A change in reactive oxygen species levels is a fundamental phenomenon in response to stress. This study provides mechanistic insight into how the stress-activated glycogen synthase kinase 3 ASKα regulates the activity of G6PD6 and is thus critical for the cellular response to salinity stress.
Abstract
Diverse stresses such as high salt conditions cause an increase in reactive oxygen species (ROS), necessitating a redox stress response. However, little is known about the signaling pathways that regulate the antioxidant system to counteract oxidative stress. Here, we show that a Glycogen Synthase Kinase3 from Arabidopsis thaliana (ASKα) regulates stress tolerance by activating Glc-6-phosphate dehydrogenase (G6PD), which is essential for maintaining the cellular redox balance. Loss of stress-activated ASKα leads to reduced G6PD activity, elevated levels of ROS, and enhanced sensitivity to salt stress. Conversely, plants overexpressing ASKα have increased G6PD activity and low levels of ROS in response to stress and are more tolerant to salt stress. ASKα stimulates the activity of a specific cytosolic G6PD isoform by phosphorylating the evolutionarily conserved Thr-467, which is implicated in cosubstrate binding. Our results reveal a novel mechanism of G6PD adaptive regulation that is critical for the cellular stress response.
INTRODUCTION
High soil salinity is a major environmental constraint for plant growth and development and negatively affects agricultural productivity (Boyer, 1982). Salinity imposes a water deficit and ion stress, which cause effects such as inhibition of essential enzymes, destabilization of cell membranes, a decrease in nutrient supply, and overproduction of reactive oxygen species (ROS) (Hasegawa et al., 2000; Zhu, 2001; Munns, 2002). ROS production is a universal feature of aerobic metabolism. Although recent evidence has uncovered a role for ROS as a signaling molecule and key physiological regulator of diverse cellular processes (Mittler et al., 2011), excess levels generated as a consequence of an insult cause oxidative damage and can ultimately lead to cell death (Møller et al., 2007). Therefore, tight regulation of excess ROS is an essential protective mechanism in all organisms.
Glc-6-phosphate dehydrogenase (G6PD; EC 1.1.1.49) catalyzes a key step of the oxidative pentose phosphate pathway (OPPP) that provides NADPH for reductive biosynthesis and maintenance of the cellular redox state. In animals, G6PD is the principal enzyme providing NADPH and is of central importance for cellular redox regulation in response to stress. For example, mouse embryonic stem cells in which G6PD activity is disrupted are extremely sensitive to oxidative stress (Pandolfi et al., 1995; Filosa et al., 2003). In addition, exogenous stresses such as pathogen infection, certain drugs, or ingestion of fava beans (Vicia faba) can trigger hemolytic anemia in humans with G6PD deficiency, which is the most common enzymopathy. Similarly, yeast mutants defective in G6PD show an increased susceptibility to oxidative stress and fail to induce adaptation (Juhnke et al., 1996; Izawa et al., 1998). In plants, G6PD activity is present in the plastids and cytosol (Debnam and Emes, 1999). G6PD activity has been positively correlated with environmental stresses such as salt stress (Valderrama et al., 2006; Wang et al., 2008), aluminum toxicity (Slaski et al., 2006), drought (Scharte et al., 2009), and infection by viral and fungal pathogens (Sindelár and Sindelárová, 2002; Scharte et al., 2009); by contrast, a decrease in chloroplastic G6PD activity has been shown to be beneficial for withstanding oxidative stress (Debnam et al., 2004). Thus, the role of G6PD in the stress response is currently unclear.
The activity of G6PD is tightly regulated. In addition to regulation at the transcriptional level, redox regulation and the cellular NADPH/NADP+ ratio modulate the activity of the different G6PD isoforms (Wendt et al., 2000; Debnam et al., 2004; Schürmann and Buchanan, 2008). G6PD is phosphorylated in animals and plants, which has been correlated with both enhanced (Ramnanan and Storey, 2006; Dieni and Storey, 2010; Gupte et al., 2011) and reduced activity (Hauschild and von Schaewen, 2003; Xu et al., 2005; Zhang et al., 2000). While different signaling pathways, including protein kinase C, protein kinase A, and Src kinase, have been implicated in regulating G6PD phosphorylation in animals under a variety of different conditions (Xu et al., 2005; Gupte et al., 2009, 2011), the upstream regulator(s) of G6PD phosphorylation in plants have yet to be identified.
Glycogen Synthase Kinase3 (GSK3) constitutes a class of evolutionarily conserved Ser/Thr protein kinases. Originally identified in mammals as a cytoplasmic modulator of glycogen metabolism, GSK3 is now recognized as a central regulator of an array of cellular events, including cell fate determination, microtubule function, cell cycle regulation, apoptosis, and inflammatory responses (Cohen and Frame, 2001; Doble and Woodgett, 2003; Jope and Johnson, 2004; Martin et al., 2005). In plants, GSK3/shaggy-like kinases (GSKs) are encoded by a gene family that directs different physiological responses (Jonak and Hirt, 2002; Saidi et al., 2012). In alfalfa (Medicago sativa), several GSKs have emerged as regulatory components in stress signaling. For example, wound-induced GSK is posttranslationally activated by wounding (Jonak et al., 2000), MsK1 is involved in innate immunity by limiting the severity of virulent bacterial infections (Wrzaczek et al., 2007), and MsK4 regulates high salt tolerance by adjusting carbohydrate metabolism in response to environmental stress (Kempa et al., 2007). In Arabidopsis thaliana, overexpression of AtGSK1/ASKι enhanced salt tolerance (Piao et al., 2001), and in rice (Oryza sativa), knockout mutants of GSK1 showed increased tolerance to salt stress (Koh et al., 2007). However, the mechanisms by which these stress-related GSKs act, including their direct targets for phosphorylation, remain elusive.
In a changing environment, cellular metabolism needs to be altered to enable an adapted physiological response. Protein phosphorylation represents an important means of fine-tuning the activity of metabolic enzymes. However, our knowledge of how signal transduction is linked to redox regulation is limited. Here, we provide direct evidence that a stress-activated GSK3 from Arabidopsis, ASKα, is a key regulatory component protecting cells against oxidative stress. Moreover, we identify G6PD as an in vivo target of ASKα and present a novel molecular mechanism for the regulation of G6PD activity.
RESULTS
ASKα Is Involved in Arabidopsis Salt Stress Resistance
To investigate the role of Arabidopsis GSKs (ASKs) in stress tolerance, we screened ASK activity mutants (i.e., ask knockout and ASK overexpressor lines) for their performance under abiotic stress conditions. This systematic study revealed that ASKα activity mutants resembled the wild type under normal growth conditions but displayed altered tolerance to high salt conditions. High soil salinity stress, imposed by watering soil-grown plants with 150 mM NaCl solution over 3 weeks, severely affected the growth of askα T-DNA insertion knockout plants (see Supplemental Figures 1A and 1B online) compared with wild-type plants (Figure 1A). No difference was observed between the different genotypes in untreated plants (see Supplemental Figure 1C online).
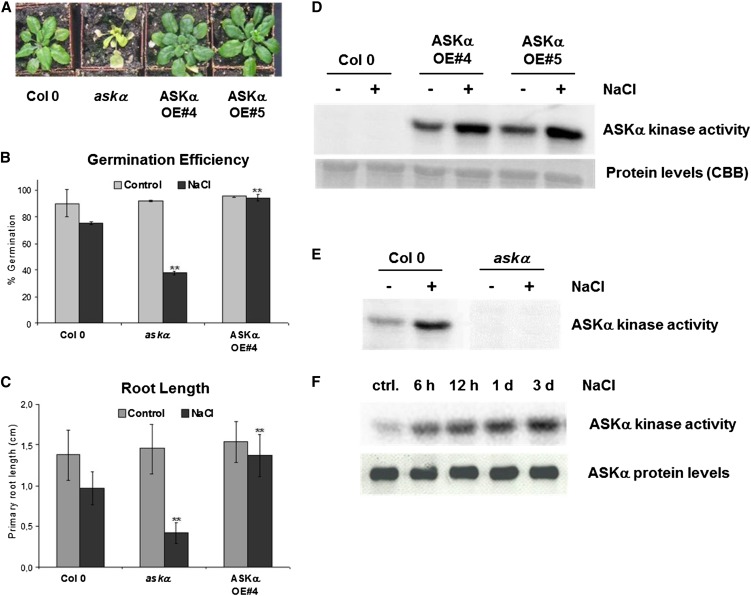
Figure 1.
ASKα Is Activated by High Salinity and Modulates Arabidopsis Salt Stress Resistance.
(A) Phenotype of ASKα activity mutants upon prolonged salt stress. Four-week-old soil-grown Arabidopsis wild-type Col-0, askα, and ASKα overexpressor lines OE#4 and OE#5 were watered with 150 mM NaCl for 3 weeks. An image of single representative individuals of the phenotypes’ mean of 30 plants is shown.
(B) and (C) Salt tolerance of ASKα activity mutants during early seedling development.
(B) Germination efficiency on half-strength MS plates and on plates supplemented with 100 mM NaCl. Data are means ± sd of three independent biological replicates with n = 100 each. Asterisks indicate a significant difference (**P < 0.01) using Student’s t test for pairwise comparison to Col-0 under stress conditions.
(C) Primary root growth on control medium and on medium supplemented with 100 mM NaCl. Root length was determined 7 d after sowing. Data are means ± sd of three independent biological replicates. Asterisks indicate a significant difference (**P < 0.01) using Student’s t test for pairwise comparison to Col-0 under stress conditions.
