Abstract
Vitiligo is an autoimmune disease presenting with progressive loss of skin pigmentation. The disease strikes 1% of the world population, generally during teenage years. The progressive loss of melanocytes from depigmenting vitiligo skin is accompanied by cellular infiltrates containing both CD4+ and CD8+ T lymphocytes. Infiltrating cytotoxic T cells with high affinity T cell receptors have likely escaped clonal deletion in the thymus, allowing such T cells to enter the circulation. Through the expression of CLA, these T cells home to the skin where they express type 1-cytokine profiles and mediate melanocyte apoptosis via the granzyme/perforin pathway. T cells found juxtapositionally apposed to remaining melanocytes can be isolated from the skin. Vitiligo T cells have demonstrated reactivity to antigens previously recognized as target antigens for T cells infiltrating melanoma tumors. In a comparison to existing melanoma-derived T cells, vitiligo T cells displayed superior reactivity towards melanoma cells. It is thought that genes encoding the TCRs expressed by vitiligo skin infiltrating T cells can be cloned and expressed in melanoma T cells, thereby generating a pool of circulating T cells with high affinity for their targets that can re-direct the immune response towards the tumor.
Keywords: Melanocytes, T cells, T cell avidity, T cell receptors, Melanosomes, Antigen presentation
1. The history of autoimmune vitiligo
Vitiligo is defined as a skin disorder characterized by progressive loss of pigmentation [1]. The age of onset is most frequently found in the 2nd decade of life, coinciding with major hormonal changes, yet a subset of patients acquires vitiligo much later in life [2]. The distribution of lesions appears to vary accordingly [3]. It is thought that as much as 1% of the world population is afflicted by vitiligo [1]. Convincing epidemiologic studies to assess the distribution of vitiligo among ethnic groups are lacking and although there is currently no evidence for a bias towards a particular skin type, the appearance of milky-white skin lesions is particularly devastating against a background of darkly pigmented skin [4]. Coincidentally, vitiligo lesions can resemble depigmented skin patches in patients with tuberculoid leprosy, a disease not yet fully eradicated [5]. Here vitiligo patients can be ostracized within their communities, with severe social and economical consequences.
Existing therapies for vitiligo are considered inadequate [6]. Patients with a recent diagnosis of vitiligo who are seeking treatment may consider their treatment effective, yet the majority of long-standing patients are not currently seeking treatment and prior treatment attempts are generally considered ineffective. We speculate that this apparent difference of opinion reflects a true difference in efficacy, as the majority of newly diagnosed patients is young and therefore likely more responsive to therapy in general, and newly diagnosed patients are more likely to receive novel and more effective therapeutic measures based on recent findings regarding vitiligo etiology.
In order to investigate whether vitiligo etiology involves an autoimmune response to melanocytes, it was necessary to first establish that milky-white skin patches were devoid of melanocytes rather than lesions being caused by melanocytes having lost the ability to generate melanin. Immunostainings with a panel of antibodies reactive with different melanocyte antigens supported the loss of differentiated melanocytes from vitiligo lesional skin [7]. The question remains whether the epidermis harbors undifferentiated melanocyte stem cells, and whether these stem cells will be left unharmed in vitiligo skin. As shown in Fig. 1, c-KIT expression is not observed in lesional vitiligo epidermis. Since c-KIT signaling is considered crucial for the proper distribution and maintenance of melanoblasts in the epidermis, these findings suggest that vitiligo skin is devoid of melanocyte precursors [7]. Thus, it may not be possible to repigment vitiligo skin through the differentiation of stem cells already present within the epidermis. However, a source of stem cell factor receptor, c-KIT expressing, melanocyte precursors is often maintained in the bulge region of the hair follicle, within vitiligo lesional skin. The stimulation of melanocyte differentiation and migration from hair follicles can thus be a successful treatment for vitiligo. This is supported by reports of ‘freckly’ repigmentation in patients undergoing PUVA therapy [8].
Fig. 1.
