Abstract
Poor oral bioavailability limits the use of many chemopreventives in the prevention and treatment of cancer. To overcome this limitation, we report an improvised implant formulation (“coated” implants) using curcumin, individual curcuminoids, withaferin A and oltipraz. This method involves the coating of blank polycaprolactone implants with 20–30 layers of 10–20% polycaprolactone solution in dichloromethane containing 0.5–2% of the test agent. The in vitro release showed that while oltipraz was released with almost zero-order kinetics over eight weeks, curcumin, individual curcuminoids and withaferin A were released with some initial burst. The in vivo release was determined by grafting implants subcutaneously in A/J mice. When delivered by coated implants, oltipraz significantly diminished lung DNA adducts in mice treated with dibenzo[a, l]pyrene compared with sham treatment (28±7 versus 54±17 adducts/109 nucleotides). Withaferin A also diminished DNA adducts, but it was insignificant. Curcumin and individual curcuminoids were ineffective. Analysis of lung, liver and brain by UPLC-fluorescence showed the presence of the three test curcuminoids indicating effectiveness of the implant delivery system. Further, based on its known antitumor activity in vivo, withaferin A given via the implants significantly inhibited human lung cancer A549 xenograft in athymic nude mice, while it was ineffective when the same total dose was administered i.p. and required over 2-fold higher dose to elicit effectiveness. Together, our data suggest that coated polymeric implants can accommodate heat-labile compounds, can furnish sustained release for long duration, and elicit DNA damage-inhibiting and anti-tumor activities.
Keywords: Polycaprolactone, Curcumin, Withaferin A, Oltipraz, DNA adducts, Lung tumor xenograft, Bioavailability
1. Introduction
Phytochemicals have proven to be a valuable source of clinically useful drugs and cancer chemopreventive agents. Chemoprophylaxis [1] or chemoprevention [2] has been a subject of numerous studies from the time these names were coined. Much progress has been made in discovering and developing agents that have promise, or have already been successfully used, to prevent various types of cancers in preclinical studies. Although this has led to a greater understanding of the prevention, it has yet to translate into a cure for the disease as the cancer incidence rate has barely declined globally. In addition, the population of cancer survivors, who are at high risk of recurrence and metastasis, is increasing steadily.
Although, tiered approach of cell culture and preclinical studies have identified several efficacious agents, bioavailability issues have hindered their use in vivo. Such agents need to be administered at high doses to reach at sufficient levels in the target organ to exert their effects. These high doses are not readily translatable due to associated toxicity concerns. Curcumin is one such compound that rendered low oral bioavailability due to extensive intestinal and hepatic first pass metabolism [3; 4]. Various studies have shown that its low solubility and high rate of metabolism results in limited plasma levels, much below its required therapeutic concentration [5]. Several other compounds including polyphenolics are poorly absorbed when taken orally, posing the greatest obstacle to their routine clinical applications. Hence, drug delivery modules are required to circumvent not only the bioavailability issues but also to decrease the effective dose.
Bioavailability can be increased by encapsulation or systemic delivery, including nanoparticles, liposomes, microparticles, micelles and implants (reviewed in [6]). Encapsulation of drugs using polymeric carriers has emerged as the workhorse solution to manage poor biodistribution and stability of bare therapeutics [7]. With the optimized encapsulation technology, therapeutic efficacy can be increased several fold. Incredible choices in the polymeric designs offer a direct route to optimal carrier design. Different types of implantable devices have been used, such as non-biodegradable silastic tubes which have encountered issues with their removal after the end of treatment [8]; use of poly(lactide-co-glycolide) (PLGA)-based implants which have shown development of fibrous encapsulation around the implant site [9]; and use of high melting-point polymers which limit their application to compounds with high thermal stability [10]. We recently reported that biodegradable polycaprolactone:F68 implants embedded with chemopreventive agents provided sustained release for long duration in vivo [6; 11]. While tested successfully for various agents, this formulation (“extrusion” method) does not apply to heat-labile compounds and generally results in an initial high burst release followed by a gradual decline.
In this manuscript, we describe an improvised method for the preparation of multilayer coated implants that can accommodate compounds of different physico-chemical properties. We further demonstrate that these implants furnish more sustained release and can elicit DNA damage-inhibiting and anti-tumor activities in animal models.