(D) and (E) ASKα in vivo kinase activity upon prolonged salt stress. Four-week-old soil-grown Arabidopsis wild type and ASKα activity mutants were either irrigated with water or with a 150 mM NaCl solution for 3 weeks. Twenty plants per line were pooled and used for immunokinase assays. ASKα was either immunoprecipitated from 100 µg leaf protein extracts with anti-myc antibodies (D) or with ASKα-specific antibodies (E). Subsequent kinase assays were performed with [γ-32P]ATP and MBP as a substrate.
(F) Kinetics of ASKα kinase activation. Soil-grown Arabidopsis Col-0 plants were exposed to high soil salinity for 6 h, 12 h, 24 h, or 3 d. Plant material was pooled from rosette leaves of 10 plants per time point. Top panel: Immunokinase assays were performed on 100 µg of leaf protein extracts. ASKα-specific antibodies were used for immunoprecipitation. Subsequently, ASKα kinase activity was determined in kinase assays with [γ-32P]ATP and MBP as a substrate. Bottom panel: ASKα protein levels were determined by immunoprecipitation of ASKα from 200 µg total protein extract and subsequent immunoblot analysis with ASKα-specific antibodies. ctrl., control.
All experiments in this figure were performed at least twice and showed similar results.
[See online article for color version of this figure.]
We next analyzed whether ASKα was also important for tolerance to salt stress during early seedling development. On medium supplemented with 100 mM NaCl, both germination efficiency and primary root length were significantly reduced in askα mutants compared with wild-type plants grown under the same high salt stress conditions (Figures 1B and 1C). By contrast, plants overexpressing ASKα from the strong constitutive 35S promoter (see Supplemental Figure 1B online) germinated equally well under normal and salt stress conditions, and root length was only slightly affected by high salt stress. In high salt conditions, both germination efficiency and root length of plants overexpressing ASKα were significantly increased compared with wild-type plants. Thus, ASKα plays an important role in sensitivity to salt stress, both during early seedling development and in adult plants.
ASKα Is Activated by High Salinity Stress
To assess whether ASKα protein kinase activity is modulated by salt stress, in vivo ASKα kinase activity was first determined from long-term salt stressed and nonstressed plants expressing ASKα-myc. Immunokinase assays using anti-myc antibodies and myelin basic protein (MBP) as a general substrate showed basal ASKα-myc kinase activity in nonstressed plants and enhanced kinase activity in plants exposed to salt stress (Figure 1D). To study the effect of salt stress on the activity of endogenous ASKα, an ASKα-specific peptide antibody (see Supplemental Figures 1D and 1E online) was used for the immunokinase assays. Consistent with the previous result, wild-type Columbia-0 (Col-0) plants grown under high salt conditions contained enhanced endogenous ASKα activity compared with plants grown under normal conditions (Figure 1E).
Plants respond to high soil salinity by a sequence of temporally successive cellular and physiological responses. To study the kinetics of ASKα activation at the onset of stress, ASKα immunokinase assays were performed on plants exposed to salt stress for 6 h, 12 h, 1 d, or 3 d. In control plants, ASKα activity was low. However, high soil salinity induced ASKα activity rapidly and persistently over an experimental period of 3 d (Figure 1F, top panel), suggesting that ASKα activation is part of a primary stress response. By contrast, the ASKα steady state protein levels remained constant (Figure 1F, bottom panel). This indicates that there is a posttranslational mechanism of ASKα activation upon salt stress.
Altered G6PD Activity and ROS Levels of ASKα Activity Mutants under Salt Stress Conditions
Our previous work indicated a role for the GSK MSK4 in metabolic regulation in alfalfa (Kempa et al., 2007), so we hypothesized that this salt stress induction of ASKα might impact cellular metabolism by regulating the activity of specific metabolic enzymes. We therefore performed a high-throughput robot-based screen for multiple enzyme activities (Gibon et al., 2004) in askα plants to identify potential downstream effectors of the stress response. This analysis revealed reduced G6PD activity in stressed askα plants. Analysis of total G6PD activity showed that prolonged salt stress enhanced G6PD activity in wild-type plants (Figure 2A). However, in askα, G6PD activity was strongly reduced by salinity. By contrast, plants overexpressing ASKα showed a stress-induced increase in G6PD activity that was higher than that in the wild type (Figure 2A).
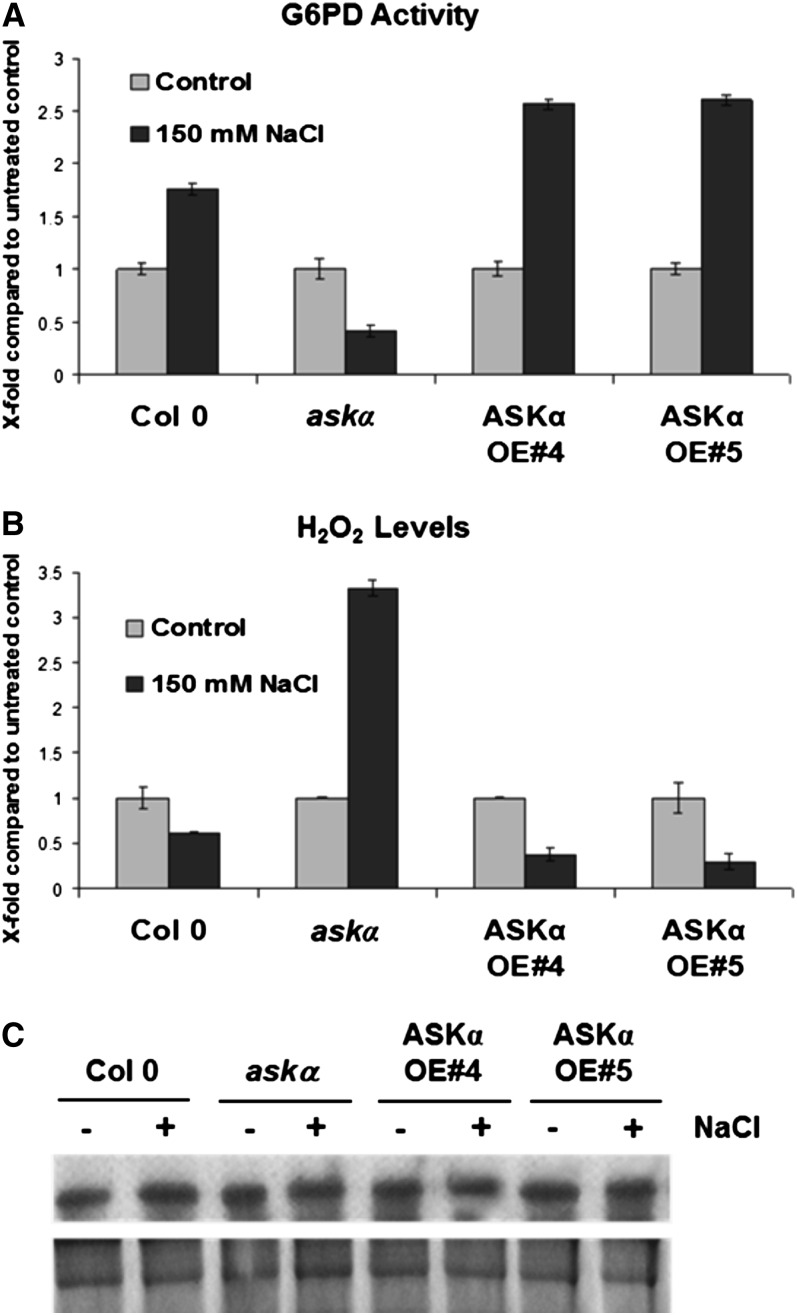
Figure 2.
G6PD Activity and H2O2 Levels Are Altered in ASKα Activity Mutants upon Salt Stress.
Four-week-old soil-grown Arabidopsis plants were watered with 150 mM NaCl for 3 weeks. The same plant material was used for (A) to (C). Analyses were performed with pools of 20 plants on three independent salt watering experiments. Data are means ± relative sd. Controls for each genotype were normalized to one ([A] and [B]).
(A) Total G6PD activity in leaf extracts of wild-type Col-0, askα, and ASKα overexpressor lines OE#4 and OE#5 grown under normal and high salinity conditions.
(B) H2O2 levels. H2O2 content was assessed spectrophotometrically in Col-0 and ASKα activity mutants.
(C) Total G6PD levels. Top lane: Total G6PD was immunoprecipitated from 50 µg protein extract and subsequently detected by immunoblot analysis using a polyclonal G6PD antibody. Bottom lane: CBB-stained gel.
G6PD activity provides reducing power important for ROS detoxification. We thus explored levels of the ROS hydrogen peroxide (H2O2) in leaves upon salt stress. Consistent with the reduced total G6PD activity, H2O2 levels were significantly elevated in askα plants exposed to high soil salinity. In stressed ASKα overexpressor plants, H2O2 levels were lower than in the wild type (Figure 2B), indicating that ASKα activity is involved in modulating stress-induced ROS accumulation.
ASKα Phosphorylates and Activates G6PD6 in Vitro
To study the mechanism by which ASKα regulates G6PD, we first analyzed total G6PD levels in leaves which were similar in all genotypes under normal and salt stress conditions (Figure 2C). Next, we assessed the possibility that ASKα might regulate G6PD activity directly by phosphorylation. The major G6PD activity in leaves originates from cytosolic G6PD isoforms (Debnam and Emes, 1999). The lack of a potential plastid targeting sequence in ASKα prompted us to focus our analyses on the two cytosolic G6PD isoforms, G6PD5 and G6PD6 (Wakao et al., 2008).