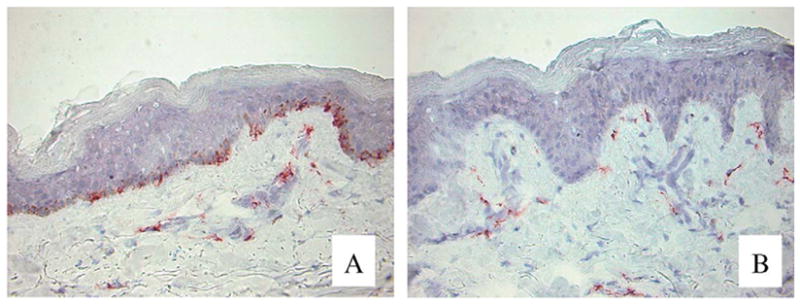
Lack of expression of c-KIT in lesional vitiligo skin. Sections of (A) non-lesional and (B) lesional vitiligo skin were subjected to indirect immunoperoxidase staining. Expression of the steel factor receptor was observed in non-lesional, but not in lesional skin. Original magnification 400×.
The concept of vitiligo as an autoimmune disease was introduced following reports of autoantibodies found in serum from patients with active disease [9]. This phenomenon has been studied in great detail, and antibodies directed against melanocytes are clearly more prevalent in patients with active vitiligo. These antibodies are generally reactive with intracellular antigens, and humoral immunity to pigment cells may constitute an epiphenomenon that occurs in response to cell damage. In this respect the discovery of the melanin-concentrating receptor-1 (MCR1) as a target for vitiligo autoantibodies is of interest [10]. This can explain at least in part why adoptive transfer of vitiligo serum can induce depigmentation of xenografted human skin in SCID mice [11]. Further evidence in support of a role for antibodies is provided by demonstrating antibody-dependent cellular cytotoxicity (ADCC) in vitro [12]. At the very least, humoral responses indicate active anti-melanocyte reactivity.
We have demonstrated the consistent presence of an inflammatory infiltrate surrounding expanding vitiligo lesions [13]. This has long been overlooked, likely because infiltrates are found only in active disease and because the infiltrates are generally small [13]. Frequently, patients report progression of their lesions to be associated with itchy skin (unpublished observation). Infiltrates consist of macrophages, as well as dendritic cells and T cells. In contrast to psoriasis, no B cells are found in vitiligo skin [14]. T cells found in perilesional skin of active lesions express the skin homing receptor CLA (cutaneous lymphocyte antigen), indicating that these T cells were ‘en route’ to the skin [13]. This finding greatly evoked our interest in cell mediated autoimmunity and its role in vitiligo pathogenesis.
It should be mentioned, that depigmentation will develop only in individuals prone to develop vitiligo. In this respect there are many reports of melanocytes with aberrant morphology in pigmented skin from vitiligo patients, suggesting that these melanocytes are increasingly sensitive to stress [15,16]. Indeed, ‘occupational vitiligo’ in response to phenolic agents will occur only in a subset of individuals [17]. It appears that melanocytes from vitiligo patients will respond differently to stress: we have demonstrated that vitiligo melanocytes will release more HSP70 in response to model stressor compound 4-TBP [18]. Stress proteins can function as peptide chaperones and are capable of activating dendritic cells [19,20]. In fact, stress protein/ tumor antigen fusion proteins are currently in clinical trials for the treatment of human tumors, including melanoma. Interestingly, it has been shown that vitiligo development in melanoma patients is an indicator of effective immunity against the tumor, and vitiligo in melanoma patients is considered a positive prognostic factor [21]. It is therefore of great importance to analyze the similarities and discrepancies among effective immunity to melanocytic cells in autoimmune vitiligo, and the lack thereof in patients with malignant melanoma [22].
2. A glance at melanocyte immunobiology
Melanocytes are unique in their ability to confer pigmentation to the skin. The process is based on melanin synthesis within specialized organelles, the melanosomes. Melanosomes contain enzymes and structural components required for effective melanization. MART-1 appears to lay the groundwork for the deposition of the melanosomal matrix, assisted by gp100 [23]. These 2 molecules are also considered the most immunogenic antigens of the melanocytic cells and T cells infiltrating melanoma tumors are most frequently reactive with either of these antigens [24]. The subsequent arrival of tyrosinase and tyrosinase-related enzymes TRP-1 and TRP-2 completes the arsenal of melanosomal enzymes. Eumelanin (brown/ black melanin) offers better protection against UV whereas in pheomelanogenesis, more radicals are generated than can be scavenged by the yellow/reddish brown end product, explaining in part why pheomelanized individuals (including orientals) will develop skin tumors more frequently than darkly pigmented individuals [25].