2. Materials and Methods
2.1 Chemicals
ε-Polycaprolactone mol. wt. 80,000 (P-80) was purchased from Sigma-Aldrich (St. Louis, MO, USA). PluronicR F68 (F68) was a gift from BASF Corp. (Florham Park, NJ, USA). Silastic tubing (1.4 mm internal dia) was purchased from Allied Biomedical (Ventura, CA, USA). Disposable syringes (5 ml) were from BD Biosciences (Franklin lakes, NJ, USA). Penicillin-streptomycin solution was from Invitrogen (Carlsbad, CA). Amber vials (20 ml) were purchased from National Scientific (Rockwood, TN, USA). Curcumin and the three curcuminoids, curcumin I, curcumin II (demethoxycurcumin) and curcumin III (bisdemethoxycurcumin) were generous gifts of Sabinsa Corp. (East Windsor, NJ, USA). Oltipraz was obtained from the National Cancer Institute chemoprevention branch (Bethesda, MD, USA). Withaferin A was isolated from standardized extract of Withania somnifera (Sabinsa Corp, East Windsor, NJ, USA) in the laboratory using column chromatography and was >97% pure. Bovine calf serum was from Hyclone (Logan, UT, USA). Dichloromethane (DCM), absolute ethanol and acetonitrile were from BDH chemicals (VWR, West Chester, PA), Pharmco-AAPER (Louisville, KY, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively. Phosphate-buffered saline (PBS) pellets and tricaprylin were from Sigma-Aldrich (St. Louis, MO, USA). Materials used in the 32P-postlabeling assay for DNA adduct analysis were as described [12]. All other chemicals were of analytical grade.
2.2 Formulation of “coated” polymeric implants
Blank P-80 implants (1.4 mm dia) were prepared by dissolving P-80:F68 (9:1) in dichloromethane, followed by the evaporation of the solvent in a Petri dish under hood and extrusion of the molten polymer through a silastic tubing mold, as described elsewhere [13]. These blank implants were coated with about 20–30 layers of 10% P-80 solution in dichloromethane containing 1.0% oltipraz, curcumin or individual curcumins I, II and III, or 0.3% or 0.5% withaferin A. For coating, one end of the silastic tube plug (6 mm long, 1.4 mm dia) was attached to a pipet tip while other end is attached to the blank implant (2.5–3.5 cm) (Figure 1B). Coatings were added by dipping the blank implant into the solution with intermittent drying with cool air using a commercial hair dryer and placing them under hood. Twenty-five to 30 layers generally resulted in 2.6 mm dia coated implants as measured by a digital caliper. Implants thus formulated had a 10% drug load of the test agents, except for withaferin A which represented 3% and 5% load. The assembly was placed under hood overnight to remove the residual DCM; implants were excised in 1 or 2 cm lengths and stored in amber vials under argon at −20°C until used.
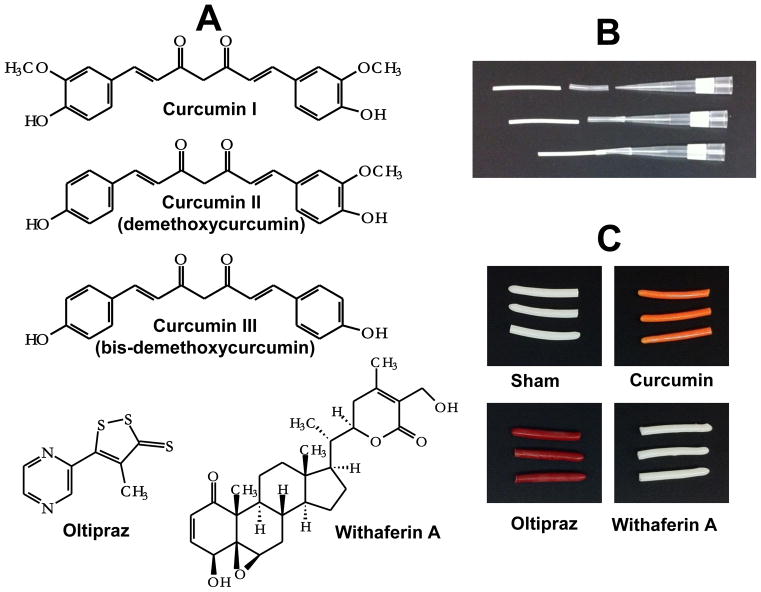
Figure 1.
Structures of selected chemopreventive agents investigated (A), insert assembled with pipette tip for coating (B) and photographs of representative coated polymeric implants (C). Implants were prepared from polycaprolactone (P80) as described in materials and methods section. Implant size: 2 cm length, 2.6 mm dia.
2.3 In vitro release
The rate of in vitro release was measured as described [13]. Briefly, 1-cm implants (n=3) were placed in 10 ml PBS containing 10% bovine calf serum and 1% penicillin-streptomycin solution in 20-ml amber vials. The vials were placed in a water bath (Julabo SW 23, Seelback, Germany) at 37°C with constant agitation at 110 rpm. The medium was changed every 24 h. Ethanol (10% final conc.) was added to the release medium to completely solubilize the compound, except for withaferin A which was extracted using acetonitrile and chloroform. The release was measured spectrophotometrically using the following absorbance maxima: oltipraz (430 nm), curcumin (430 nm), curcumin I (429 nm), curcumin II (424 nm), curcumin III (419 nm), and withaferin A (215 nm). The concentrations were calculated against standard curves.