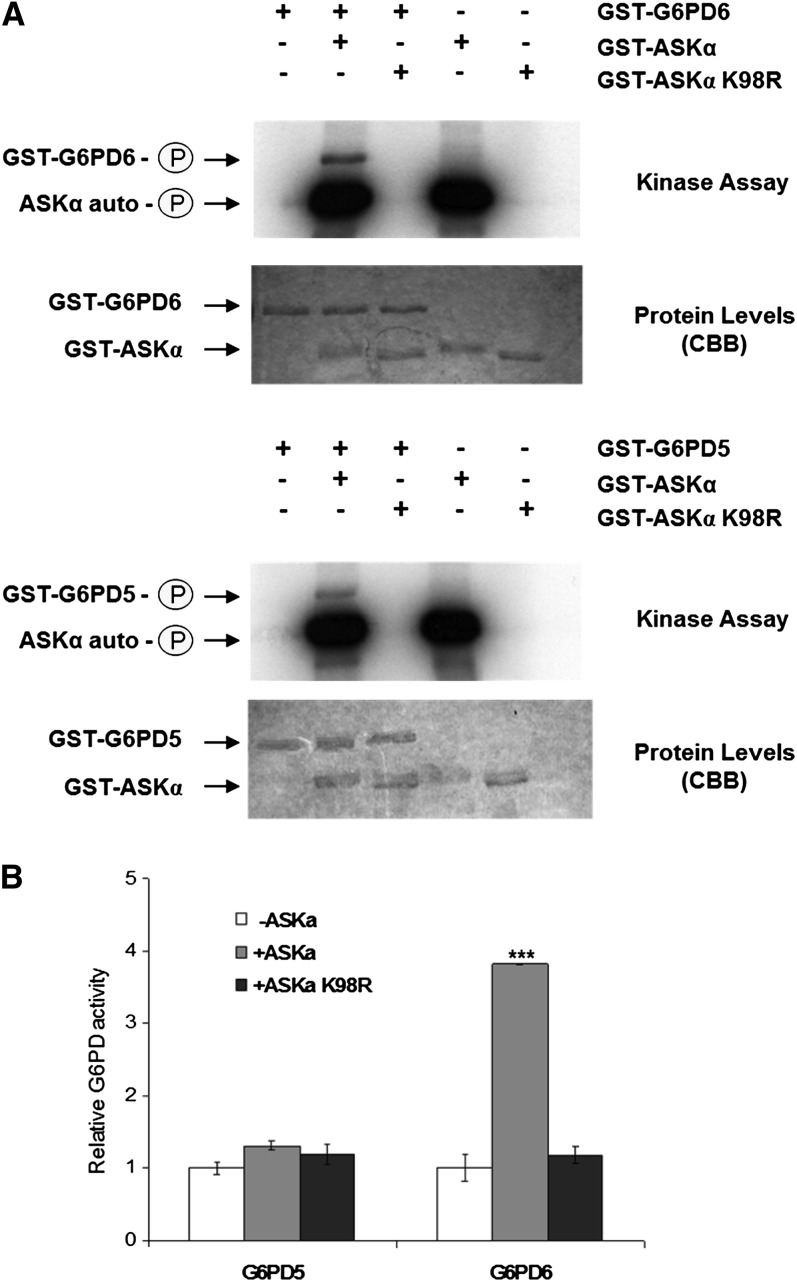
In an in vitro kinase assay with recombinant proteins, ASKα was able to phosphorylate G6PD5 and G6PD6 (Figure 3A). Subsequent analysis of G6PD activity revealed that phosphorylation by active ASKα stimulated the activity of G6PD6 (Figure 3B), whereas a kinase-dead version of ASKα (ASKα K98R) could not phosphorylate G6PD6 and also failed to modify its activity. Interestingly, ASKα did not enhance G6PD5 activity, indicating that the two G6PD isoforms might be regulated by distinct mechanisms.
Figure 3.
ASKα Phosphorylates and Thereby Activates G6PD6 in Vitro.
(A) Phosphorylation of G6PD5 and G6PD6 by ASKα. Kinase assays were performed with purified GST-ASKα and GST-G6PD5 or GST-G6PD6. ASKα did not phosphorylate GST alone.
(B) Enzymatic activities of G6PD5 and G6PD6. G6PD activity was quantified after a phosphorylation reaction of GST-G6PD5 and GST-G6PD6 without kinase or with GST-ASKα or with GST-ASKα K98R. Data are means ± relative sd. The assay was performed three times using fresh proteins from independent purifications. Asterisks indicate a significant difference (***P < 0.005) tested by Student’s t test for pairwise comparison to the unphosphorylated control.
Thr-467 Is Necessary for G6PD6 Activation by ASKα
To investigate the molecular mechanism of G6PD6 activation by ASKα, liquid trap quadrupole mass spectrometry analysis was performed on recombinant G6PD phosphorylated by ASKα in vitro. Thr-467 at the C terminus of G6PD6 was identified as the ASKα target site (see Supplemental Figure 2 online). Thr-467, and the adjacent amino acids, are highly conserved in eukaryotes (see Supplemental Figure 3 online), suggesting an important role for this residue in regulating G6PD6 activity.
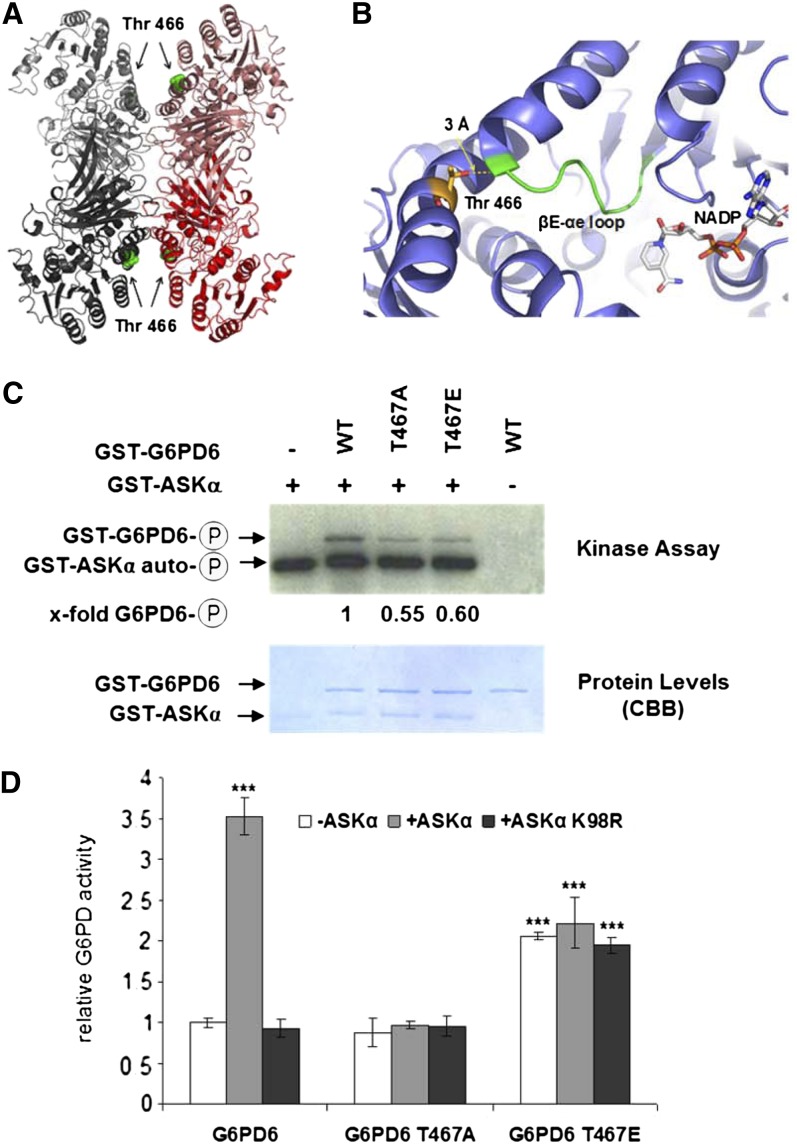
To understand the molecular effects of Thr-467 phosphorylation, we analyzed the homologous crystal structure of human G6PD (Au et al., 2000; Kotaka et al., 2005). The corresponding human Thr residue (Thr-466) is exposed on the G6PD surface and thus is readily accessible by the kinase (Figure 4A). Active G6PD exists in a dimer-tetramer equilibrium (Au et al., 2000). Human Thr-466 is located remotely from the dimer and tetramer interfaces of the G6PD molecule, suggesting that this residue would not affect the oligomeric state (Figure 4A). Interestingly, human Thr-466 is in close proximity (3 Å) to the βE–αe loop, which is part of the NADP binding site (Figure 4B). Phosphorylation of Thr-467 in G6PD6 might thus introduce structural changes in the coenzyme binding site connected with an enhanced G6PD activity.
Figure 4.
Thr-467 Is Necessary for G6PD6 Activation by ASKα in Vitro.
(A) and (B) The crystal structure of human G6PD suggests that Thr-467 phosphorylation influences coenzyme binding.
(A) Crystal structure of human G6PD with G6PD dimers are colored in red and gray and Thr-466 highlighted in ball representation (green).
(B) Human Thr-466 (stick representation with carbon atoms colored in yellow) is positioned in close proximity to the βE–αe loop (in green), which is part of the NADP binding region (NADP highlighted in stick representation with carbon atoms colored in white). Representations were generated with PyMOL (DeLano, 2002) using the PDB codes 2BH9 and 1QKI (Au et al., 2000; Kotaka et al., 2005).