The melanosome is unique in particular because it is transferred to neighboring cells in a process that remains poorly understood to date. This process is of particular interest to immunologists. Organelle transfer has otherwise been described only for exosomes, small lipid vesicles serving to discard or transfer membrane proteins. Exosomes are considered important for DC activation and the induction of immune responsiveness to antigens contained within these organelles [26]. Interestingly, organelles transferred from melanocytes to keratinocytes are in fact equivalent to lysosomes in other cells. It is well possible that lysosome transfer can likewise occur between cells as well but cannot be easily observed for lack of a marker as readily detectable as melanin. The transfer of lysosome-like organelles may allow for sharing of antigens destined for presentation in the context of MHC class II. It should be noted that ripe melanosomes do not contain intact melanogenic enzymes as shown by melanocyte fractionation [27]. But peptide fragments may be generated within the acidic environment of the melanosome. Resulting peptides may retain their immunogenicity when transferred to dendritic cells. Thus peptides derived from gp100, MART-1 and other melanosomal proteins, may be transferred to infiltrating dendritic cells in the absence of melanocyte death within the skin. This may explain why melanocytes are highly immunogenic whereas this cell type is otherwise very resistant to apoptosis [28].
Melanocyte immunogenicity has also been ascribed to their Langerhans cell-like morphology and distribution. Melanocytes synthesize primary cytokines, and are thus capable of eliciting a local immune response [29]. Moreover, we have previously demonstrated that melanocytes are capable of phagocytosis and can process and present antigens in the context of MHC class II to CD4+ proliferative and cytotoxic T cells [30,31]. As melanocytes lack the mobility of Langerhans cells and other professional antigen presenting cells, it is conceivable that antigen presentation within the skin renders the melanocyte a ‘sitting duck’ for an immune attack. Of course, this situation will arise only when the melanocyte expresses MHC class II molecules, which happens to be the case only in vitiligo and in melanoma [32]. These are also situations where T cell responses to melanocytes prevail. In conclusion, a role for melanocytes within the Skin Immune System cannot be overlooked as we assess the etiology of vitiligo.
3. Involvement of T cells in depigmentation
T cells are more prevalent in vitiligo perilesional skin than in surrounding non-lesional or lesional skin. An occasional T cell remains in lesional skin but most of the infiltrate appears to migrate with the depigmenting epidermal border. Among the T cells, the infiltrate consists mainly of CD8 but also of CD4 T cells [33]. Since cytotoxic T cells are especially prevalent and colocalize with remaining melanocytes, it was postulated that these T cells are actively cytolytic towards remaining melanocytes [34]. T cells express granzyme and the prevailing theory is that cytotoxicity is mediated by the granzyme/perforin pathway rather than through Fas/FasL in vitiligo (unpublished observations). It is well possible that cytotoxicity is executed in part by CD4+ T cells as HLA-DR expression by melanocytic cells appears to be limited to melanocytes in vitiligo marginal skin and melanoma cells within tumors. In this respect it is of interest that the T cell clones originally isolated from a patient with leprosy that were used to demonstrate reactivity towards MHC class II expressing melanocytes were reactive with mycobacterial stress protein hsp65 [31]. This is noteworthy, particularly since Schwann cells harbor the causative M. leprae organism in leprosy, whereas Schwann cells are developmentally very closely related to melanocytes [35]. This similarity may ultimately contribute to cross reactivity or epitope spreading from Schwann cell antigens to melanocytes, explaining the similarity among lesions observed in tuberculoid leprosy and autoimmune vitiligo.
The involvement of T cells in vitiligo is further supported by the observation that depigmentation is accompanied by the expression of type 1 cytokines. We found that IFN-γ inducible CDw60, representative of the ganglioside GD3, was upregulated in perilesional skin [36]. The type 1 cytokine profile observed for T cells isolated directly from depigmenting lesions likewise supports a T cell mediated autoimmune response.
