Abstract
Here we investigated the effect of Beauveria bassiana infection on the survival of A. aegypti mosquitoes and the modulation of their susceptibility to dengue virus infection. Application of B. bassiana caused a reduction in the life span of A. aegypti and hindered dengue virus replication in the mosquito midgut. Fungus infection induced the expression a variety anti-microbial and dengue virus restriction factor genes. Transient reverse genetic analyses showed that the JAK-STAT pathway is implicated anti-fungal defense of Aedes mosquitoes. Our data suggest that this B. bassiana-mediated anti-dengue activity is likely to be at least partly indirectly mediated through the activation of the mosquito's anti-dengue Toll and JAK-STAT pathways.
Keywords: Aedes aegypti, dengue virus, Beauveria bassiana, innate immunity
1. Introduction
Dengue fever has become the most important arboviral disease worldwide, with a dramatic increase in its incidence and negative impact on public health in recent decades (Gibbons and Vaughn, 2002; WHO, 2011). The World Health Organization has estimated that about half of the world's population is at daily risk of contracting dengue and that some 50 million cases occur every year worldwide (Mackenzie et al., 2004; WHO, 2011).
Dengue is a positive single-stranded RNA virus that is primarily transmitted through the A. aegypti blood feeding, but A. albopictus also plays a relevant role in dengue transmission, especially in areas where A. aegypti is not present (Teyssou, 2009). After the mosquito has ingested an infected blood, the dengue virus invades the mosquito's midgut epithelium, where it undergoes replication and is then disseminated to other tissues such as the trachea, fat body, and finally the salivary glands. This whole process usually takes approximately 7-10 days, although in a minority of cases dengue virus can be detected in the mosquito's salivary glands as early as 4 days post-infection. Once in the salivary glands, the virus can be transmitted during probing and blood feeding for the rest of the mosquito's adult life (Salazar et al., 2007).
The lack of an effective vaccine and the mosquitoe's resistance to insecticides has accentuated the need for the development of novel control strategies that can prevent dengue virus transmission by the mosquito vectors (Gubler, 2005; Guzman, 2005; Kay and Vu, 2005; Mackenzie et al., 2004; Ritchie et al., 2004; WHO, 2011). The entomopathogenic fungi, such as Metarhizium anisopliae and Beauveria bassiana, have gained interest as candidate bio-control agents of mosquitoes vectors mainly because of their ability to shorten the mosquitoes life span or reduce instantaneous blood feeding of mosquitoes (Blanford et al., 2005; Howard et al., 2010b; Knols et al., 2010; Knols and Thomas, 2006; Michalakis and Renaud, 2005; Scholte et al., 2004; Scholte et al., 2006; Scholte et al., 2005; Scholte et al., 2003; Thomas and Read, 2007), and more importantly that pyrethroid resistance A. gambiae mosquitoes have increased susceptibility to these entomopathogenic fungi (Howard et al., 2010a). B. bassiana is a soil-borne fungus that infects soil-dwelling insects, including both Anopheles and Aedes mosquitoes (Boucias DG, 1998). While B. bassiana is not as virulent to Aedes larvae as to anopheline larvae, the dried conidia are highly virulent to A. aegypti adult mosquitoes, which kill mosquitoes in only 5 days which suggests that application of conidia to adult Aedes mosquitoes is more effective (Clark et al., 1968;). Laboratory bioassays of two development stages of B. bassiana have shown that blastospores (BS) have significant higher larvicidial potency than conidiospores (CS) (Miranpuri and Khachatourians, 1990). A recent publication has shown that exposure of A. aegypti to a filter paper impregnated with conidia is highly effective in infecting and killing mosquitoes, and the fungus can be transmitted between mosquitoes through mating (Garcia-Munguia et al., 2011).
Similar to other insects, mosquitoes use their innate immune system to fight pathogenic organisms and the three immune signaling cascades, Toll, Immune Deficiency (IMD), and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathways play key roles in these defenses (Cherry and Silverman, 2006; Dimopoulos, 2003; Dostert et al., 2005; Garver et al., 2009; Hoffmann and Reichhart, 2002; Pinheiro and Ellar, 2006; Souza-Neto et al., 2009; Xi et al., 2008). The Toll pathway is more specific for Gram-positive bacteria, fungi, and viruses, whereas the Imd pathway is mainly active against Gram-negative bacteria and Plasmodium falciparum (Cirimotich et al., 2010; Shin et al., 2006; Shin et al., 2005). The lesser studied JAK-STAT pathway has been implicated in anti-viral defenses (Souza-Neto et al., 2009). The Toll pathway has been shown to be activated in response to fungal infection, resulting in the transcriptional activation of several anti-microbial peptides and other immune genes (i.e. Cecropin A, Defensin A, Serpin 27A) (Shin et al., 2005). We have previously shown that the Toll pathway is also activated in response to dengue virus infection in A. aegypti and that such activation, in turn, controls viral proliferation in the mosquito midgut (Xi et al., 2008). Another key player in the A. aegypti anti-dengue defense is the JAK-STAT pathway, presumably through one or several STAT-regulated dengue virus restriction factors (DVRFs) (Souza-Neto et al., 2009). Here we show that B. bassiana infection shortens the mosquito's life span and induces the Toll and JAK-STAT pathways which are known to exert anti-dengue defense. We establish the optimal dosage and route of infection of B. bassiana spores for reducing the life span of Aedes mosquitoes and the vector competence for dengue virus.