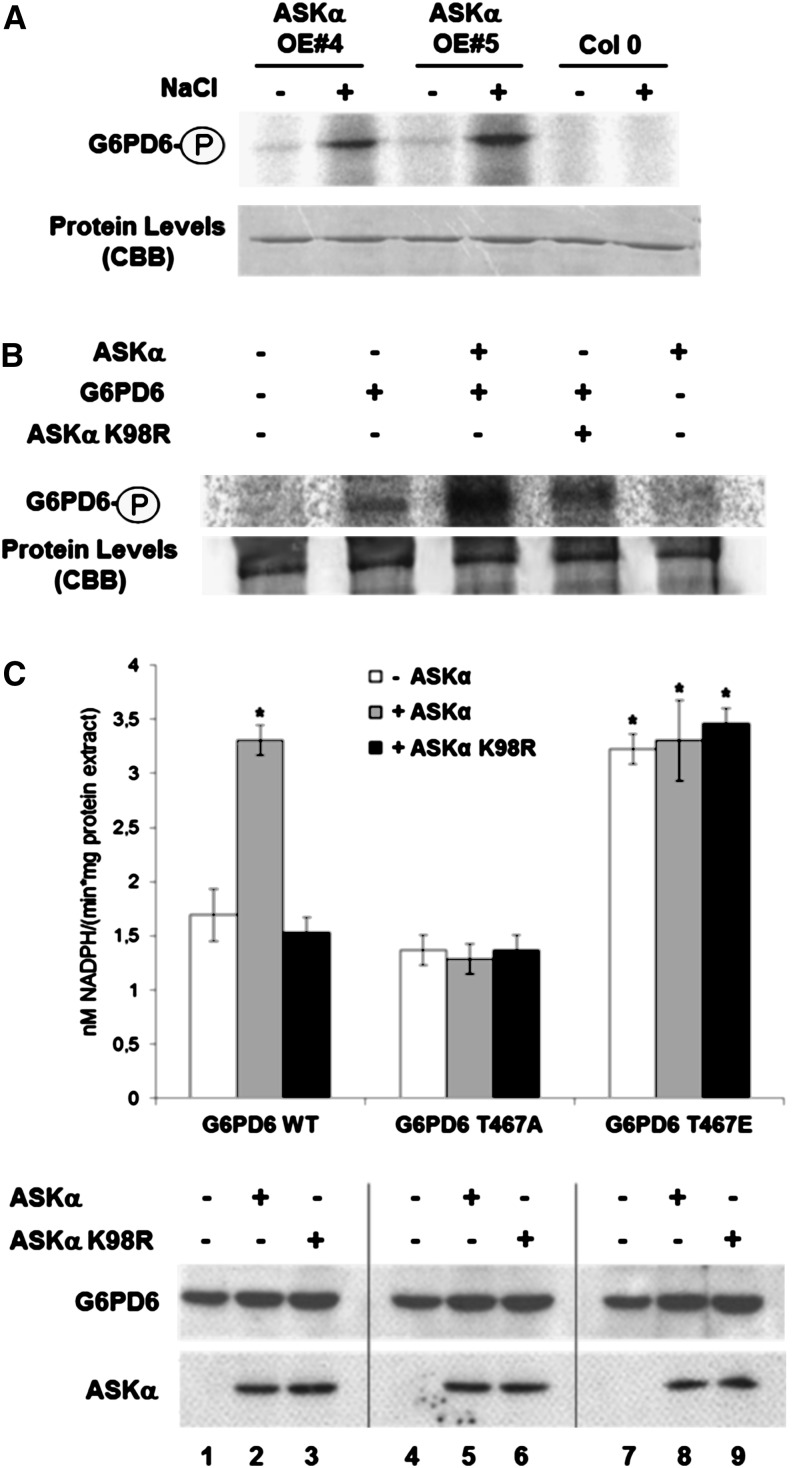
(C) In vitro kinase assay with GST-ASKα and GST-G6PD6, GST-G6PD6 T467A, or GST-G6PD6 T467E. The experiment was repeated three times, showing comparable results. WT, wild type.
(D) Enzymatic activity of G6PD6, G6PD6 T467A, and G6PD6 T467E. G6PD6 activity was quantified after a phosphorylation reaction with ASKα or ASKα K98R, or without kinase. Data are means ± relative sd. The assay was performed four times using fresh proteins from independent purifications. Asterisks indicate a significant difference (***P < 0.005) tested by Student’s t test for pairwise comparison to the unphosphorylated control.
To explore the significance of Thr-467 modification for G6PD6 activity further, the residue was mutated either to Ala (T467A), which cannot be phosphorylated, or to Glu (T467E), mimicking the phosphorylation event. In ASKα kinase assays, phosphorylation of the T467A (nonphosphorylatable) mutant was reduced compared with wild-type protein, suggesting that Thr-467 is a central target site for ASKα phosphorylation (Figure 4C). The observed residual phosphorylation of G6PD6 T467A and G6PD6 T467E indicates that ASKα phosphorylates additional sites, at least in vitro. Importantly, consistent with the results of the kinase assays, analyses of the impact of ASKα on the activity of G6PD6 mutant versions revealed that the activity of G6PD6 T467A could no longer be enhanced by ASKα, whereas the G6PD6 T467E phosphomimic mutant displayed constitutively elevated enzyme activity (Figure 4D). Thus, phosphorylation of G6PD6 on Thr-467 is necessary for stimulating G6PD6 activity.
G6PD6 Is Phosphorylated and Activated by ASKα in Vivo
We have shown that G6PD activity is modified in ASKα activity mutants upon salt stress. To assess whether G6PD6 might be a direct target of ASKα in vivo, we first tested whether ASKα activity immunoprecipitated from control and stressed plants could phosphorylate recombinant G6PD6. Consistent with the data shown in Figure 1, ASKα immunoprecipitated from salt-stressed ASKα-overexpressing plants showed a higher kinase activity toward G6PD6 than ASKα immunoprecipitated from plants grown under normal conditions (Figure 5A).
Figure 5.
ASKα Activates G6PD6 in Vivo.
(A) Phosphorylation of G6PD6 by immunoprecipitated ASKα. Immunokinase assay using anti-myc antibodies (Santa Cruz) to purify ASKα-myc from nonstressed and high salinity–stressed ASKα-myc–expressing plants (same plant material as in Figure 1D) using GST-G6PD6 as a substrate.
(B) G6PD6 is an in vivo phosphoprotein. Arabidopsis protoplasts were transformed with G6PD6-HA or cotransformed with G6PD6-HA and ASKα-myc or ASKα K98R-myc and labeled with 32P-orthophosphate. The autoradiogram shows G6PD6 immunoprecipitated from 200 µg of protein extracts with HA antibodies. ASKα K98R-myc and untransformed protoplasts were used as specificity controls for phosphorylation and immunoprecipitation.
(C) In vivo activity of G6PD6, G6PD6 T467A, and G6PD6 T467E. G6PD activity (top) and protein levels (bottom) of Arabidopsis protoplasts transformed solely with G6PD6-HA, G6PD6-HA T467A, and G6PD6-HA T467E (lanes 1, 4, and 7, respectively), or cotransformed either with ASKα-myc (lanes 2, 5, and 8) or with ASKα K98R-myc (lanes 3, 6 and 9). Data are means ± sd. The assay was repeated three times. Asterisks indicate a significant difference (*P < 0.05) using Student’s t test for pairwise comparison to cells solely transformed with wild-type G6PD6-HA. WT, the wild type.
We next determined whether G6PD6 exists as an in vivo phosphoprotein. Hemagglutinin (HA)-tagged G6PD6 was transiently expressed in radiolabeled Arabidopsis protoplast cells and immunoprecipitated with anti-HA antibodies. As shown in Figure 5B, G6PD6 is phosphorylated in vivo. Cotransformation of G6PD6-HA with ASKα further enhanced G6PD6 phosphorylation, also compared with coexpression of the kinase-dead mutant ASKα K98R.
To verify whether ASKα might modulate G6PD6 activity in vivo, G6PD activity was quantified in protoplast cells transformed with G6PD6 in the presence or absence of ASKα (Figure 5C). Indeed, G6PD activity was enhanced by ASKα but not by ASKα K98R in cells expressing G6PD6. However, when protoplasts were transformed with the nonphosphorylatable mutant G6PD6 T467A, ASKα was unable to stimulate G6PD activity. Cells expressing the phosphomimicking mutant G6PD6 T467E showed constitutively high G6PD activity, which could not be further stimulated by ASKα. Collectively, these results suggest that Thr-467 phosphorylation by ASKα is necessary and sufficient for activation of G6PD6 in vivo.