4. Promising aspects of high affinity autoimmune TCR
Skin infiltrating T cells can be isolated from tissue biopsies of interest and grown in bulk. These T cells can be collected and propagated without antigen selection in presence of IL-2 and anti-CD3/anti-CD28 antibody coated beads. T cells have been isolated from perilesional skin of an HLA-A*0201+ patient with active vitiligo. These unselected T cells were found to have similar reactivity towards HLA-A*0201+ melanocytes when compared with established T cell lines from melanoma patients that are >90% melanoma reactive (unpublished observation). Importantly, our vitiligo T cells demonstrated superior reactivity towards HLA-A*0201+ melanoma cells. We have since determined that >15% of our T cells were reactive with the gp100 209–217 epitope as shown in Fig. 2. Interestingly, among six T cell clones reactive with the gp100 209–217 epitope we have thus far identified two different TCRs (unpublished). Our data support the current hypothesis, that T cells isolated from vitiligo skin are highly reactive with melanoma-associated antigens [37]. High affinity TCRs, once expressed in PBMC from melanoma patients, can more effectively target the tumor [38]. It is well possible that high affinity T cells previously escaped clonal deletion in skin-draining lymph nodes and were inadvertently allowed to enter the circulation, emigrating to the skin to inflict damage to the melanocyte population [39].
Fig. 2.

Abundance of gp100 reactive T cells among lymphocytes from perilesional vitiligo skin. Lymphocytes from a progressive generalized vitiligo patient were expanded in presence of CD3/CD28 coated beads and IL-2, then reacted with fluorescently detected HLA-A2 dimers loaded with either (A) gp100 209–217 or (B) irrelevant human papillomavirus-derived immunogenic peptide, HPV16 E7 86–93, plus a fluorescent antibody to CD8, and analyzed by flow cytometry.
An ongoing autoimmune response may be allowed to further develop and mature in the absence of functional T regulatory cells (T regs) [40]. These cells actively mediate suppression of the immune system generally by secreting IL-10 and TGF-β to prevent autoimmunity [40]. Very recent data showing a decrease in the number of T regs in vitiligo skin indicate that this may apply to vitiligo as well (unpublished observation). In the absence of regulatory T cells, cytotoxic T cells with increasing affinity for their targets enter the skin and continuously proliferate and migrate towards novel target cells, causing depigmentation. This unfortunate event of disfiguring depigmented skin patches left in vitiligo patients may turn out to be a blessing in disguise if the T cell receptors expressed by high affinity T cells provide an effective treatment for melanoma patients.
Take-home messages
Vitiligo is characterized by progressive loss of melanocytes from the epidermis accompanied by elevated titers of anti-melanocyte antibodies and by inflammatory skin infiltrates.
Melanosomes are lysosome-like organelles where melanogenic enzymes reside that form prime target antigens for T cells infiltrating melanoma tumors or depigmenting vitiligo skin.
Melanocytes secrete cytokines, and can phagocytoze, process and present antigens to T cells in the context of MHC class II, accounting in part for their immunogenicity.
Depigmenting vitiligo skin is characterized by MHC class II expressing melanocytes by granzyme-expressing, skin infiltrating T cells with type 1 cytokine profiles.
Polyclonal cytotoxic T cells derived from vitiligo skin are highly reactive toward melanoma cells and may serve as a superior source of high affinity TCR genes to treat melanoma.
Acknowledgments
The authors gratefully acknowledge funding from the National Vitiligo Foundation (USA), from NCI RO1 CA109636 and from NIAMS RO3 AR50137 to ICLP from NCI CA90873, to MIN, and from The Netherlands Organization for Scientific Research NOW-VIDI016.056.337 to RML.