2.4 Animal studies
2.4.1 Effect on DNA adducts
Following acclimation, 5–6 week-old female A/J mice (Harlan Laboratories, Indianapolis, IN) were treated with sham or coated polymeric implants (two 1.5 cm implants, 60 mg/implant) of oltipraz, curcumin, individual curcuminoids (curcumin I, curcumin II and curcumin III) and withaferin A. Drug load in the implants was @10%, except for withaferin A which was @5%. All groups of animals were also treated with DBP via polymeric implants (2-cm, 1.4 mm dia, 5% DBP load) at the same time; DBP implants were prepared as described [14]. Implants were grafted subcutaneously as described [15]. Animal were euthanized after 3 weeks. Two additional groups of mice received only withaferin A implants to determine the rate of release in vivo, and animals were euthanized after 2 and 8 weeks. Animals were euthanized by CO2 asphyxiation, and selected tissues (lung, liver and brain) were collected and stored at −80°C until processing. Implants were also recovered, dried under vacuum and stored in amber vials under argon at 4°C until analysis.
2.4.2 Toxicity studies
Blood was collected separately at the time of euthanasia, and hematological parameters were analyzed using whole blood by Cell Dyn 3500 hematology analyzer (Abbott laboratories, Santa Clara, CA). Serum was used to analyze the liver and kidney function test. An ion selective electrode was used for various electrolytes analysis, and in-built spectrophotometric techniques were used for all other biochemical parameters analysis on automated AU640 Chemistry analyzed (Beckman Coulter, Inc., Brea, CA).
2.4.3 Effect on tumor xenograft
Five- to 6-week old female athymic nude mice (Harlan Laboratories, Indianapolis, IN) were acclimated for a week and then inoculated with human lung cancer A549 cells (3×106 cells) in matrigel. Following inoculation, two groups of animals (n=10), were treated i. p. with vehicle (tricaprylin) or withaferin A (8 mg/kg, b.wt.) on alternate days, while two additional groups were treated with sham or withaferin A implants (two 1.5-cm, 5% drug load) after tumor grew to ~50 mm3. Animals were weighed, and the tumors were measured by a digital caliper weekly. At the end of 6–8 weeks, animals were euthanized by CO2 asphyxiation, and tumors were collected and measured. Implants were also recovered, dried under vacuum and stored in amber vials under argon at 4°C until analysis.
2.5 Measurement of residual oltipraz, curcumin, individual curcuminoids and withaferin A in the implants
Initial and residual contents of the test agent in the implants (n=3) were analyzed by dissolving the implants in 5 ml DCM, diluted with ethanol (1:1), and finally centrifuged at 12,000×g and discard the polymer pellet. The supernatants were diluted with ethanol:DCM (80:20); their concentrations were measured spectrophotometrically at maximal absorbance; and the concentrations were calculated against standard curves of individual compounds.
The stability of the test agents was confirmed by ultra performance liquid chromatography (UPLC) monitoring the peak purity and by co-chromatography with reference compounds. Analyses were carried out on a Shimadzu UPLC system (Kyoto, Japan), using Shim-Pack XR-ODS II C18 column (150 × 3.0 mm i.d., 2.2μm).
Withaferin A was analyzed using acetonitrile and water at a flow rate of 0.7 ml/min with a linear gradient elution in which acetonitrile concentration was increased from 5 to 60% from 1.3 to 5.1 min, followed by an increase to 80% from 5.1 to 7.7 min and finally to 100% in 10 min. The latter ratio was then maintained till 10.9 min and finally decreased to 5% in 12 min. The withaferin A was detected at 215 nm by PDA-UV and total concentration was calculated against the standard curve of withaferin A. Stability of the oltipraz was also analyzed in the same system and it was monitored at 430 nm. Curcumin and individual curcuminoids were analyzed as described later in this manuscript.
2.6 Analysis of DNA adducts
DNA was isolated by a solvent extraction procedure of crude nuclei as described [16]. Briefly, lung tissue (50 mg) was homogenized in 50 mM Tris.HCl/10 mM EDTA, pH 8.0 with a polytron homogenizer. The homogenate was centrifuged at 3000 × g for 10 min and crude nuclear pellet was collected by gently pouring off the supernatant. DNA was isolated by the treatment of the isolated crude nuclei with RNases and proteinase K, followed by extractions with phenol, phenol:Sevag and Sevag. DNA was recovered by ethanol precipitation, and its concentration was measured spectrophotometrically after dissolving in HPLC-grade water considering 1 A260 equals 50 μg DNA. DNA adducts were analyzed by nuclease P1-mediated 32P-postlabeling/TLC assay and expressed as adducts per 109 nucleotides, as described [12].