Loss of G6PD6 Alters the Cellular Redox State and Renders Plants More Sensitive to High Salt Stress
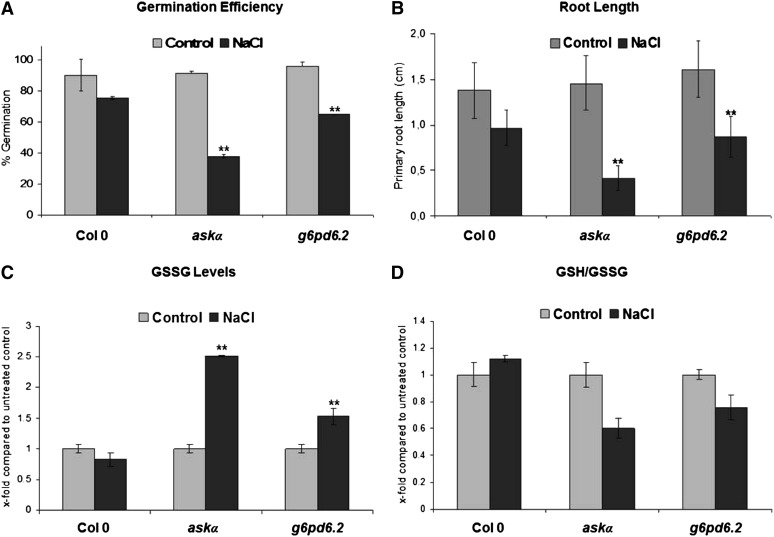
Given that G6PD activity is modulated by high salt conditions, we postulated that it plays a direct role in stress tolerance. To test this, g6pd6.2 knockout plants (see Supplemental Figures 4A and 4B online) were assayed for their stress sensitivity. Wild-type seeds germinated nearly as well on medium supplemented with 100 mM NaCl as on normal growth medium. However, the germination efficiency of g6pd6.2 was significantly reduced under high salinity conditions (Figure 6A). Similarly, root growth of g6pd6.2 was more strongly affected by NaCl compared with the wild type (Figure 6B). Thus, G6PD6 is indeed important for tolerance to high salt.
Figure 6.
g6pd6.2 Shows an Altered Redox Status and Enhanced Sensitivity to Salt Stress.
(A) Germination efficiency of wild-type Col-0 and askα and g6pd6.2 mutants under normal and salt stress conditions. Germination rate on control plates and on plates supplemented with 100 mM NaCl. Data are means ± sd of three independent biological replicates with n = 100 each. Asterisks indicate a significant difference (**P < 0.01) using Student’s t test for pairwise comparison to Col-0 under stress conditions.
(B) Wild-type Col-0, askα, and g6pd6.2 were exposed to salt stress during early seedling development. Primary root growth under control conditions and on medium supplemented with 100 mM NaCl. Root length was determined 7 d after sowing. Data are means ± sd of three independent experiments. Asterisks indicate a significant difference (**P < 0.01) using Student’s t test for pairwise comparison to wild-type Col-0 under stress conditions.
(C) and (D) Glutathione redox status. GSSG levels (C) and the ratio of GSH to GSSG (D) in leaf extracts from Col-0, askα, and g6pd6.2 grown in soil under normal or high salinity conditions. Analyses were performed with pools of 20 plants on three independent salt watering experiments. Data are means ± relative sd. Asterisks indicate a significant difference (**P < 0.01) using Student’s t test for pairwise comparison to wild-type Col-0 under stress conditions.
Glutathione is a key marker of the intracellular redox state. Consistent with a role of G6PD6 in redox regulation, levels of GSSG were elevated (Figure 6C) and the ratio of reduced GSH to oxidized glutathione was altered in g6pd6.2 under salt stress conditions (Figure 6D). Early seedling development and the glutathione redox status of askα plants were more strongly affected by high salinity conditions than in g6pd6.2 plants, suggesting that ASKα also regulates other targets in addition to G6PD6 for successful acclimation to stress.
We also analyzed a previously described G6PD6 T-DNA insertion line, which was unaffected by salt stress (Wakao et al., 2008). In this T-DNA insertion line, G6PD6 is disrupted downstream of the ASKα phosphorylation site Thr-467, underlining the significance of Thr-467 for adaptive regulation of G6PD.
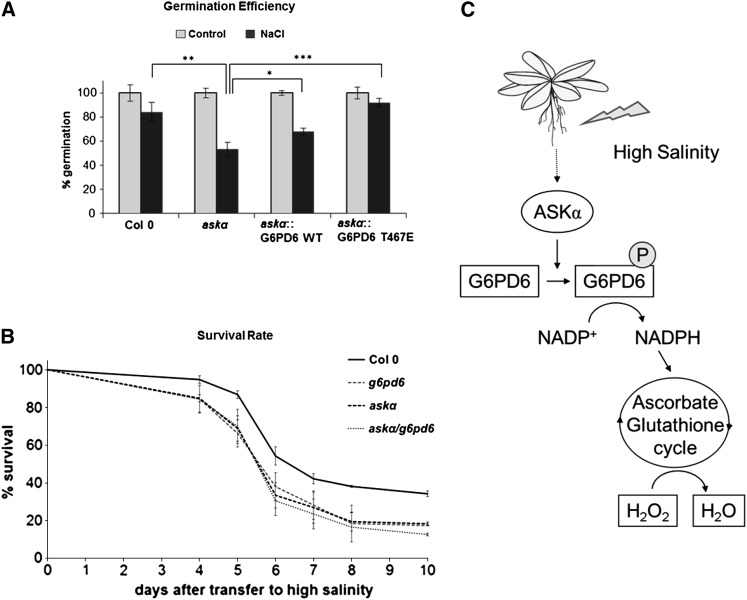
Finally, to assess whether the effect of ASKα on stress tolerance is indeed mediated by phosphorylation of G6PD6, we used the askα knockout plants, which are hypersensitive to salt stress. This hypersensitivity was restored by expression of the constitutively phosphorylated mutant G6PD6 T467E (Figure 7A; see Supplemental Figure 4C online). In addition, askα g6pd6.2 double knockout plants showed a similar sensitivity to salt as askα and g6pd6.2 (Figure 7B), suggesting that they are indeed part of the same pathway. Thus, our results support a role for T467 phosphorylation of G6PD6 by ASKα in protecting plants against salt stress.
Figure 7.
ASKα Regulates Salt Stress Tolerance by Phosphorylating G6PD6.
(A) Phosphorylation of G6PD6 is important for salt stress tolerance. Germination efficiency of Col-0, askα, and askα mutants transformed with either G6PD6 or G6PD6 T467E on half-strength MS plates supplemented with 100 mM NaCl. Data are means ± relative sd of three independent biological replicates with at least 75 plants each. Asterisks indicate a significant difference compared with askα under stress conditions (*P < 0.05, **P < 0.01, and ***P < 0.005) using Student’s t test for pairwise comparison. WT, wild type.
(B) ASKα and G6PD6 function in the same signaling pathway. Survival rate of Col-0, askα, g6pd6, and askα g6pd6 double knockout plants on medium supplemented with 200 mM NaCl. Data are means ± relative sd of three independent biological experiments with at least 75 plants each.
(C) Model: ASKα-mediated phosphorylation of G6PD6 contributes to maintaining cellular redox balance under high salinity conditions.
DISCUSSION
A change in ROS levels is a key phenomenon in response to abiotic and biotic stresses (Mittler, 2002; Apel and Hirt, 2004). ROS can act as a physiological signal, but when overproduced causes oxidative damage; therefore, ROS formation and removal must be tightly regulated. Plants, like other organisms, have the potential to adapt to environmental stress, and successful protection against oxidative stress is a major determinant in coping with stressful conditions. In this study, we identified the Arabidopsis GSK3, ASKα, as a regulator of G6PD6 activity, glutathione redox status, and ROS levels and showed that ASKα is important for acclimation to salt stress.
ASKα is a novel component regulating the high salinity response. Plant GSK3s are encoded by a gene family, and different GSK3 proteins have diverse functions (Jonak and Hirt, 2002; Saidi et al., 2012). In addition to ASKα, other GSK3 family members have been implicated in the response of plants to salinity. Salt stress–induced transcriptional upregulation has been reported for some GSK3 family members in different plant species (Piao et al., 1999; Charrier et al., 2002; Chen et al., 2003; Koh et al., 2007). Overexpression of Arabidopsis ASKι and alfalfa MsK4 enhanced salt stress tolerance (Piao et al., 2001; Kempa et al., 2007), whereas knockout mutants of rice OsGSK1 showed increased tolerance to salt stress (Koh et al., 2007), suggesting that different GSK3s have distinct, either positive or negative, regulatory functions in salt stress tolerance. Furthermore, different subcellular localization of stress-related GSK3s indicates that distinct GSK3s might be involved in different aspects of the high-salinity response.
ASKα has previously been implicated in flower development (Dornelas et al., 2000); however, using askα knockout and ASKα-overexpressing lines, we did not observe the flower phenotype described in ASKα antisense plants, which might be due to the different approaches used to generate plants deficient in ASKα. Our results suggest that ASKα is activated by salt stress at the posttranslational level. The activity of mammalian GSK3 is modulated by various mechanisms, including phosphorylation and differential protein complex formation (Cohen and Frame, 2001; Woodgett, 2001; Jope and Johnson, 2004). We have shown that ASKs are phosphorylated in vivo and that Tyr-230 phosphorylation is necessary for ASKα activity (de la Fuente van Bentem et al., 2008). An important role of Tyr phosphorylation for ASK activity was confirmed in studies on BIN2/ASKη. BIN2 is phosphorylated at the corresponding Tyr (Tyr-200). Brassinosteroid-triggered signaling led to inhibition of active BIN2 via dephosphorylation by BSU1 phosphatase (Kim et al., 2009). Although Tyr phosphorylation is essential for ASKα activity (de la Fuente van Bentem et al., 2008), preliminary data indicate that salinity-induced activation of ASKα involves other mechanisms in addition to Tyr-230 phosphorylation. Future studies will aim to identify the molecular mechanism of ASKα activation during stress.