2. Material and methods
2.1. Mosquito rearing
Aedes aegypti mosquitoes of the Rockefeller strain were maintained in an insectary at 27°C and 80% humidity on a 12-h light/dark cycle according to standard rearing procedures. Larval stages were fed on pulverized fish food, and adults were provided with a 10% sucrose solution.
2.2. Culture of B. bassiana
Beauveria bassiana strain 80.2 (kindly provided by Dr. Neal Silverman, University of Massachusetts Medical School) was used for all assays (Lemaitre et al., 1997). Prior to use, a glycerol stock of conidia stored at −80°C was inoculated on malt-agar medium and allowed to grow at room temperature for 2 weeks. Spores and hyphae were harvested according to standard procedures (Broekaert et al., 1990) by using a sterile spatula and resuspended in PBS containing 0.05% Tween 20. The concentration of conidia was measured using a hemocytometer and adjusted to the following concentrations at 2.3×108, 108, 107, 105, and 103 conidia/ml.
2.3. Infection of mosquitoes with B. bassiana and survival assays
Different concentrations of conidia were applied to A. aegypti mosquitoes either through filter papers or by dipping mosquitoes in the conidia suspensions. For each trial of a bioassay with filter paper, 4 ml of diluted conidia was pipetted evenly onto a piece of filter paper (173 cm2) attached to the side of an autoclaved 8-oz cardboard cup. This fungus-containing cup was allowed to dry at room temperature for 10 min while the filter paper is still damp, after which the mosquitoes were placed inside the cups. For the dipping method, mosquitoes were cold-anesthetized and briefly dipped into the appropriate conidia suspension for 1 min, and then transferred to 8-oz cardboard paper cups. For both methods, at least three biological replicates (of 40 2-day-old females each) were used; control mosquitoes were treated with a PBS/0.05% Tween 20 solution (referred as PBS thereafter). The survival of B. bassiana-infected and control mosquitoes was monitored daily for up to 30 days. After this period, the LT50 for each group was determined (Clements and Paterson, 1981). The mosquito survival rate was analyzed by Kaplan–Meier pairwise comparison (Everitt, 1994).
2.4. Propagation of DENV-2 and infection assays
The A. albopictus cell line C6/36 was grown in minimal essential medium (MEM) with 10% heat-inactivated FBS, 1% L-glutamine, and 1% non-essential amino acids in a Thermo tissue culture incubator at 32°C supplied with 5% CO2 The propagation of the New Guinea C strain of DENV-2 in C6/36 cells essentially followed the methods described in (Troyer et al., 2001; Xi et al., 2008). In brief, 80% confluent C6/36 cells were infected with the virus in a 75-cm2 flask, at a multiplicity of infection (MOI) of 3.5 virus particles per cell. Infected cells were incubated for 5 to 7 days, harvested with a cell scraper, and lysed by repeated freezing and thawing in dry CO2 and a 32°C water bath to release the viral particles. The virus suspension was then mixed with an equal amount of commercial human blood and 10% human serum and maintained at 37°C for 30 min prior to feeding. Two days before the blood feeding, 2-day-old female mosquitoes were treated with fungus at 2.3×108 conidia/ml as described above (both filter paper and dipping methods). All infections with DENV-2 and manipulation of DENV-2-infected mosquitoes were carried out in a Biological Safety Level-3 insectary.
2.5. DENV-2 virus titration
At 7 days after blood feeding, mosquitoes were briefly washed in 70% ethanol and then rinsed in sterile PBS five times. The midgut was dissected in sterile PBS, and a pool of five midguts was homogenized in 150 ml of MEM using a Kontes pellet pestle motor in a sterile environment, and five data points were collected for each replicate. Virus titers in the tissue homogenates were measured as previously described (http://www.jove.com/index/Details.stp?ID=220). The virus-containing homogenates were serially diluted, inoculated into 80% confluent C6/36 cells in 24-well plates and kept for 5-7 days at 32°C and 5% CO2. Plaque-forming units (PFU) were visualized by peroxidase immunostaining, using mouse hyperimmune ascitic fluid (MHIAF) specific for DENV-2 and a goat anti-mouse HRP conjugate as the primary and secondary antibodies, respectively. All procedures involving DENV-2 infections were carried out in a Biological Safety Level 2 laboratory.
