y., Zhou R.G. (2004). Ca(2+) and calmodulin modulate DNA-binding activity of maize heat shock transcription factor in vitro. Plant Cell Physiol. 45: 627–634 [DOI] [PubMed] [Google Scholar]
- Li M., Doll J., Weckermann K., Oecking C., Berendzen K.W., Schöffl F. (2010). Detection of in vivo interactions between Arabidopsis class A-HSFs, using a novel BiFC fragment, and identification of novel class B-HSF interacting proteins. Eur. J. Cell Biol. 89: 126–132 [DOI] [PubMed] [Google Scholar]
- Liu H.-C., Liao H.-T., Charng Y.-Y. (2011). The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 34: 738–751 [DOI] [PubMed] [Google Scholar]
- Liu H.T., Gao F., Li G.L., Han J.L., Liu D.L., Sun D.Y., Zhou R.G. (2008). The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J. 55: 760–773 [DOI] [PubMed] [Google Scholar]
- Liu H.-T., Li G.-L., Chang H.U.I., Sun D.-Y., Zhou R.-G., Li B. (2007). Calmodulin-binding protein phosphatase PP7 is involved in thermotolerance in Arabidopsis. Plant Cell Environ. 30: 156–164 [DOI] [PubMed] [Google Scholar]
- Liu H.T., Un D.Y., Zhou R.G. (2005). Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in Arabidopsis. Plant Cell Environ. 28: 1276–1284 [Google Scholar]
- Lobell D.B., Schlenker W., Costa-Roberts J. (2011). Climate trends and global crop production since 1980. Science 333: 616–620 [DOI] [PubMed] [Google Scholar]
- Luján R., Lledías F., Martínez L.M., Barreto R., Cassab G.I., Nieto-Sotelo J. (2009). Small heat-shock proteins and leaf cooling capacity account for the unusual heat tolerance of the central spike leaves in Agave tequilana var. Weber. Plant Cell Environ. 32: 1791–1803 [DOI] [PubMed] [Google Scholar]
- Maathuis F.J.M. (2011). Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol. 191: 84–91 [DOI] [PubMed] [Google Scholar]
- Malik M.K., Slovin J.P., Hwang C.H., Zimmerman J.L. (1999). Modified expression of a carrot small heat shock protein gene, hsp17. 7, results in increased or decreased thermotolerancedouble dagger. Plant J. 20: 89–99 [DOI] [PubMed] [Google Scholar]
- Mäser P., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri D., Tazat K., Cohen-Peer R., Farchi-Pisanty O., Aviezer-Hagai K., Avni A., Breiman A. (2010). Involvement of Arabidopsis ROF2 (FKBP65) in thermotolerance. Plant Mol. Biol. 72: 191–203 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2006). Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11: 15–19 [DOI] [PubMed] [Google Scholar]
- Mittler R., Blumwald E. (2010). Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 61: 443–462 [DOI] [PubMed] [Google Scholar]
- Mittler R., Finka A., Goloubinoff P. (2012). How do plants feel the heat? Trends Biochem. Sci. 37: 118–125 [DOI] [PubMed] [Google Scholar]
- Mogk A., Schlieker C., Friedrich K.L., Schönfeld H.-J., Vierling E., Bukau B. (2003). Refolding of substrates bound to small Hsps relies on a disaggregation reaction mediated most efficiently by ClpB/DnaK. J. Biol. Chem. 278: 31033–31042 [DOI] [PubMed] [Google Scholar]
- Mori I. (1999). Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu. Rev. Genet. 33: 399–422 [DOI] [PubMed] [Google Scholar]
- Nilius B., Owsianik G. (2011). The transient receptor potential family of ion channels. Genome Biol. 12: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V., Cornelius T., Morimoto R.I. (2008). Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320: 811–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C., Hong S.W., Vierling E., Lindquist S. (2000). Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12: 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D., MacInnis B.L., Goodman M.B. (2008). Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 11: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S., Wünnenberg P., Lee J., Becker D., Dunkel M., Hedrich R., Scheel D., Dietrich P. (2008). Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 53: 287–299 [DOI] [PubMed] [Google Scholar]
- Rizhsky L., Liang H., Shuman J., Shulaev V., Davletova S., Mittler R. (2004). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi Y., Domini M., Choy F., Zryd J.P., Schwitzguebel J.P., Goloubinoff P. (2007). Activation of the heat shock response in plants by chlorophenols: Transgenic Physcomitrella patens as a sensitive biosensor for organic pollutants. Plant Cell Environ. 30: 753–763 [DOI] [PubMed] [Google Scholar]
- Saidi Y., Finka A., Chakhporanian M., Zrÿd J.P., Schaefer D.G., Goloubinoff P. (2005). Controlled expression of recombinant proteins in Physcomitrella patens by a conditional heat-shock promoter: a tool for plant research and biotechnology. Plant Mol. Biol. 59: 697–711 [DOI] [PubMed] [Google Scholar]
- Saidi Y., Finka A., Goloubinoff P. (2011). Heat perception and signalling in plants: A tortuous path to thermotolerance. New Phytol. 190: 556–565 [DOI] [PubMed] [Google Scholar]
- Saidi Y., Finka A., Muriset M., Bromberg Z., Weiss Y.G., Maathuis F.J.M., Goloubinoff P. (2009). The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21: 2829–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi Y., Peter M., Finka A., Cicekli C., Vigh L., Goloubinoff P. (2010). Membrane lipid composition affects plant heat sensing and modulates Ca2+-dependent heat shock response. Plant Signal. Behav. 5: 1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M.E. (2008). Association of Rubisco activase with chaperonin-60β: A possible mechanism for protecting photosynthesis during heat stress. J. Exp. Bot. 59: 1923–1933 [DOI] [PubMed] [Google Scholar]