2.7 Analysis of tissue curcumin/curcuminoids levels
Tissue curcumin levels were measured by solvent extraction as described [11], except that the eluates were analyzed by UPLC. Briefly, lung, liver and brain tissues (200, 500 and 400 mg, respectively) were analyzed from individual animals except for lung where it was pooled from two animals. Tissues were homogenized in 3 ml PBS (pH 7.4) containing 200 μl of 0.5 M sodium acetate, and extracted with ethyl acetate. After evaporation of the pooled extracts, the residue was reconstituted in 100 μl acetonitrile and analyzed by UPLC system with a Shim-Pack XR-ODS II reverse phase column (Shimadzu; 150 × 3.0 mm i.d., 2.2 μm). The three curcuminoids, curcumin I, demethoxycurcumin and bisdemethoxycurcumin (75:25:5), were separated by using acetonitrile and 1% citric acid (pH 2.5) at a flow rate of 0.75 ml/min with a linear gradient elution in which acetonitrile concentration was increased from 2 to 30% in first 2 min, followed by an increase to 45% from 2 to 6.4 min; the latter ratio was then maintained till 11.5 min and finally decreased to 2% in 13 min. The curcuminoids were detected using 411 and 500 nm as excitation and emission maxima, respectively, in the fluorescence detector and total curcumin concentration was calculated from standard curves of individual curcuminoids.
2.8 Statistical analyses
Statistical analyses were performed using the student’s t-test and a p-value of <0.05 was considered significant. All analyses were carried out using the GraphPad Prizm statistical software, ver. 4.3 (La Jolla, CA).
3. Results and Discussion
The efficacy assessment of chemopreventive agents has almost always relied on oral administration. While it is most desirable and preferred, the oral route of administration has its own limitations. Only a handful of compounds identified through cell culture systems have been used in pre-clinical studies and still fewer agents have been tested in clinical studies. Chemopreventives, such as curcumin and green tea polyphenols, because of their wide-spread use in the diet (curcumin as part of the yellow spice turmeric) and common drink (green tea and black tea), have been the subject of numerous clinical trials. However, both these compounds have elicited little success in clinical studies despite bolus doses (e.g., 8–12 g per day for curcumin) [17]. The lack of success with curcumin, green tea polyphenols and several other chemopreventives has resulted from their lack of oral bioavailability (reviewed in [6]).
We recently reported that polymeric implants embedded with curcumin, green tea polyphenols and many other chemopreventives when grafted subcutaneously in rodents provide controlled systemic delivery for long duration [13]. This polymeric implant formulation (the “extrusion” method), however, does not apply to heat-labile compounds. Further, implants prepared by this method generally accompany an initial burst release of the compound. The coated implant formulation, which circumvents the limitations of the implants formulated by the extrusion method, is the subject of this study.
3.1 Coated polymeric implants
The improvised polymeric implant formulation was developed using structurally diverse compounds, namely, oltipraz, curcumin and its three constituents (curcumin I, II and III), and withaferin A (Figure 1A). Oltipraz, an analogue of dithiol-3-thione and a constituent of cruciferous vegetables, has been reported as an effective chemopreventive agent in pre-clinical studies and has been used extensively as an antischistosomal drug in Europe [18; 19]. All previously reported pre-clinical studies with curcumin have been done with commercial curcumin which is a mixture of three curcuminoids. Withaferin A, a triterpenoid, represents the principal bioactive of the herb Withania somnifera, commonly known as “ashwagandha”, as part of the ‘Ayurvedic’ folklore medicine in India.
The coated implants were formulated by coating multiple layers of the polymer (P80) solution in DCM, with or without test agents, with intermittent drying with cool air on blank P80 implants (1.4 mm dia). Twenty-five or 30 coatings of 20% and 10% polymer solution respectively resulted in cylindrical implants with about 2.6 mm diameter (Figure 1C). The number of coatings depended upon the polymer solution concentration which, in turn, depended upon the solubility of the test agent in DCM or other appropriate solvent.
3.2 In vitro release
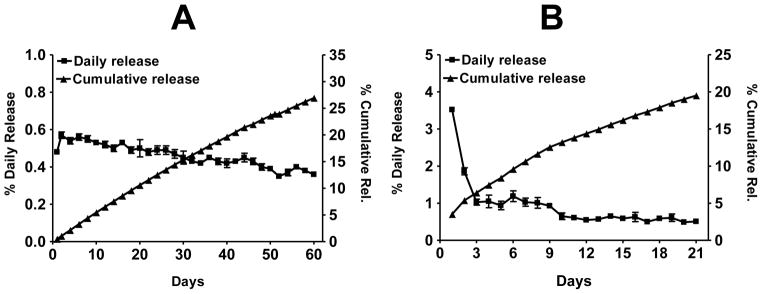
When coated implants of oltipraz, curcumin and withaferin A were agitated in PBS alone at 37°C, there was essentially no release. However, the presence of 10% bovine serum in the release medium, which mimics the in vivo scenario of extracellular fluid, showed that all test agents were released from the implants at controlled rates [11; 13]. Oltipraz implants showed essentially zero-order kinetics over a period of 60 days, with a cumulative release of about 12% and 27% after 21 and 60 days, respectively (Figure 2A). However, withaferin A was released with some initial burst. The initial burst release phenomenon for this compound lasted for only two days; subsequently, the release was more sustained, with a accumulative release of about 20% over 3 weeks (Figure 2B). On the other hand, withaferin A implants formulated by the extrusion method resulted in a cumulative release of about 35% over 3 weeks, largely because the burst release effect on this type of implant formulation was more pronounced and lasted for several days [13] compared with the coated implants.
Figure 2.