2.6. RNA sample preparation, semi quantitative RT-PCR and real-time RT-PCR (qRT-PCR)
At various time points (2, 5, 7, 9 days) after exposure to B. bassiana spores (via the filter paper and dipping method) at 108/ml or PBS (control), six mosquitoes from each replicate were collected and homogenized in lysis buffer. RNA was extracted using the RNeasy kit (Qiagen). Reverse transcription was carried out at 42°C for 2 h using a Superscript II kit (Invitrogen) and 20-μL reaction mixtures containing oligo (dT) primers and 2 μg of total RNA. The PCR cycles were controlled to produce a non-saturated amount of PCR products, indicating linear amplification. For the semi-quantitative RT-PCR optimal cycle numbers were empirically determined from a test PCR run, and three replicates were obtained for each gene. The relative -fold induction or repression of gene expression in the experimental samples was determined by comparing these values to their respective controls after normalizing the transcript levels with the A. aegypti ribosomal S7 gene (Dong et al., 2006). The qRT-PCR assays were performed according to standard protocol (Dong et al., 2006) by using Sybr Green PCR Master mix (Applied Biosystems) and ABI StepOne real-time PCR system. The primers used for the RT-PCR were: attacin (AAEL003389) ATT-F: 5′-TTGGCAGGCACGGAATGTCTTG-3′, ATT-R: 5′-TGTTGTCGGGACCGGGAAGTG-3′; cecropin G (AAEL015515) CECG-F: 5′-TCACAAAGTTATTTCTCCTGATCG-3′, CECG-R: 5′-GCTTTAGCCCCAGCTACAAC-3′; defensin A (AAEL003841) DEFA-F: 5′-CTGCCGGAGGAAACCTATCAG-3′, DEFA-R: 5′- GCAATGCAATGAGCAGCACAAG-3′; defensin C (AAEL003832), DEFC-F: 5′-TTGTTTGCTTCGTTGCTCTTT-3′, DEFC-R: 5′-ATCTCCTACACCGAACCCACT 3′; diptericin (AAEL004833)DIP-F: 5′-ATCCGATTCAGAATTCGCTTT-3′, DIP-R: 5′-TTTACCGTCTCCCTGAAATCC-3′; ribosomal protein S7 (AAEL009496) S7-F: 5′-GGGACAAATCGGCCAGGCTATC-3′, S7-R: 5′-TCGTGGACGCTTCTGCTTGTTG-3′. Primers for DOME, PIAS, DVRF1, and DVRF2 qRT-PCR were from (Souza-Neto et al., 2009). All PCR reactions were performed in triplicate; to check for the specificity of the PCR reations, melting curves were analyzed for each data point. The fold-change of the gene expression levels were determined by normalizing cDNAs using the AeS7 ribosomal gene and comparing to controls without B. bassiana challenge. P-values were calculated using either a one-way ANOVA as described in (Lajoie et al., 2010) to determine the significance of the gene expression among different time points, or t-test to determine the significance of a single time point as described in (Garver et al., 2009).
2.7 RNAi-mediated gene silencing and survival assays
RNAi assays were carried out according to previously established methodology with modifications (Blandin et al., 2002; Dong et al., 2006). Briefly, sense and antisense RNAs were synthesized from ∼300 bp PCR-amplified fragments using the HiScribe T7 In Vitro Transcription kit (New England Biolabs) by using the same primers as listed in (Souza-Neto et al., 2009). About 69 nl dsRNA in DEPC-water was introduced into the thorax of cold-anesthetized 3-5 days old female mosquitoes nano-microinjector (Drummond) with glass capillary needles. Both dsGFP- (control) and gene specific dsRNA-injected (experimental) groups were kept in the regular insectary conditions as described above. The dsRNA injection assay of different genes was repeated at least three times with at least 35 mosquitoes in each experiment, and the GFP dsRNA-injected mosquitoes served as controls. Verification of gene silencing was done through qRT-PCR by using the same verification primers as listed in (Souza-Neto et al., 2009).
Two days post dsRNA injection, the conidia suspension (1010 conidia/ml) was applied to A. aegypti mosquitoes through a filter paper method or by rolling the mosquitoes in the petri dish with fully grown fungi as described earlier (Lemaitre et al., 1997). The mortality of the mosquitoes was recorded up to 10 days, and at least 3 biological replicates were included in each assay. The significance of gene silencing on the mortality of mosquitoes to B. bassiana infection, compared with dsGFP-treated controls, was determined by Kaplan-Meier survival analysis by GraphPad Prism 5.0 and p-values were calculated through Wilcoxon test (Dong et al., 2006).