In mammals, GSK3 is a central regulatory component modulating a multitude of cellular targets (Cohen and Frame, 2001; Woodgett, 2001; Jope and Johnson, 2004). Similarly, the localization of ASKα to the cytoplasm and the nucleus implies that ASKα might act on different organelle-specific targets. G6PD6 appears to be one of the cytosolic in vivo targets of ASKα. Notably, ASKγ, the closest homolog of ASKα, was unable to enhance G6PD6 activity in cells (see Supplemental Figure 5 online), thus emphasizing the specificity of ASKα on G6PD6 in vivo regulation.
G6PD is subject to complex control, and regulation of G6PD transcript and protein levels has been reported (Salvemini et al., 1999; Nemoto and Sasakuma, 2000; Scharte et al., 2009). Additionally, redox regulation and product inhibition participate in modulating G6PD activity (Wendt et al., 2000; Debnam et al., 2004; Schürmann and Buchanan, 2008). It is interesting to note that G6PD6 was shown to be inactivated by oxidation (Wakao and Benning, 2005) and that total G6PD activity was reduced in salt-stressed askα mutants. Thus, in salt-stressed plants, ASKα might play a crucial role in counterbalancing oxidative inhibition of G6PD6 by phosphorylating and thereby enhancing G6PD6 activity.
Previous studies in plants suggested a negative regulation of G6PD by phosphorylation (Zhang et al., 2000; Hauschild and von Schaewen, 2003; Xu et al., 2005). However, we provide direct evidence that G6PD6 activity can be enhanced by phosphorylation. Consistent with our data, aestivation-induced phosphorylation of G6PD in the snail Olata lactea also positively correlated with G6PD activity (Ramnanan and Storey, 2006).
The ASKα phosphorylation site Thr-467 of G6PD6 is conserved in eukaryotes. Interestingly, structural analyses show that Thr-467 is in close proximity to the βE–αe loop that is part of the NADP binding region. Phosphorylation of Thr-467 might thus introduce defined structural rearrangements in the active site cleft required for proper cosubstrate binding. Consistently, mutations in the βE–αe loop were shown to affect the catalytical activity of G6PD in vitro and in vivo (Roos et al., 1999). Notably, changing Thr-467 of G6PD5 to Glu enhanced G6PD activity to a similar extent to that of G6PD6 (see Supplemental Figure 6B online), suggesting that Thr-467 phosphorylation might be a common mechanism regulating G6PD activity. Since ASKα does not phosphorylate G6PD5 on this residue (see Supplemental Figure 6A online), future studies are aimed at identifying the protein kinase targeting G6PD5.
In animals and yeast, the pentose phosphate pathway is the main source of NADPH for ROS removal (Pandolfi et al., 1995; Juhnke et al., 1996); however, in plants, the role of the OPPP during salt stress is unclear. Under normal growth conditions, photosynthesis supplies reducing equivalents; however, under stress conditions, when photosynthesis may be impaired, the OPPP might deliver reducing power for maintenance of the cellular redox balance. Several lines of evidence indicate that the OPPP is important during salt stress responses in plants. The activity of G6PD, a key enzyme of the OPPP, increased in Arabidopsis plants upon high salinity stress. Consistently, G6PD activity was reported to be induced by NaCl treatment in other plant species (Valderrama et al., 2006; Wang et al., 2008). Importantly, loss of G6PD6 impinged on the redox status of glutathione and rendered plants more sensitive to salt stress. These data, together with the observation that ectopic overexpression of a plastidic G6PD isoform in the cytosol enhanced drought tolerance of tobacco (Nicotiana tabacum; Scharte et al., 2009), not only establish an important (positive) role of the OPPP in stress defense in plants but also provide strong evidence for a function for the cytoplasmic OPPP in stress acclimation.
In summary, our data indicate a model in which ASKα is an important regulator of ROS detoxification and, thus, acclimation to salt stress (Figure 7C). High salinity activates ASKα, which in turn phosphorylates G6PD6 on Thr-467, thereby stimulating its activity. Enhanced G6PD activity provides NADPH for the antioxidant system to remove excess ROS. Reduction of H2O2 to water can then be mediated by the glutathione peroxidase cycle or by the ascorbate-glutathione cycle.
G6PD is a major determinant of cellular redox homeostasis, which plays a pivotal role in determining cellular responsiveness to stress. ROS generation and redox imbalance are closely linked to aging and a wide range of diseases, including inflammation, cancer, and neurodegenerative disorders. Our data not only provide novel mechanistic insights into the regulation of G6PD6 activity by phosphorylation, but also offer a starting point for future studies beyond metabolic adaptation of plants to adverse environments.
METHODS
Plant Growth and Stress Treatments
Arabidopsis thaliana ecotype Col-0 was germinated on half-strength Murashige and Skoog (MS) medium (Duchefa). After 10 d, seedlings were transferred to soil and cultivated in a 16-h-light/8-h-dark regime at 150 µmol⋅m−2⋅s−1 light intensity and 60% relative humidity. For high soil salinity stress, plants were watered with a 150 mM NaCl solution. For germination and root length assay under high salt conditions, seeds were allowed to germinate on half-strength MS or half-strength MS supplemented with 100 mM NaCl in vertical plates in a 16-h-light/8-h-dark regime. Seeds used for one experiment were propagated in the same growth chamber at the same time.
Plant Material and Plasmid Constructs
The askα mutant (SAIL_1055_F02) was genotyped using primers ASKα1 5′ and ASKα 9ex 3′ (see Supplemental Table 1 online). The g6pd6.2 mutant (Gabi_KAT_142G07) was genotyped using primers G6PD6 9ex and G6PD6 13ex (see Supplemental Table 1 online). The cDNA of ASKα-Myc and ASKαK98R-Myc were amplified by PCR using the primers ASKα cDNA forward and reverse (see Supplemental Table 1 online) and were cloned as BamI fragments into the expression vector pGreenII0029 under the control of the 35S promoter. G6PD6-HA and its mutated variants were cloned into pGWR8 (Rozhon et al., 2010). Arabidopsis Col-0 plants were transformed using the floral dipping method (Clough and Bent, 1998).
Gene Expression Analysis
Total RNA was extracted from plant material using the RNeasy plant mini kit (Qiagen) and treated with RNase-free DNase following the manufacturer’s protocol. RT-PCR experiments were performed with cDNAs generated from 2 µg of total RNA using oligo(dT) primers and M-MuLV reverse transcriptase (Q-BIOgene). The RT-PCR exponential phase was determined on 22 to 30 cycles. Oligonucleotide primers used for RT-PCR were designed based on the 3′ untranslated region of control and selected genes (see Supplemental Table 1 online).
For real-time PCR, first-strand cDNA synthesis from 1 μg of total RNA was done using qScript cDNA SuperMix (Quanta). Samples were then diluted 1:5 with water. Five microliters of the diluted samples was used for real-time PCR reactions using the SensiMix SYBR and Fluorescein kit (Peqlab) on an IQ5 multicolor real-time PCR detection system (Bio-Rad). All experiments were performed three times with independent RNA samples under the following cycling conditions: a 95°C hold for 10 min followed by 45 cycles at 95°C for 15 s, 58°C for 15 s, and 72°C for 15 s. Nonspecific PCR products were identified by dissociation curves. Relative expression values were calculated using the 2−ΔΔCT (for cycle threshold) method (Livak and Schmittgen, 2001). Primer efficiencies were calculated by relative standard curves. PP2A was used as a normalization control as described by Czechowski et al. (2005). Normalized gene expression was represented relative to a wild-type control. The oligonucleotide primers used were designed based on the 3′ untranslated region for G6PD6 and G6PD5, or in case of ASKα, spanning the junction between exon 11 and 12 (see Supplemental Table 1 online).
Total G6PD Activity Measurement
Fifty milligrams of plant material was extracted in 0.5 mL of enzyme extraction buffer (10% glycerol, 0.25% BSA, 0.1% Triton X-100, 50 mM HEPES-KOH, pH 7.5, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM benzamidine, 1 mM 6-aminocaproic acid, 1 mM phenylmethylsulfonyl fluoride, and 10 µM leupeptin). G6PD total activity was determined as described (Stanton et al., 1991) with some modifications. Five microliters of enzyme extract was used in a 100-μL total reaction volume. Absorbance was measured with a microplate reader spectrophotometer (GeniosPro; Tecan). In vivo G6PD activities from plants were calculated in nmol NADPH/(min*mg fresh weight) and from protoplast cells in nmol NADPH/(min*µg protein). A minimum of four technical replicas was performed per experiment. For determination of G6PD activities from recombinant proteins, several enzyme concentrations were tested per measurement and G6PD activities were calculated in nmol NADPH/(min*µg protein).
H2O2 Level Measurement
H2O2 levels were measured according to Wolff (1994). Briefly, 50 mg of freshly pulverized plant material was extracted with 0.5 mL of 25 mM H2SO4. Samples were centrifuged at 4°C, and 100 μL of clear supernatant was added to 900 μL of reagent solution [0.1 mM Xylenol Orange tetrasodium salt, 0.25 mM ammonium-iron(II)sulfate, 100 mM sorbitol, and 25 mM H2SO4]. Reactions were incubated for 1 h at room temperature in the dark. Absorbance was measured at 580 nm with a single beam spectrophotometer (Ultrospec 3100 pro; Amersham Biosciences). A calibration curve with dilutions from 0 to 10 nmol H2O2 in 100 μL of 25 mM H2SO4 was recorded and used for quantification. To avoid oxidation of plant material, no more than 12 samples were measured at a time.
Glutathione Measurement
Oxidized and reduced forms of glutathione were determined with a spectrophotometric plate reader assay as described by Queval and Noctor (2007). Assays were performed on a BioTek’s Synergy 4 Multi-Mode Microplate Reader using Corning 96-well UV-transparent plates. Standards and sample extracts where assayed as triplicates.