Percent average in vitro daily and cumulative release of oltipraz (A), withaferin A (B) (2 cm length, 2.6 mm dia) implants. Drug load was 20% for oltipraz and 3% for withaferin A. The release was measured by agitating implants in a shaker incubator in PBS supplemented with 10% bovine serum, and the media was changed daily. Data represent average ± SD of 3 implants.
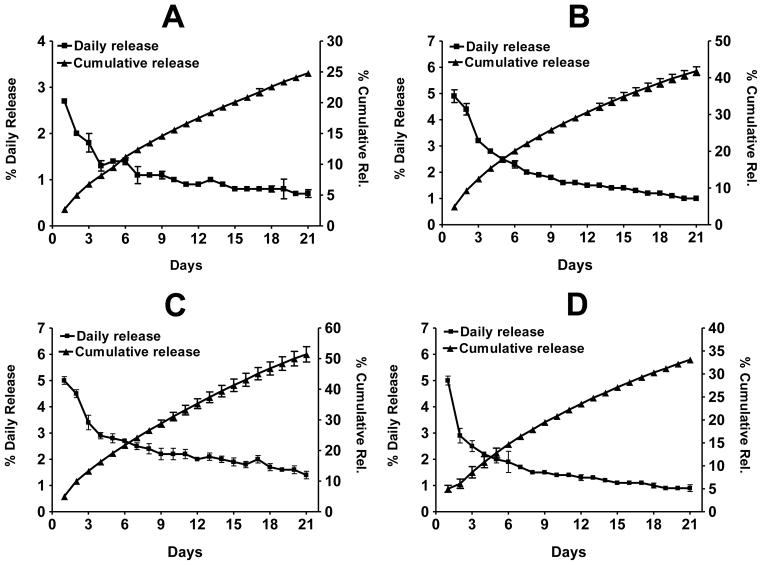
To determine the effect of substituents on the rate of release, coated implants were prepared from individual constituents of curcumin, i.e., curcumin I, curcumin II (demethoxycurcumin) and curcumin III (bisdemethoxy-curcumin). While no noticeable differences were found during the formulation of the coated implants, the three curcuminoids were released at different rates, with the cumulative release over 3 weeks being in the following descending order: curcumin III (51.4%), curcumin II (41.8%) and curcumin I (24.8%) (Figure 3). Thus, the presence of none (curcumin III), one (curcumin II) and two (curcumin I) methoxy groups in the curcuminoid structures (Figure 1A) significantly influenced the rates of release. These data suggest that the presence of lipophilic group(s) in the structure can reduce the rate of release of the compound from the implants. Curcumin implants resulted in a cumulative release of about 37% over 3 weeks.
Figure 3.
Percent average in vitro daily and cumulative release of curcumin I (A), curcumin II (B), curcumin III (C), and curcumin (D) from coated polymeric implants (2 cm, 2.6 mm dia; 10% drug load). The Implants were agitated in a shaker incubator in PBS supplemented with 10% bovine serum, and the medium was changed daily. The release was measured spectrophotometrically against the standard curve of individual compounds. Data represent average ± SD of 3 implants.
3.3 In vivo release
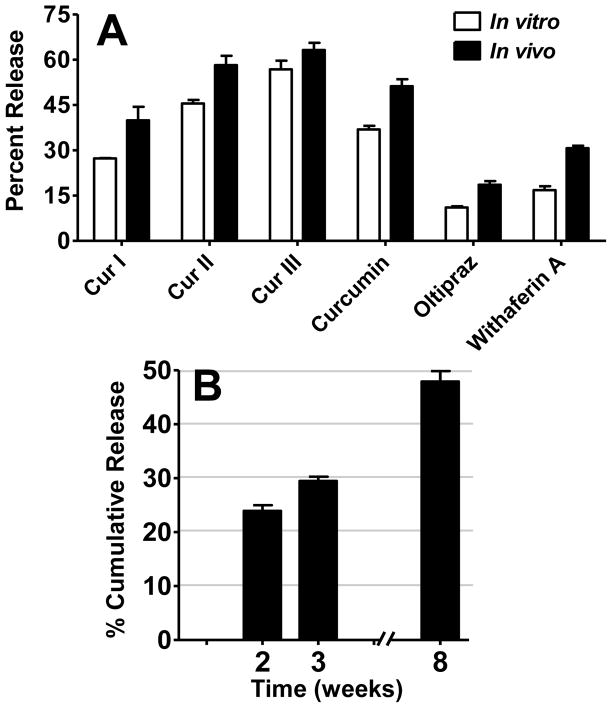
Measurement of the residual amounts in the implants recovered from the animals at the end of the 3 week study showed that the total release was in the following descending order: curcumin III (56.8%), Curcumin II (45.5%), curcumin (36.9%), withaferin A (30.7%), curcumin I (27.3%), and oltipraz (18.6%) (Figure 4A). Withaferin A release was also measured at additional time points, i.e., by euthanizing animals after 2 weeks and 8 weeks, in addition to the 3 weeks. The data presented in Figure 4B show that withaferin A continued to be released for 8 weeks, with over 50% of the compound still present in the implants. This release pattern suggests that withaferin A release could last for several more months. As expected, no physical change was observed in the withaferin A implants at the end of 8 weeks, since degradation of P80 implants can take over two years based on the time-dependent decrease in the molecular weights reported for this polymeric material [20].