3. Results and discussion
3.1. Survival of A. aegypti mosquitoes after fungal infection
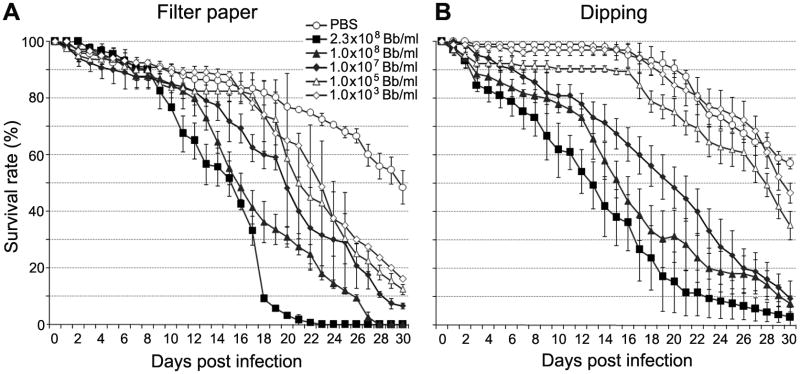
Previous studies have shown that the application of the entomopathogenic fungus M. anisopliae to female A. aegypti mosquitoes at 1010 conidia/m2 results in the killing of half the treated mosquitoes by 4 days after fungal infection (Scholte et al., 2007). The main objective of this study was to investigate the use of a fungus-infection as a way to induce mosquito resistance to the dengue virus in addition to shortening its life span. To avoid extensive mosquito mortality in our co-infection assays with B. bassiana and the dengue virus, we first performed experiments to determine the optimal dosage of the fungus by treating the mosquitoes with five different concentrations of B. bassiana. Mosquitoes were exposed to the fungus via two routes: (i) continuous exposure to immobilized fungus on a filter paper and (ii) one-time exposure by a single dip into a conidial fungus solution. After challenge, we monitored daily mosquito survival for up to 30 days (Fig. 1). For both filter paper and dipping methods, all the B. bassiana-treated mosquitoes died significantly earlier than the PBS-treated control mosquitoes (see statistical analysis in Table S1), except of those exposed to 103 conidia/ml B. bassiana through the dipping method, and the half-survival rate (LT50) was significantly lower for the B. bassiana-treated mosquitoes compared to the controls (Table S1), regardless of fungal dosage. However, the mosquitoes exposed to fungal spores through the dipping method died at a slower rate compared to those exposed through the filter paper method when treated with a lower dosage of the B. bassiana conidia solution (from 103 to 105 conidia/ml) (Figs. 1A and 1B, Table S1), suggesting that a one-time exposure of fungi spores requires longer time for developing pathogenicity to mosquitoes. However, when mosquitoes were treated at higher concentrations of B. bassiana spores (from 107 to 2.3 × 108 conidia/ml), the mortality after fungus exposure through the two different methods were similar but the mortality of mosquitoes through dipping methods had a large variation between different biological replicates (Fig. 1B). This is most likely because the one-time treatment does not result in a homogenous infection with the fungal spores. In order to confirm that the mosquitoes were dying from fungal infection, dead mosquitoes were transferred to a Petri dish containing a distilled water-moisturized piece of filter paper, which revealed the growth of vigorous B. bassiana hyphae at 24 h and sporulation at 3 days after exposure (Fig S1).
Fig. 1.
Survival curves of 2-day-old female mosquitoes treated with different concentrations of B. bassiana conidia through the filter paper method (A) or dipping method (B). Three independent experiments were performed and all cohorts were monitored daily for survival until day 30th. The survival percentage that represents the mean survival percentage for all three biological replicates of 40 mosquitoes each, along with the standard errors of means shown. Statistical significance was determined by Kaplan-Meier survival analysis with GraphPad Prism5 software, and p-values were determined by Wilcoxon test (the detailed information is listed in Table 1).
Our data show that regardless of the application method of fungal spores, infection with B. bassiana results in a significant increase in mosquito mortality, suggesting that this fungus could be a suitable candidate for use as a bio-control agent for dengue vectors. Our results are similar to those of previous laboratory and field studies that used B. bassiana and M. anisopliae to shorten the lifespan of the African malaria vector A. gambiae (Blanford et al., 2005; Scholte et al., 2005). In sum, we show that a prolonged exposure to immobilized fungus on a filter paper is more effective than a one-time exposure method in infecting and killing the Aedes mosquitoe.
3.2. B. bassiana infection renders mosquitoes more resistant to dengue virus infection
Blanford and colleagues (Blanford et al., 2005) have shown that treatment of anopheline mosquitoes with B. bassiana not only reduces the lifespan of Plasmodium-infected mosquitoes but also dramatically decreases the prevalence of mosquitoes carrying sporozoites in the salivary glands, suggesting that the fungal infection is directly or indirectly (possibly through the activation of the mosquito's immune system) inhibit Plasmodium development in the mosquito. In order to investigate the hypothesis that B. bassiana can have a deleterious effect on dengue virus replication in A. aegypti, we treated mosquitoes with the fungus (1×108 conidia/ml) and fed them 2 days later on a DENV2-containing blood meal. Similarly to the survival assays, mosquitoes were exposed to B. bassiana conidial solution through the two routes. Seven days after the DENV-2 infected blood meal, the midguts were removed, and the viral load was measured by plaque assays and compared to that of non-fungus-exposed PBS-treated control mosquitoes (Fig. 2). Dengue infection was significantly reduced by a 2-fold (p < 0.01) in the midguts of mosquitoes continuously exposed to B. bassiana using the filter paper method (Fig. 2). The viral load was also approximately a 2-folder lower in the mosquitoes dipped once into the conidial solution, but this effect was variable between different individual mosquitoes and was therefore statistically insignificant (p = 0.22) (Fig. 2), indicating that a prolonged exposure to the fungus is necessary to achieve an efficient suppression of viral infection. In order to determine whether the fungus could directly inhibit dengue virus replication, we conducted an in vitro study in which the virus was incubated for 4 h with B. bassiana spores. No significant influence of the fungus on virus viability was observed (data not shown), suggesting that the effect of B. bassiana on dengue virus infection is indirect and likely to be at least partly mediated by the activation of the mosquito's immune response to fungus infection.