References
- 1.Le Poole IC, van den Wijngaard RMJGJ, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100(6):816–33. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- 2.Liu JB, Li M, Yang S, Gui JP, Wanf HY, Du WH, et al. Clinical profiles of vitiligo in China: an analysis of 3742 patients. Clin Exp Dermatol. 2005;30(4):327–31. doi: 10.1111/j.1365-2230.2005.01813.x. [DOI] [PubMed] [Google Scholar]
- 3.Dogra S, Parsad D, Handas S, Kanwar AJ. Late onset vitiligo: a study of 182 patients. Int J Dermatol. 2005;44(3):193–6. doi: 10.1111/j.1365-4632.2004.01948.x. [DOI] [PubMed] [Google Scholar]
- 4.Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(S6):S143–8. doi: 10.1067/mjd.2003.274. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi SK, Singh G, Gupta N. Stigma experience in skin disorders: an Indian perspective. Dermatol Clin. 2005;23 (4):635–42. doi: 10.1016/j.det.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Firooz A, Bouzari N, Fallah N, Ghazisaidi B, Firoozabadi MR, Dowlati Y. What patients with vitiligo believe about their condition. Int J Dermatol. 2004;43(11):811–4. doi: 10.1111/j.1365-4632.2004.02059.x. [DOI] [PubMed] [Google Scholar]
- 7.Steel KP, Davidson DJ, Jackson JJ. TRP-2/DT, a new early melanoblast marker, shows that steel factor (ckit ligand) is a survival factor. Development. 1992;115(4):1111–9. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- 8.Abdel Naser MB, Wollina U, El Okby M, El Shiemy S. Psoralens plus ultraviolet A irradiation-induced lentigines arising in vitiligo: involvement of vitiliginous and normal appearing skin. Clin Exp Dermatol. 2004;29(4):380–2. doi: 10.1111/j.1365-2230.2004.01536.x. [DOI] [PubMed] [Google Scholar]
- 9.Harning P, Cui J, Bystryn JC. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol. 1991;97(6):1078–80. doi: 10.1111/1523-1747.ep12492607. [DOI] [PubMed] [Google Scholar]
- 10.Kemp EH, Waterman EA, Hawes BE, O'Neill K, Gottumukkala RV, Gawkrodger DJ, et al. The melanin-concentrating receptor 1, a novel target of autoantibody responses in vitiligo. J Clin Invest. 2002;109(7):923–30. doi: 10.1172/JCI14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilhar A, Zelickson B, Ulman Y, Etzioni A. In vivo destruction of melanocytes by the IgG fraction of serum from patients with vitiligo. J Invest Dermatol. 1995;105(5):683–6. doi: 10.1111/1523-1747.ep12324456. [DOI] [PubMed] [Google Scholar]
- 12.Norris DA, Kissinger RM, Naughton GM, Bystryn JC. Evidence for immunologic mechanisms in human vitiligo: patients' sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity. J Invest Dermatol. 1988;90(6):783–9. doi: 10.1111/1523-1747.ep12461505. [DOI] [PubMed] [Google Scholar]
- 13.Van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Invest. 2000;80(8):1299–309. doi: 10.1038/labinvest.3780138. [DOI] [PubMed] [Google Scholar]
- 14.De Boer OJ, van der Loos CM, Hamerlinck F, Bos JD, Das PK. Reappraisal of in situ immunophenotypic analysis of psoriatic skin: interaction of activated HLA-DR+ immunocompetent cells and endothelial cells is a major feature of psoriatic lesions. Arch Dermatol Res. 1994;286(2):87–96. doi: 10.1007/BF00370733. [DOI] [PubMed] [Google Scholar]
- 15.Boissy RE, Liu YY, Medrano EE, Nordlund JJ. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991;97(3):395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- 16.Im S, Hann SK, Kim HI, Kim NS, Park YK. Biologic characteristics of cultured human melanocytes. Int J Dermatol. 1994;33(8):556–62. doi: 10.1111/j.1365-4362.1994.tb02895.x. [DOI] [PubMed] [Google Scholar]
- 17.Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17(3):208–14. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol. 2005;124(4):798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asea A. Stress proteins and initiation of immune response: chaperone activity of hsp72. Exerc Immunol Rev. 2005;11:34–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167(9):4844–52. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- 21.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, et al. Prognostic significance of autoimmunity during treatment with interferon. N Engl J Med. 2006;254(7):709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 22.Luiten RM, Kueter EW, Mooi W, Gullee MP, Rankn EM, Gerritsen WR, et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. J Clin Oncol. 2005;23(35):8978–91. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- 23.Hoashi T, Watabe H, Muller J, Yamaguchi Y, Vieira VD, Hearin VJ. MART-1 is required for the function of the melanosomal matrix protein Pmel17/gp100 and the maturation of melanosomes. J Biol Chem. 2005;230(14):14006–16. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami Y, Rosenberg SA. Immunobiology of human melanoma antigens MART-1 and gp100 and their use for immunogene therapy. Int Rev Immunol. 1997;14(2–3):173–92. doi: 10.3109/08830189709116851. [DOI] [PubMed] [Google Scholar]