Figure 4.
Rate of in vitro-in vivo release of curcuminoids (Cur-I, -II, and -III), curcumin, oltipraz and withaferin A (A) from coated implants grafted subcutaneously (two 1.5 cm, 2.6 mm dia; drug load 10% for curcuminoids and curcumin, 20% for oltipraz and 5% for withaferin A, respectively) in female A/J mice. Animals were euthanized after 3 weeks, and the implants recovered were solvent extracted to measure the residual amount as described in text.
In vivo cumulative release of withaferin A (B) from coated implants grafted subcutaneously (two 1.5 cm, 2.6 mm dia; 5% loads) in female A/J mice. Animals were euthanized after 2, 3 and 8 weeks, and the implants recovered were solvent extracted to measure the residual amount by UPLC as described in materials and methods section.
Comparison of the cumulative release over a period of 3 weeks in vivo and in vitro (Figures 4A) for all the test agents indicate that the in vivo release was 1.1 – 1.8-fold higher than the respective in vitro release. The higher release in the animal system presumably results from the differences in ‘sink’ conditions where large volume of circulating body fluid may play a major role in higher release followed by accumulation of the released compounds in the tissue environment. Further, the involvement of enzymes and other sources cannot be completely ruled out for the observed higher release.
3.4 The implant delivery is effective in diminishing DBP-induced tissue DNA adducts
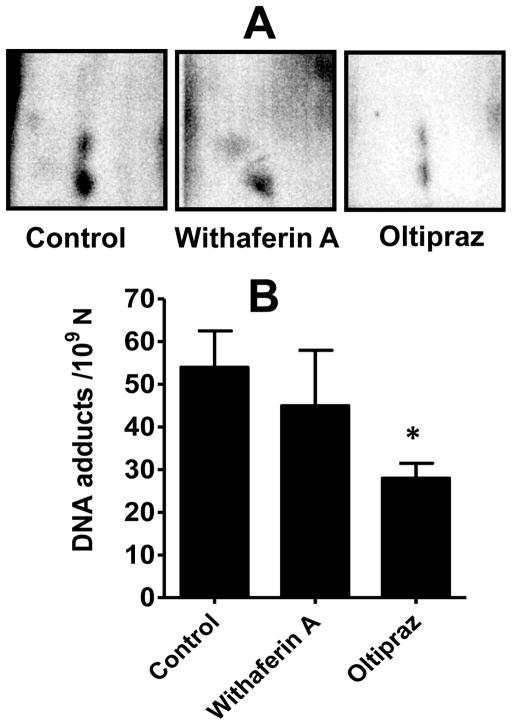
To determine the effect of chemopreventives delivered by the coated polymeric implants, as well as to determine their rates of release in vivo, groups of female A/J mice (n=4–5) were acclimated and then grafted subcutaneously with two (1.5 cm, 2.6 mm dia) implants each of oltipraz, withaferin A, curcumin and individual curcuminoids, and simultaneously challenged with low dose of the carcinogen, DBP via polymeric implants. Analysis of lung DNA by 32P-postlabeling showed multiple adducts (Figure 5A); no such adduct spots were detected in the sham-treated sample (maps not shown). The identity of DNA adducts has previously been established as guanine and adenine derivatives of the electrophilic metabolites, DBP-11,12-diol-13,14-epoxides [21]. No qualitative differences were observed in the adduct profiles in the absence or presence of test chemopreventives. However, quantitative differences were observed. Both withaferin A and oltipraz delivered via the implants showed reduction in the DNA adduct levels, but the reduction was statistically significant only with oltipraz (p<0.05) (Figure 5B). Intervention with curcumin and the individual curcuminoids showed virtually no effect on the adduct levels (not shown).
Figure 5.
Inhibition of DBP-DNA adducts in lung tissue of female A/J mice by chemopreventives delivered via implant route. Animals were treated with dibenzo[a, l]pyrene (DBP) by subcutaneous polymeric implants or implants of oltipraz and withaferin A (two 1.5 cm, 2.6 mm dia; 20% and 5% loads, respectively). Three weeks later animals were euthanized, and lung DNA adducts were analyzed by 32P-postlabeling. Data represent average ± SD of four animals. *p < 0.05.