- Sangwan V., Orvar B.L., Beyerly J., Hirt H., Dhindsa R.S. (2002). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 31: 629–638 [DOI] [PubMed] [Google Scholar]
- Schaefer D.G., Zrÿd J.P. (1997). Efficient gene targeting in the moss Physcomitrella patens. Plant J. 11: 1195–1206 [DOI] [PubMed] [Google Scholar]
- Schär C., Vidale P.L., Lüthi D., Frei C., Häberli C., Liniger M.A., Appenzeller C. (2004). The role of increasing temperature variability in European summer heatwaves. Nature 427: 332–336 [DOI] [PubMed] [Google Scholar]
- Shacklock P.S., Read N.D., Trewavas A.J. (1992). Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 358: 753–755 [Google Scholar]
- Shakeel S., Haq N.U., Heckathorn S.A., Hamilton E.W., Luthe D.S. (2011). Ecotypic variation in chloroplast small heat-shock proteins and related thermotolerance in Chenopodium album. Plant Physiol. Biochem. 49: 898–908 [DOI] [PubMed] [Google Scholar]
- Sharma S.K., De los Rios P., Christen P., Lustig A., Goloubinoff P. (2010). The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat. Chem. Biol. 6: 914–920 [DOI] [PubMed] [Google Scholar]
- Snyman M., Cronjé M.J. (2008). Modulation of heat shock factors accompanies salicylic acid-mediated potentiation of Hsp70 in tomato seedlings. J. Exp. Bot. 59: 2125–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel F., Baldwin A.J., Painter A.J., Jaya N., Basha E., Kay L.E., Vierling E., Robinson C.V., Benesch J.L.P. (2010). Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc. Natl. Acad. Sci. USA 107: 2007–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöver B.C., Müller K.F. (2010). TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P.H., Li H.M. (2008). Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol. 146: 1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.D., et al. (2004). Extinction risk from climate change. Nature 427: 145–148 [DOI] [PubMed] [Google Scholar]
- Tsvetkova N.M., Horváth I., Török Z., Wolkers W.F., Balogi Z., Shigapova N., Crowe L.M., Tablin F., Vierling E., Crowe J.H., Vigh L. (2002). Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. USA 99: 13504–13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca R.A., de Pinto M.C., Valenti D., Passarella S., Marra E., De Gara L. (2004). Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol. 134: 1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera S., Suzuki N., Miller G., van de Cotte B., Morsa S., Ravanat J.-L., Hegie A., Triantaphylidès C., Shulaev V., Van Montagu M.C., Van Breusegem F., Mittler R. (2011). Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. USA 108: 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinger L., Diamant S., Buchner J., Goloubinoff P. (1998). The small heat-shock protein IbpB from Escherichia coli stabilizes stress-denatured proteins for subsequent refolding by a multichaperone network. J. Biol. Chem. 273: 11032–11037 [DOI] [PubMed] [Google Scholar]
- Venkatachalam K., Montell C. (2007). TRP channels. Annu. Rev. Biochem. 76: 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E. (1991). The roles of heat-shock proteins in plants. Annu. Rev. Plant Physiol. 42: 579–620 [Google Scholar]
- Volkov R.A., Panchuk I.I., Mullineaux P.M., Schöffl F. (2006). Heat stress-induced H(2)O (2) is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 61: 733–746 [DOI] [PubMed] [Google Scholar]
- von Koskull-Döring P., Scharf K.D., Nover L. (2007). The diversity of plant heat stress transcription factors. Trends Plant Sci. 12: 452–457 [DOI] [PubMed] [Google Scholar]
- Wahid A., Gelani S., Ashraf M., Foolad M.R. (2007). Heat tolerance in plants: An overview. Environ. Exp. Bot. 61: 199–223 [Google Scholar]
- Weiss Y.G., Bromberg Z., Raj N., Raphael J., Goloubinoff P., Ben-Neriah Y., Deutschman C.S. (2007). Enhanced heat shock protein 70 expression alters proteasomal degradation of IkappaB kinase in experimental acute respiratory distress syndrome. Crit. Care Med. 35: 2128–2138 [DOI] [PubMed] [Google Scholar]
- Wheeler G.L., Brownlee C. (2008). Ca2+ signalling in plants and green algae—Changing channels. Trends Plant Sci. 13: 506–514 [DOI] [PubMed] [Google Scholar]
- Wu H.-C., Hsu S.-F., Luo D.-L., Chen S.-J., Huang W.-D., Lur H.-S., Jinn T.-L. (2010). Recovery of heat shock-triggered released apoplastic Ca2+ accompanied by pectin methylesterase activity is required for thermotolerance in soybean seedlings. J. Exp. Bot. 61: 2843–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Fukao Y., Hayashi M., Fukazawa M., Suzuki I., Nishimura M. (2007). Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J. Biol. Chem. 282: 37794–37804 [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhou R.-G., Gao Y.-J., Zheng S.-Z., Xu P., Zhang S.-Q., Sun D.-Y. (2009). Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 149: 1773–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Zagotta W.N. (2004). Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron. 42: 411–421 [DOI] [PubMed] [Google Scholar]