Fig. 2.
Effect of B. bassiana infection on DENV-2 replication in A. aegypti midguts. Dengue titers in the midguts of B. bassiana-treated mosquitoes were determined by plaque assays in C6/36 cells at 7 days post-feeding on DENV-2 infected blood. Mosquitoes were treated with B. bassiana 2 days before dengue infection by the filter paper method (left panel) or dipping method (right panel). Data for control mosquitoes (PBS) and those treated with B. bassiana at 108 conidia/ml (B.b.) are shown. At least three biological replicates are included, and each point indicate the absolute number of DENV-2 PFU in an individual midgut, and the horizontal lines indicate the median values. The statistical significance of the effects of fungal treatment on dengue replication was determined by Mann-Whitney test.
3.3. B. bassiana infection induces key factors and downstream effectors of the toll and JAK-STAT pathways
In response to fungal infection, the Toll pathway is activated in both Aedes and Drosophila, and this immune pathway regulates the transcription of a number of antimicrobial peptide and other immune genes (Lemaitre et al., 1996; Xi et al., 2008). In A. aegypti, the Toll pathway is also stimulated in response to dengue virus infection, and the transient activation of this pathway prior to virus infection increases the mosquito's resistance to the virus (Visintin et al., 2010; Xi et al., 2008). The prominent activation of the A. aegypti Toll pathway in response to fungal, bacterial, and dengue virus infections suggests that this pathway controls multiple immune-related functions and is therefore able to defend against diverse pathogens. We therefore hypothesized that the activation of the Toll pathway during mosquito infection with B. bassiana could be at least partly responsible for the inhibition of dengue virus proliferation in the mosquito midgut.
To assess the Toll pathway activation after the exposure of mosquitoes to B. bassiana, we performed preliminary experiments by using semi-quantitative PCR to monitor the transcript abundance of the Toll pathway marker genes attacin, defensin, and cecropin, and other antimicrobial peptide genes (diptericin and lysozyme C) after fungal infection. At 48 h after exposure to B. bassiana through the filter paper method, the expression of these genes (attacin, cecropin G, defensin A and C, diptericin) was significantly up-regulated except lysozyme C. Cecropin and Defensin displayed the strongest induction (Fig. S2) which agrees with previous published data where cecA and defA were strongly induced upon B. bassiana challenge (Shin et al., 2005).
Given that a one-time exposure of mosquitoes to the fungus yielded a lesser suppression of dengue viral loads compared to the prolonged exposure to the fungus, suggests that the latter type of exposure might induce a stronger anti-dengue immune response. In order to compare the changes of immune gene transcript abundance after two different modes of exposure to the fungus temporally and quantitatively, we also conducted real-time qRT-PCR–based analyses. As shown in Fig. 3, all assayed genes showed a stronger induction when mosquitoes were exposed to fungus through the filter paper method, especially Rel1, the transcription factor of the Toll pathway. Rel1 was induced as soon as 2 days after fungal exposure through the filter paper method, while the one-time exposure method resulted in Rel1 induction at 7 days post fungal challenge. Marked increases in transcript abundance of immune genes were observed at 5 to 9 days post exposure to fungal challenge, which is also the time when the dengue virus reaches the highest titres (from 3 to 7 days post dengue infected blood feeding). This result provides confirmatory evidence that B. bassiana infection activates the Toll pathway in mosquitoes, corroborating the hypothesis that fungus infection mediates resistance to the virus, at least partly, by the activation of an immune response. The prolonged exposure to fungus induced the Toll pathway -mediated immune responses stronger and earlier, which might have contributed to the observed stronger anti-dengue effect. These results show a synergy between anti-fungal and anti-dengue defenses, as previously suggested (Xi et al., 2008). We can however not exclude the possibility that fungal infection also induces some other physiological changes that restricts dengue virus infection.
Fig. 3.