Arabidopsis Protoplast Transformation
Protoplasts from an Arabidopsis cell suspension culture were transformed as described (Cardinale et al., 2002).
In Vivo Labeling Assay
HA-tagged G6PD6 was transiently expressed in Arabidopsis protoplasts. One hour after transformation, [32P] orthophosphoric acid (0.1 mCi/mL) (carrier free; Hartmann Analytic) was added and protoplasts were incubated overnight in the dark. Immunoprecipitation was performed using HA antibodies (Santa-Cruz).
Expression and Purification of Recombinant GST-Fusion Proteins
ASKα cDNA was cloned as a BamHI fragment into pGEX5x3, and cDNAs from G6PD5 and G6PD6 were cloned as EcoRI fragments into pGEX4T1. Recombinant proteins were expressed as GST fusion proteins in the Escherichia coli BL21codon plus strain. Proteins were purified using the Sepharose beads affinity method (Glutathione Sepharose 4B; GE Healthcare).
In Vitro Mutagenesis
PCR was performed using 2.5 U of Pfu Ultra (Stratagene) with the primers listed in Supplemental Table 1 online. PCR mixtures were digested with the enzyme DpnI for 2 h at 37°C and transformed into E. coli. In vitro mutagenesis was verified by sequencing.
Antibody Production and Specificity Tests
A rabbit polyclonal ASKα-specific antibody was raised against the synthetic peptide (PNPGARDSTGVDKL) from the N terminus of ASKα. Crude serum antibody was used for immunoprecipitation of the kinase. An antibody specificity test for immunoprecipitation was performed using ASKα, and its two closest homologs, ASKγ and ASKε in vitro translated with the T3/T7 coupled translation kit using the T7 primer (Amersham Biosciences). Kinases were immunoprecipitated with the ASKα antibody as described for immunokinase assays.
For the peptide-competition assay, 10 µg of ASKα antibody was incubated with 5 µg of synthetic ASKα peptide at 4°C overnight. After immunoprecipitation of ASKα with ASKα-specific antibodies from 20 µg total protein extract, immunoblot analysis was performed with ASKα-specific antibodies with and without prior peptide incubation.
Immunokinase and in Vitro Kinase Assay
Immunokinase assays were performed as described (Jonak et al., 2000). G6PD or MBP was used as the specific or general substrate, respectively (Jonak et al., 2000; De Rybel et al., 2009). In vitro kinase assays using recombinant proteins were performed in a total volume of 20 μL of kinase buffer (20 mM HEPES, pH 7.5, 15 mM MgCl2, and 5 mM EGTA). The reaction was started with 2 µCi [γ-32P]ATP and incubated at room temperature for 30 min. The reaction was stopped by the addition of 5 μL of 4× SDS loading buffer. Proteins were resolved by 8% SDS-PAGE. The gel was dried and exposed overnight to a phosphor imager screen.
Mass Spectrometry and Phosphopeptide Analysis
Following the in vitro kinase assay between ASKα and G6PD6, proteins were separated by SDS-PAGE. Polyacrylamide gels were stained with a colloidal Coomassie Brilliant Blue (CBB) staining solution (Roti-Blue; Carl Roth). Excised bands were processed and putative phosphopeptides were identified by liquid chromatography–tandem mass spectrometry analysis as previously described (Penkner et al., 2009). Raw spectra were interpreted by Discoverer 1.3 (Thermo Scientific). Peptide tolerance was set to 2 D, and fragment ion tolerance was set to 0.8 D. Carbamidomethylcysteine was set as static, and oxidation of Met residues, phosphorylation of Ser, Thr, and Tyr residues as variable modifications. Phospho RS 2.0 was used to evaluate phosphorylation sites, and the spectra of possibly phosphorylated peptides were evaluated manually. An in-house generated FASTA database was used for the search containing the sequences of the target proteins, common contaminants, and proteolytic enzymes. Peptides were filtered according to the XCorr/Charge state values; false positive rate was set to 5%.
Protein Extraction and Immunoblot Analysis
Total proteins from Arabidopsis leaves and protoplasts were extracted and blotted on polyvinylidene fluoride membranes as described (Wrzaczek et al., 2007). Protein concentration was assessed using the Bradford method. Membranes were probed with a 1:5000 dilution of anti-ASKα, anti-Myc (Santa-Cruz), or anti-HA (Santa-Cruz) antibodies. To detect total G6PD protein levels, a 1:6000 dilution of polyclonal antibody against Saccharomyces cerevisiae G6PD (Sigma-Aldrich) was used as described for plants (Valderrama et al., 2006). Alkaline phosphatase–conjugated goat anti-rabbit IgG (Santa-Cruz) was used as the secondary antibody. The reaction was detected using the CDP-Star detection reagent (GE Healthcare).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under the following accession numbers: ASKα, At5g26751; G6PD6, AT5G40760; G6PD5, AT3G27300; β-tubulin, At5g12250; GAPDH, AT1G13440; and PP2A, AT1G13320.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. askα T-DNA Insertion Mutant and Specificity of the Anti-ASKα Antibody.
Supplemental Figure 2. Mass Spectrometry Analysis of G6PD6 Phosphorylation by ASKα.
Supplemental Figure 3. G6PD6 Thr-467 Is Evolutionary Conserved.
Supplemental Figure 4. ASKα and G6PD6 Function in the Same Signaling Pathway.
Supplemental Figure 5. ASKγ Does Not Enhance G6PD6 Activity in Vivo.
Supplemental Figure 6. Characterization of Thr-467 of G6PD5.
Supplemental Table 1. List of Primers.
Supplementary Material
Acknowledgments
We thank Anneliese Auer, Andrey Belokurow, and Bettina Dekrout for technical assistance, Edina Csaszar for mass spectrometry, and Manuela Baccarini for critical comments on the article. This work was supported by grants from the Austrian Science Foundation (P20375-B03) and the Vienna Science and Technology Fund (LS 123).
AUTHOR CONTRIBUTIONS
S.D.S. and H.S. analyzed the stress tolerance and performed the protein kinase and enzyme activity assays. S.D.S. identified the phosphorylation site. H.S. and J.K. analyzed the cellular redox status. S.K. and Y.G. performed the robot-based screen for enzyme activities. E.P. and W.R. generated transgenic plants. A.H. and T.C. performed the structural analyses. C.J. conceived, designed, and evaluated the experiments and wrote the article.
Glossary
- ROS
reactive oxygen species
- G6PD
Glc-6-phosphate dehydrogenase
- OPPP
oxidative pentose phosphate pathway
- GSK
GSK3/shaggy-like kinase
- MBP
myelin basic protein
- H2O2
hydrogen peroxide
- Col-0
Columbia-0
- MS
Murashige and Skoog
- HA
to be defined
References
- Apel K., Hirt H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Au S.W., Gover S., Lam V.M., Adams M.J. (2000). Human glucose-6-phosphate dehydrogenase: The crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure 8: 293–303 [DOI] [PubMed] [Google Scholar]
- Boyer J.S. (1982). Plant productivity and environment. Science 218: 443–448 [DOI] [PubMed] [Google Scholar]
- Cardinale F., Meskiene I., Ouaked F., Hirt H. (2002). Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases. Plant Cell 14: 703–711 [PMC free article] [PubMed] [Google Scholar]
- Charrier B., Champion A., Henry Y., Kreis M. (2002). Expression profiling of the whole Arabidopsis shaggy-like kinase multigene family by real-time reverse transcriptase-polymerase chain reaction. Plant Physiol. 130: 577–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.P., Ma W.S., Huang Z.J., Xu T., Xue Y.B., Shen Y.Z. (2003). Isolation and characterization of TaGSK1 involved in wheat salt tolerance. Plant Sci. 165: 1369–1375 [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cohen P., Frame S. (2001). The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2: 769–776 [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M.K., Scheible W.R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam P.M., Emes M.J. (1999). Subcellular distribution of enzymes of the oxidative pentose phosphate pathway in root and leaf tissues. J. Exp. Bot. 50: 1653–1661 [Google Scholar]
- Debnam P.M., Fernie A.R., Leisse A., Golding A., Bowsher C.G., Grimshaw C., Knight J.S., Emes M.J. (2004). Altered activity of the P2 isoform of plastidic glucose 6-phosphate dehydrogenase in tobacco (Nicotiana tabacum cv. Samsun) causes changes in carbohydrate metabolism and response to oxidative stress in leaves. Plant J. 38: 49–59 [DOI] [PubMed] [Google Scholar]
- de la Fuente van Bentem S., et al. (2008). Site-specific phosphorylation profiling of Arabidopsis proteins by mass spectrometry and peptide chip analysis. J. Proteome Res. 7: 2458–2470 [DOI] [PubMed] [Google Scholar]
- DeLano W.L. (2002). The PyMOL Molecular Graphics System. (Palo Alto, CA: DeLano Scientific)
- De Rybel B., et al. (2009). Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chem. Biol. 16: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni C.A., Storey K.B. (2010). Regulation of glucose-6-phosphate dehydrogenase by reversible phosphorylation in liver of a freeze tolerant frog. J. Comp. Physiol. B 180: 1133–1142 [DOI] [PubMed] [Google Scholar]
- Doble B.W., Woodgett J.R. (2003). GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116: 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornelas M.C., Van Lammeren A.A., Kreis M. (2000). Arabidopsis thaliana SHAGGY-related protein kinases (AtSK11 and 12) function in perianth and gynoecium development. Plant J. 21: 419–429 [DOI] [PubMed] [Google Scholar]