3.5 Tissue distribution of curcumin and curcuminoids
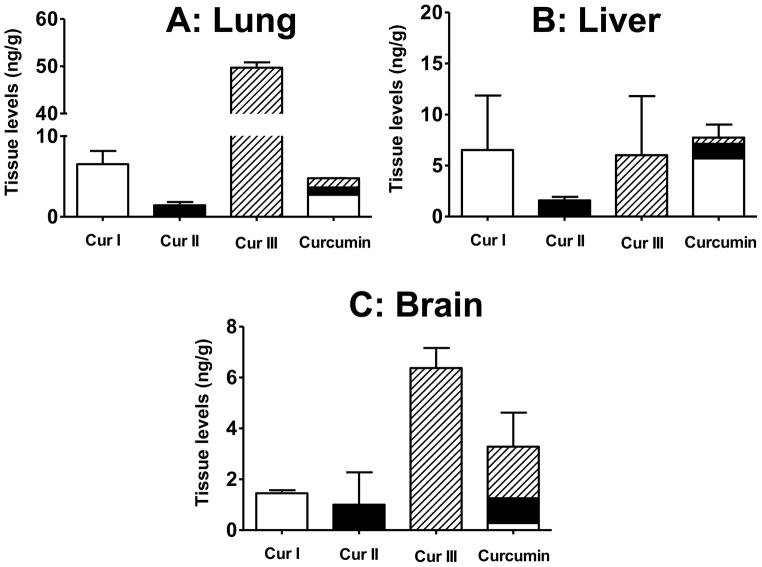
It is well known that when curcumin is administered orally, it exhibits poor bioavailability [17] and rapid metabolism both in intestine and liver [4; 22]. We failed to see an effect of curcuminoids on reduction of DNA adducts even when delivered by the implant route. To determine if this ineffectiveness was due to bioavailability issues, curcuminoids were measured in the lung; liver and brain were included for comparison. Curcuminoids were extracted from the lung, liver and brain tissues by solvent extraction and analyzed by UPLC using fluorescence detector. All the curcuminoids were readily detectable even 21 days after grafting the implants. When treated with implants of the individual curcuminoids, curcumin I was found at a level of 6.5 ± 1.6 ng/g lung whereas curcumin III was almost 8 fold higher (49.7 ± 1.3 ng/g lung tissue). However, curcumin II was present at the lowest level (1.4 ± 0.4 ng/g lung tissue). The three curcuminoids were, however, present at essentially the same compositions in the lung tissue when animals were treated with curcumin implants (Figure 6A). In the liver, curcumin I and III were found at similar levels (5–6 ng/g tissue); however, curcumin II was present in nearly 5 times lower levels (Figure 6B), suggesting this analog underwent more rapid metabolism. Interestingly, however, the three curcuminoids were found essentially in the same ratio (74:18:8) in the liver when animals were treated with curcumin.
Figure 6.
Curcuminoids levels in lung (A), liver (B) and brain (C) of female A/J mice treated with curcumin via polymeric implants (two, 1.5-cm implants; 10% drug load) for 21 days. Samples were analyzed by ultra performance liquid chromatography (UPLC) as described in materials and methods. Data represent average ± SD of 4 animals.
To determine if the individual curcuminoids administered by the implant could cross the blood-brain-barrier (BBB), the brain tissues were also analyzed. Data presented in Figure 6C demonstrate while curcumin I and II accumulated to similar levels (1.4 and 1 ng/g tissue, respectively), the levels of curcumin III were substantially higher (6.4 ng/g tissue). The higher accumulation of curcumin III in the brain was also evident when animals were treated with curcumin implants as curcumin I, II and III were found to be present in the ratios of 16:11:73, while in the original curcumin they were present in the ratios of 75:20:5. The higher levels of curcumin III in the brain may be a result of either readily crossing of the BBB and/or due to its lesser metabolism in the brain tissue. The higher accumulation of curcumin III in the brain may have biologically important consequences in view of the report that curcumin III is protective against Alzheimer’s in a cell culture model [23].
3.6 Toxicity studies
Animals were observed for the possible toxicity after subcutaneous polymeric implants embedded with chemopreventive agents (withaferin A and curcumin). There was no significant effect of sham or drug implants on the body weight. No significant differences in various hematological parameters (white blood cells, red blood cells, hemoglobin), liver enzymes (like aspartate transaminase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, amylase, or lipase), or biochemical parameters of kidney function (like blood urea nitrogen, creatinine, Ca2+, Na+, and Cl− levels) were observed with any of the compound administered via the implant compared with the sham implant 3 weeks after grafting. Furthermore, neutrophils, lymphocytes, monocytes, eosinophils, and basophils were also analyzed, and no statistical difference was observed in the animals treated with polymeric implants (data not shown).