Temporal expression analysis of Toll and JAK-STAT pathway marker genes after exposure of A. aegypti to B. bassiana through the filter paper method (A) or dipping method (B). Bars indicate fold change (Log2 transformed) in expression of the assayed genes at 2, 5, 7, 9 days after B. bassiana infection, compared to PBS-treated control mosquitoes. Each bar indicates the mean value from at least 3 replicates, and the error bar indicates standard error among three biological replicates. A ribosomal RNA gene S7 was used as internal control. P-values (<0.05, <0.01, <0.001, <0.0001) calculated from one-way ANOVA analysis are indicated at the bottom of the bars. The p-values from pair wise comparisons (compared to the PBS-treated controls) were calculated through a student's t-test and indicated on each bar (a: p<0.05; b: p<0.01; c: p<0.001; d: p<0.0001). Dome: Domeless; DVRF1: dengue virus restriction factor #1 (AAEL008492); CecG: Cecropin G; DefA: Defensin A; DefC: Defensin C; Dipt: Diptericin.
To investigate whether the JAK-STAT pathway-controlled anti-dengue immune genes are responsive to fungal infection, we used qRT-PCR to assess changes in gene transcript abundance of Dome and DVRFs at different time points (day 2, 5, 7, 9) post exposure to a B. bassiana conidial solution. Our previous study showed that the transcription factor of JAK-STAT (Dome) and STAT-regulated anti-dengue restriction factors (DVRF- 1 and -2) were strongly induced upon dengue infection (Souza-Neto et al., 2009). As shown in Fig. 3, both Dome and DVRF1 transcript abundance significantly increased at 5 and 7 days post exposure to B. bassiana through the filter paper method. DVRF1 was only induced at 9 days post exposure to fungus, which might explain part of the fungal-mediated anti-dengue effect when application of fungus occurred through the dipping method. DVRF2 also showed a trend to be induced after fungal exposure through the filter paper method (data not shown, due to the lesser number of replicates). Future experiments will be necessary to elucidate the molecular mechanisms involved in the interplay of fungus induced infection responses and antiviral activity in a greater detail.
3.4. The JAK-STAT pathway exerts an anti-fungal defense
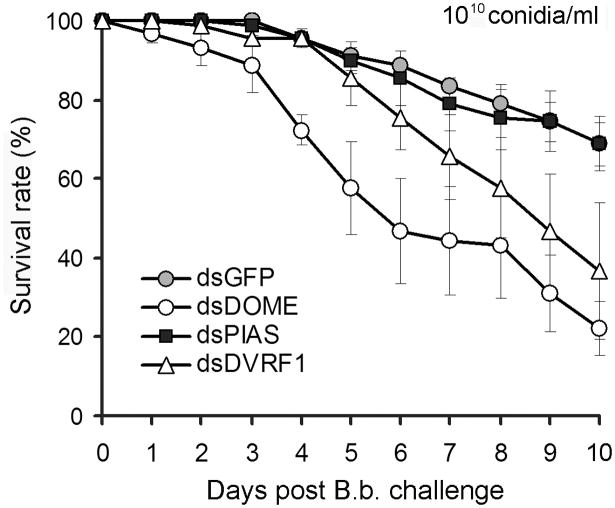
Our gene expression analyses showed that Dome and DVRF1 are induced upon B. bassiana challenge at 5 and 7 days post infection, suggesting that the JAK-STAT pathway may play a role in the mosquito's anti-fungal defense. To address this hypothesis we monitored the susceptibility of Dome, PIAS and DVRF1 gene silenced mosquitoes to fungal challenge. After 2 to 3 days recovery following dsRNA-injection, the treated mosquitoes were exposed to a B. bassiana conidial solution of 1010 conidia/ml to assure that the mortality of mosquitoes would take place within the time-window when the dsRNA-mediated gene silencing is still efficient, since the B. bassiana strain used here is the least virulent strain to mosquitoes (half of the mosquitoes were killed at around 16 days after exposure to a 108 conidia/ml dosage). The Dome dsRNA-treated mosquitoes (65% silencing efficiency) were significantly more sensitive to the fungal infection compared to the GFP dsRNA injected mosquitoes, and the median LT50 was around 6 days, which is significantly lower than that of the control groups. At the end of the survival monitoring time (10 days) at least 70% of dsGFP-injected control mosquitoes had survived (p<0.001) and the the LT50 could therefore not be determined (Fig. 4, Table S1). DVRF1 depletion upon fungus challenge resulted in a trend of higher mortality compared to the control group, but this effect was only significant in 2 replicates, (Table S2). In accordance to our previous study, Dome and DVRF1 gene silencing resulted in a 3-fold increase of virus loads at 7 days post dengue virus infected blood meal, suggesting a relationship between anti-fungal and anti-dengue immune defences and pathways. Interestingly, gene silencing of PIAS, the negative regulator of JAK-STAT pathway, did not influence survival of mosquitoes upon fungus challenge, while we have previously shown that it renders mosquitoes more resistant to dengue infection. This may indicate some as yet unknown of complexity of this pathway or that the pathway can't be further boosted to cope with a fungal infection at these conditions. The timing of infection assays and gene silencing are also different between the two experiments with fungus and dengue infection.
Fig. 4.