- 25.Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. MC1R and the response of melanocytes to ultraviolet radiation. Mutat Res. 2005;571(1–2):133–52. doi: 10.1016/j.mrfmmm.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Taieb J, Chaput N, Zitvogel L. Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol. 2005;25 (3):215–23. doi: 10.1615/critrevimmunol.v25.i3.30. [DOI] [PubMed] [Google Scholar]
- 27.Orlow SJ, Boissy RE, Moran DJ, Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: implications for melanosomal biogenesis. J Invest Dermatol. 1993;100(1):55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- 28.van den Wijngaard RMJGJ, Aten J, Scheepmaker A, Le Poole IC, Tigges AJ, Westerhof W, et al. Expression and modulation of apoptosis regulatory molecules in human melanocytes: significance in vitiligo. Br J Dermatol. 2000;143(3):573–81. doi: 10.1111/j.1365-2133.2000.03712.x. [DOI] [PubMed] [Google Scholar]
- 29.Kruger-Krasagakes S, Krasagakis S, Garbe C, Diamantstein T. Production of cytokines by human melanoma cells and melanocytes. Recent Results Cancer Res. 1995;139:155–68. doi: 10.1007/978-3-642-78771-3_11. [DOI] [PubMed] [Google Scholar]
- 30.Le Poole IC, van den Wijngaard RM, Westerhof W, Verkruisen RP, Dutrieux RP, Dingemans KP, et al. Phagocytosis by normal human melanocytes in vitro. Exp Cell Res. 1993;205(2):388–95. doi: 10.1006/excr.1993.1102. [DOI] [PubMed] [Google Scholar]
- 31.Le Poole IC, Mutis T, van den Wijngaard RMJGJ, Westerhof W, Ottnhof T, de Vries RR, et al. A novel, antigen-presenting function of melanocytes and its possible relationship to hypopigmentary disorders. J Immunol. 1993;151(12):7284–92. [PubMed] [Google Scholar]
- 32.Overwijk WW, Restifo NP. Autoimmunity and immunotherapy of cancer: targeting the ‘self’ to destroy the ‘other’. Crit Rev Immunol. 2000;20(6):433–50. [PMC free article] [PubMed] [Google Scholar]
- 33.Le Poole IC, van den Wijngaard R, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148 (4):1219–28. [PMC free article] [PubMed] [Google Scholar]
- 34.Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, et al. Immunopolarization of CD4+ and CD8+ T cells to type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest. 2003;83(5):683–95. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- 35.Dupin E, Real C, Clavieux-Paranaud C, Vargot P, Le Douarin NM. Reversal of developmental restrictions in neural crest lineages: transition from Schwann cells to glial-melanocytic precursors in vitro. Proc Natl Acad Sci U S A. 2003;100 (9):5229–33. doi: 10.1073/pnas.0831229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Poole IC, Stennett LS, Bonish BK, Dee L, Robinson JK, Hernandez C, et al. Expansion of vitiligo lesions is associated with reduced epidermal CDw60 expression and increased expression of HLA-DR in perilesional skin. Br J Dermatol. 2003;149(4):739–48. doi: 10.1046/j.1365-2133.2003.05539.x. [DOI] [PubMed] [Google Scholar]
- 37.Das PK, van den Wijngaard RM, Wankowicz-Kalinska A, Le Poole IC. A symbiotic concept of autoimmunity and tumour immunity: lessons from vitiligo. Trends Immunol. 2001;22 (3):130–6. doi: 10.1016/s1471-4906(00)01844-5. [DOI] [PubMed] [Google Scholar]
- 38.Duval L, Schmidt H, Kaltoft K, Fode K, Jenssen JJ, Sorensen SM, et al. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma. Clin Cancer Res. 2006;12(4):1229–36. doi: 10.1158/1078-0432.CCR-05-1485. [DOI] [PubMed] [Google Scholar]
- 39.Palermo B, Garbelli S, Mantovani S, Scoccia E, DaPrada GA, Bernabei P, et al. Qualitative difference between the cytotoxic T-lymphocyte responses to melanocyte antigens in melanoma and vitiligo. Eur J Immunol. 2005;35(11):3153–62. doi: 10.1002/eji.200535110. [DOI] [PubMed] [Google Scholar]
- 40.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schermer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