- Filosa S., Fico A., Paglialunga F., Balestrieri M., Crooke A., Verde P., Abrescia P., Bautista J.M., Martini G. (2003). Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem. J. 370: 935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y., Blaesing O.E., Hannemann J., Carillo P., Höhne M., Hendriks J.H., Palacios N., Cross J., Selbig J., Stitt M. (2004). A Robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R.S., Ata H., Rawat D., Abe M., Taylor M.S., Ochi R., Gupte S.A. (2011). Glucose-6-phosphate dehydrogenase is a regulator of vascular smooth muscle contraction. Antioxid. Redox Signal. 14: 543–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R.S., Floyd B.C., Kozicky M., George S., Ungvari Z.I., Neito V., Wolin M.S., Gupte S.A. (2009). Synergistic activation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase by Src kinase elevates superoxide in type 2 diabetic, Zucker fa/fa, rat liver. Free Radic. Biol. Med. 47: 219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa P.M., Bressan R.A., Zhu J.K., Bohnert H.J. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51: 463–499 [DOI] [PubMed] [Google Scholar]
- Hauschild R., von Schaewen A. (2003). Differential regulation of glucose-6-phosphate dehydrogenase isoenzyme activities in potato. Plant Physiol. 133: 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Maeda K., Miki T., Mano J., Inoue Y., Kimura A. (1998). Importance of glucose-6-phosphate dehydrogenase in the adaptive response to hydrogen peroxide in Saccharomyces cerevisiae. Biochem. J. 330: 811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C., Beisteiner D., Beyerly J., Hirt H. (2000). Wound-induced expression and activation of WIG, a novel glycogen synthase kinase 3. Plant Cell 12: 1467–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C., Hirt H. (2002). Glycogen synthase kinase 3/SHAGGY-like kinases in plants: An emerging family with novel functions. Trends Plant Sci. 7: 457–461 [DOI] [PubMed] [Google Scholar]
- Jope R.S., Johnson G.V. (2004). The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29: 95–102 [DOI] [PubMed] [Google Scholar]
- Juhnke H., Krems B., Kötter P., Entian K.D. (1996). Mutants that show increased sensitivity to hydrogen peroxide reveal an important role for the pentose phosphate pathway in protection of yeast against oxidative stress. Mol. Gen. Genet. 252: 456–464 [DOI] [PubMed] [Google Scholar]
- Kempa S., Rozhon W., Samaj J., Erban A., Baluska F., Becker T., Haselmayer J., Schleiff E., Kopka J., Hirt H., Jonak C. (2007). A plastid-localized glycogen synthase kinase 3 modulates stress tolerance and carbohydrate metabolism. Plant J. 49: 1076–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.W., Guan S., Sun Y., Deng Z., Tang W., Shang J.X., Sun Y., Burlingame A.L., Wang Z.Y. (2009). Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 11: 1254–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S., Lee S.-C., Kim M.-K., Koh J.H., Lee S., An G., Choe S., Kim S.-R. (2007). T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 65: 453–466 [DOI] [PubMed] [Google Scholar]
- Kotaka M., Gover S., Vandeputte-Rutten L., Au S.W., Lam V.M., Adams M.J. (2005). Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr. D Biol. Crystallogr. 61: 495–504 [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Martin M., Rehani K., Jope R.S., Michalek S.M. (2005). Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6: 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. (2011). ROS signaling: The new wave? Trends Plant Sci. 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Møller I.M., Jensen P.E., Hansson A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58: 459–481 [DOI] [PubMed] [Google Scholar]
- Munns R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25: 239–250 [DOI] [PubMed] [Google Scholar]
- Nemoto Y., Sasakuma T. (2000). Specific expression of glucose-6-phosphate dehydrogenase (G6PDH) gene by salt stress in wheat (Triticum aestivum L.). Plant Sci. 158: 53–60 [DOI] [PubMed] [Google Scholar]
- Pandolfi P.P., Sonati F., Rivi R., Mason P., Grosveld F., Luzzatto L. (1995). Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 14: 5209–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkner A.M., Fridkin A., Gloggnitzer J., Baudrimont A., Machacek T., Woglar A., Csaszar E., Pasierbek P., Ammerer G., Gruenbaum Y., Jantsch V. (2009). Meiotic chromosome homology search involves modifications of the nuclear envelope protein Matefin/SUN-1. Cell 139: 920–933 [DOI] [PubMed] [Google Scholar]
- Piao H.L., Lim J.H., Kim S.J., Cheong G.W., Hwang I. (2001). Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J. 27: 305–314 [DOI] [PubMed] [Google Scholar]
- Piao H.L., Pih K.T., Lim J.H., Kang S.G., Jin J.B., Kim S.H., Hwang I. (1999). An Arabidopsis GSK3/shaggy-like gene that complements yeast salt stress-sensitive mutants is induced by NaCl and abscisic acid. Plant Physiol. 119: 1527–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G., Noctor G. (2007). A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 363: 58–69 [DOI] [PubMed] [Google Scholar]
- Ramnanan C.J., Storey K.B. (2006). Glucose-6-phosphate dehydrogenase regulation during hypometabolism. Biochem. Biophys. Res. Commun. 339: 7–16 [DOI] [PubMed] [Google Scholar]
- Roos D., et al. (1999). Molecular basis and enzymatic properties of glucose 6-phosphate dehydrogenase volendam, leading to chronic nonspherocytic anemia, granulocyte dysfunction, and increased susceptibility to infections. Blood 94: 2955–2962 [PubMed] [Google Scholar]
- Rozhon W., Mayerhofer J., Petutschnig E., Fujioka S., Jonak C. (2010). ASKtheta, a group-III Arabidopsis GSK3, functions in the brassinosteroid signalling pathway. Plant J. 62: 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi Y., Hearn T.J., Coates J.C. (2012). Function and evolution of ‘green’ GSK3/Shaggy-like kinases. Trends Plant Sci. 17: 39–46 [DOI] [PubMed] [Google Scholar]
- Salvemini F., Franzé A., Iervolino A., Filosa S., Salzano S., Ursini M.V. (1999). Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J. Biol. Chem. 274: 2750–2757 [DOI] [PubMed] [Google Scholar]
- Scharte J., Schön H., Tjaden Z., Weis E., von Schaewen A. (2009). Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants. Proc. Natl. Acad. Sci. USA 106: 8061–8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B.B. (2008). The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid. Redox Signal. 10: 1235–1274 [DOI] [PubMed] [Google Scholar]
- Sindelár L., Sindelárová M. (2002). Correlation of viral RNA biosynthesis with glucose-6-phosphate dehydrogenase activity and host resistance. Planta 215: 862–869 [DOI] [PubMed] [Google Scholar]
- Slaski J.J., Zhang G., Basu U., Stephens J.L., Taylor G.J. (2006). Aluminum resistance in wheat (Triticum aestivum) is associated with rapid, Al-induced changes in activities of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in root apices. Physiol. Plant. 98: 477–487 [Google Scholar]
- Stanton R.C., Seifter J.L., Boxer D.C., Zimmerman E., Cantley L.C. (1991). Rapid release of bound glucose-6-phosphate dehydrogenase by growth factors. Correlation with increased enzymatic activity. J. Biol. Chem. 266: 12442–12448 [PubMed] [Google Scholar]
- Valderrama R., Corpas F.J., Carreras A., Gómez-Rodríguez M.V., Chaki M., Pedrajas J.R., Fernández-Ocaña A., Del Río L.A., Barroso J.B. (2006). The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ. 29: 1449–1459 [DOI] [PubMed] [Google Scholar]
- Wakao S., Andre C., Benning C. (2008). Functional analyses of cytosolic glucose-6-phosphate dehydrogenases and their contribution to seed oil accumulation in Arabidopsis. Plant Physiol. 146: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao S., Benning C. (2005). Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. 41: 243–256 [DOI] [PubMed] [Google Scholar]
- Wang X., Ma Y., Huang C., Wan Q., Li N., Bi Y. (2008). Glucose-6-phosphate dehydrogenase plays a central role in modulating reduced glutathione levels in reed callus under salt stress. Planta 227: 611–623 [DOI] [PubMed] [Google Scholar]
- Wendt U.K., Wenderoth I., Tegeler A., Von Schaewen A. (2000). Molecular characterization of a novel glucose-6-phosphate dehydrogenase from potato (Solanum tuberosum L.). Plant J. 23: 723–733 [DOI] [PubMed] [Google Scholar]
- Wolff S.P. (1994). Ferrous ion oxidation in presence of ferric ion indicator xenol orange for measurment of hydroperoxides. Methods Enzymol. 233: 182–189 [Google Scholar]
- Woodgett J.R. (2001). Judging a protein by more than its name: GSK-3. Sci. STKE 2001: re12. [DOI] [PubMed] [Google Scholar]
- Wrzaczek M., Rozhon W., Jonak C. (2007). A proteasome-regulated glycogen synthase kinase-3 modulates disease response in plants. J. Biol. Chem. 282: 5249–5255 [DOI] [PubMed] [Google Scholar]
- Xu Y., Osborne B.W., Stanton R.C. (2005). Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am. J. Physiol. Renal Physiol. 289: F1040–F1047 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Apse K., Pang J., Stanton R.C. (2000). High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J. Biol. Chem. 275: 40042–40047 [DOI] [PubMed] [Google Scholar]
- Zhu J.K. (2001). Plant salt tolerance. Trends Plant Sci. 6: 66–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.