The JAK-STAT pathway modulates Aedes mosquitoes' susceptibility to B. bassiana infection. Survival rates of PIAS dsRNA-, DOME dsRNA-, DVRF1 (AAEL008492) dsRNA-, and GFP dsRNA-injected mosquitoes upon B. bassiana infection. The significance of gene silencing on the mortality of mosquitoes upon B. bassiana infection, compared to GFP dsRNA-treated controls, was determined by Kaplan-Meier survival analysis and p-values are listed in Table S2. The mean survival percentage for all three biological replicates along with the standard errors are shown.
The increased susceptibility to fungal infection of Dome and DVRF1 depleted mosquitoes indicate a role of the JAK-STAT pathway anti-fungal immune defense, and that fungus infected mosquitoes may exert their anti-dengue activity partly through STAT-regulated effector molecules including the DVRF1. Our study points at a synergy between anti-fungal and anti-dengue defenses through the Toll and JAK-STAT pathways.
4. Conclusions
We demonstrate that B. bassiana can significantly shorten the life span of A. aegypti mosquitoes at infection with as low as 103 conidia/ml. Furthermore, we have for the first time we have shown that fungal treatment can significantly suppress the dengue virus in the mosquitoes' midgut, at least partly through the immune activation of the Toll and JAK-STAT pathway-controlled effector genes. We also show for the first time that the JAK-STAT pathway plays a role in the Aedes mosquito's anti-fungal defense. The fact that both these events can influence the dynamics of dengue transmission renders B. bassiana a potentially promising agent for the development of dengue control strategies. B. bassiana has previously been used for the bio-control of agricultural pests, is commercially available, and is harmless to humans (reviewed in Kanzok and Jacobs-Lorena, 2006). A field study carried out in Tanzania suggests that application of M. anisopliae-impregnated black cotton sheets to mosquito's resting places could lead to a reduction of 75% in malaria transmission (Scholte et al., 2005). Considering that A. aegypti, like A. gambiae, is highly anthropophilic and endophilic (Harrington et al., 2001), the use of entomopathogenic fungus to infect field populations of A. aegypti appears to be a promising approach for reducing dengue transmission and therefore deserves further investigation.
Supplementary Material
Acknowledgments
We thank the personnel at the Johns Hopkins Malaria Research Institute Insect Facility for assistance with mosquito rearing. We also thank the Arbovirus Diseases Branch at the CDC for providing the anti-dengue antibodies (mouse hyperimmune ascitic fluid). This work has been supported by the National Institutes of Health/National Institute of Allergy and Infectious Disease 1R01AI061576-01A1, a Johns Hopkins School of Public Health Faculty Innovation Grant, and the Johns Hopkins Malaria Research Institute. We also thank Dr. Deborah McClellan for editorial assistance. J.C.M. was supported by Johns Hopkins Diversity Research Internship Program, J.L.R. was supported by NIH individual NRSA training grant F31 AI080161-01 A1 and by the American Society for Microbiology Robert D. Watkins Graduate Research Fellowship and JA.S.-N. was supported by the Johns Hopkins Malaria Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford S, Chan BH, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- Boucias DG, P J. Principles of insect pathology. Kluwer Academic Publishers; 1998. [Google Scholar]
- Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J. An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett. 1990;69:55–60. [Google Scholar]
- Cherry S, Silverman N. Host-pathogen interactions in drosophila: new tricks from an old friend. Nat Immunol. 2006;7:911–917. doi: 10.1038/ni1388. [DOI] [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TB, Kellen WR, Fukuda T, Lindegren JE. Field and laboratory studies on the pathogenicity of the fungus Beauveria bassiana to three genera of mosquitoes. J Invertebr Pathol. 1968;11:1–7. doi: 10.1016/0022-2011(68)90047-5. [DOI] [PubMed] [Google Scholar]
- Clements AN, Paterson GD. The analysis of mortality and survival rates in wild populations of mosquitoes. J Appl Ecol. 1981;18:373–399. [Google Scholar]
- Dimopoulos G. Insect immunity and its implication in mosquito-malaria interactions. Cell Microbiol. 2003;5:3–14. doi: 10.1046/j.1462-5822.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Everitt BS. Statistical Methods for Medical Investigations. Halsted Press; New York: 1994. [Google Scholar]
- Garcia-Munguia AM, Garza-Hernandez JA, Rebollar-Tellez EA, Rodriguez-Perez MA, Reyes-Villanueva F. Transmission of Beauveria bassiana from male to female Aedes aegypti mosquitoes. Parasit Vectors. 2011;4:24. doi: 10.1186/1756-3305-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RV, Vaughn DW. Dengue: an escalating problem. Bmj. 2002;324:1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D. The emergence of epidemic dengue fever and dengue hemorrhagic fever in the Americas: a case of failed public health policy. Rev Panam Salud Publica. 2005;17:221–224. doi: 10.1590/s1020-49892005000400001. [DOI] [PubMed] [Google Scholar]
- Guzman MG. Global voices of science. Deciphering dengue: the Cuban experience. Science. 2005;309:1495–1497. doi: 10.1126/science.1115177. [DOI] [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- Howard AF, Koenraadt CJ, Farenhorst M, Knols BG, Takken W. Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malar J. 2010a;9:168. doi: 10.1186/1475-2875-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AF, N'Guessan R, Koenraadt CJ, Asidi A, Farenhorst M, Akogbeto M, Thomas MB, Knols BG, Takken W. The entomopathogenic fungus Beauveria bassiana reduces instantaneous blood feeding in wild multi-insecticide-resistant Culex quinquefasciatus mosquitoes in Benin, West Africa. Parasit Vectors. 2010b;3:87. doi: 10.1186/1756-3305-3-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzok SM, Jacobs-Lorena M. Entomopathogenic fungi as biological insecticides to control malaria. Trends Parasitol. 2006;22:49–51. doi: 10.1016/j.pt.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kay B, Vu SN. New strategy against Aedes aegypti in Vietnam. Lancet. 2005;365:613–617. doi: 10.1016/S0140-6736(05)17913-6. [DOI] [PubMed] [Google Scholar]
- Knols BG, Bukhari T, Farenhorst M. Entomopathogenic fungi as the next-generation control agents against malaria mosquitoes. Future Microbiol. 2010;5:339–341. doi: 10.2217/fmb.10.11. [DOI] [PubMed] [Google Scholar]
- Knols BGJ, Thomas MB. Fungal entomopathogens for adult mosquito control—a look at the prospects. Outlooks Pest Manag. 2006;17:257–259. [Google Scholar]
- Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Michalakis Y, Renaud F. Malaria: fungal allies enlisted. Nature. 2005;435:891–893. doi: 10.1038/435891a. [DOI] [PubMed] [Google Scholar]
- Miranpuri GS, Khachatourians GG. Larvicidal activity of blastospores and conidiospores of Beauveria bassiana (strain GK 2016) against age groups of Aedes aegypti. Vet Parasitol. 1990;37:155–162. doi: 10.1016/0304-4017(90)90070-r. [DOI] [PubMed] [Google Scholar]
- Pinheiro VB, Ellar DJ. How to kill a mocking bug? Cell Microbiol. 2006;8:545–557. doi: 10.1111/j.1462-5822.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Long S, Smith G, Pyke A, Knox TB. Entomological investigations in a focus of dengue transmission in Cairns, Queensland, Australia, by using the sticky ovitraps. J Med Entomol. 2004;41:1–4. doi: 10.1603/0022-2585-41.1.1. [DOI] [PubMed] [Google Scholar]
- Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte EJ, Knols BG, Samson RA, Takken W. Entomopathogenic fungi for mosquito control: a review. J Insect Sci. 2004;4:19. doi: 10.1093/jis/4.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte EJ, Knols BG, Takken W. Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. J Invertebr Pathol. 2006;91:43–49. doi: 10.1016/j.jip.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Scholte EJ, Ng'habi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BG. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science. 2005;308:1641–1642. doi: 10.1126/science.1108639. [DOI] [PubMed] [Google Scholar]
- Scholte EJ, Njiru BN, Smallegange RC, Takken W, Knols BG. Infection of malaria (Anopheles gambiae s.s.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae. Malar J. 2003;2:29. doi: 10.1186/1475-2875-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte EJ, Takken W, Knols BG. Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae. Acta Trop. 2007;102:151–158. doi: 10.1016/j.actatropica.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Shin SW, Bian G, Raikhel AS. A toll receptor and a cytokine, Tol15A and Spz1C, are involved in toll antifungal immune signaling in the mosquito Aedes aegypti. J Biol Chem. 2006;281:39388–39395. doi: 10.1074/jbc.M608912200. [DOI] [PubMed] [Google Scholar]
- Shin SW, Kokoza V, Bian G, Cheon HM, Kim YJ, Raikhel AS. REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem. 2005;280:16499–16507. doi: 10.1074/jbc.M500711200. [DOI] [PubMed] [Google Scholar]
- Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssou R. Dengue fever: from disease to vaccination. Med Trop (Mars) 2009;69:333–334. [PubMed] [Google Scholar]
- Thomas MB, Read AF. Can fungal biopesticides control malaria? Nat Rev Microbiol. 2007;5:377–383. doi: 10.1038/nrmicro1638. [DOI] [PubMed] [Google Scholar]
- Troyer JM, Hanley KA, Whitehead SS, Strickman D, Karron RA, Durbin AP, Murphy BR. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg. 2001;65:414–419. doi: 10.4269/ajtmh.2001.65.414. [DOI] [PubMed] [Google Scholar]
- Visintin AM, Laurito M, Stein M, Ramirez P, Molina G, Lorenzo PR, Almiron WR. Two new mosquito species and six new provincial records in Argentina. J Am Mosq Control Assoc. 2010;26:91–94. doi: 10.2987/09-5955.1. [DOI] [PubMed] [Google Scholar]
- WHO. Dengue and dengue haemorrhagic fever. 2011 WHO available at http://www.who.int/csr/disease/dengue/en/
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.