3.7 Withaferin A delivery via polymeric implants significantly improves the therapeutic response
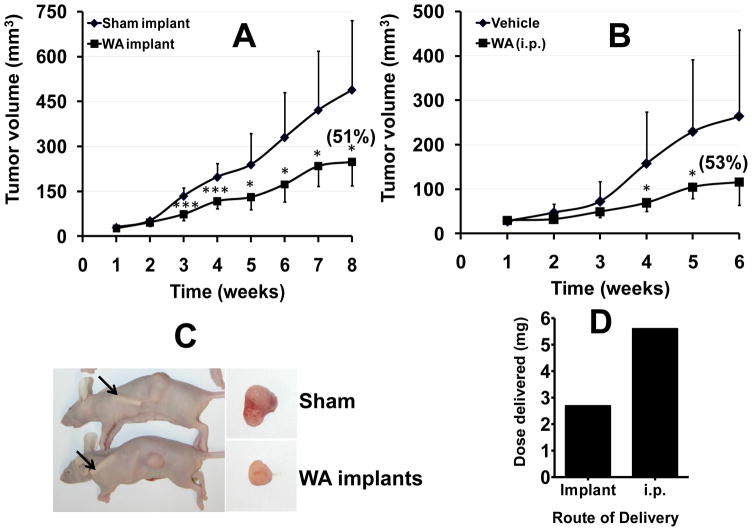
Newly discovered drugs are tested for their therapeutic activity generally in nude mouse xenograft models, and, in most studies reported, the compounds have been administered intraperitoneally or, in some cases, by gavage. Withaferin A has been reported to show potent therapeutic activity in our previous study [24], and from several other labs [25; 26; 27]. Even though withaferin A showed only modest reduction of carcinogen-induced DNA adducts, we tested its antitumor efficacy to determine if the required therapeutic dose can be reduced by the implant delivery. Thus, withaferin A was administered via the implants or i.p. Control groups received respective sham treatments. Measurement of the body weights weekly showed that sham and withaferin A-treated animals had essentially the same body weight at the end of the 8-week study, indicating that the drug via implants was well tolerated. Weekly measurements of the tumor volume showed a significant reduction of 51% in the growth of the tumor in the group receiving withaferin A via the implants (Figure 7A). Similar (53%) inhibition of the tumor was observed when withaferin A was given intraperitoneally (8 mg/kg) (Figure 7B); no reduction was observed when a lower dose of withaferin A (4 mg/kg) was administered intraperitoneally (not shown). The total dose of withaferin A administered via the two implants (2.69 mg), calculated based on the residual amount in the implants, was less than one half of the total dose (5.6 mg) given every other day i.p. (8 mg/kg). This difference in the total dose is expected to be significantly higher had withaferin A was delivered orally. Intraperitoneal route is used commonly in animal models to test efficacy of compounds but it is not applicable in humans, whereas implants can be potentially used for cancer patients. These data demonstrate that the implant delivery can achieve therapeutic dose of withaferin A and that the effective implant dose was 2.1-fold lower than the dose given intraperitoneally (Figure 7D).
Figure 7.
Polymeric implants of withaferin A (WA) inhibit human lung cancer A549 cell xenograft in nude mice. Following inoculation with human lung cancer A549 cells (3 × 106 cells), when tumor xenografts grew to over 50 mm3, animals were grafted with polymeric implants (two 1.5-cm implants, 2.6 mm dia; 5% load) (A). Two other groups, following inoculation with A549 cells, were treated intraperitoneally (i.p.) on alternate days with vehicle or withaferin A (8 mg/kg) two days after inoculation (B). Representative animals at the time of euthanasia and excised tumors (C). Total dose delivered by implant route and intraperitoneally (D). Control groups received respective sham treatments. Data represent average ± SD (n=8–10). *p < 0.05, **p <0.005, and ***p < 0.001.
Delivery of chemopreventive agents directly into systemic circulation offers multiple benefits including bypassing gut absorption and hepatic first-pass effect. These polymeric devices will reduce the effective dose by enhancing bioavailability as the drug is released directly in systemic circulation thereby reducing toxicity generally associated with high oral doses. Since multiple implants can be grafted, it will also help deliver a combination of chemopreventive agents targeting multiple pathways.
4. Conclusions
We conclude on a technical note that coated polymeric implant formulation allows the embedding of theoretically any compound in the polymeric matrix as long as the test agent is soluble in DCM or any other solvent (e.g., tetrahydrofurane, ethanol and acetone) which is miscible with DCM. Since the in vitro release of test agents correlate with the in vivo release, one can manipulate the in vivo dose based on the drug load or implant size or both. The release from the coated polymeric implants is either almost sustained (e.g., oltipraz), or it can be sustained by adding 6-8 coatings of blank polymers (data not shown). The polymeric implant formulation opens several new avenues as it accommodates heat-labile compounds, circumvents the problem of oral bioavailability, can deliver continuous (“24/7”) low doses for long duration (months), and allows in vivo efficacy assessment of minor plant constituents and synthetic metabolites which otherwise remains uninvestigated in vivo due to their insufficient quantities available.
Acknowledgments
This work was supported from the USPHS grants CA-118114, CA-125152 and CA-90892, Kentucky Lung Cancer Research Program, and the Agnes Brown Duggan Endowment. R.C.G. holds the Agnes Brown Duggan Chair in Oncological Research. We thank Sabinsa Corp. (East Windsor, NJ) for generously providing curcumin, individual curcuminoids and standardized extract of Withania somnifera. Mr. Adam Robinson, Associate Director of the University of Louisville Writing Center is acknowledged for editorial corrections.
Footnotes
Conflict of interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wattenberg LW. Chemoprophylaxis of carcinogenesis: a review. Cancer Res. 1966;26:1520–1526. [PubMed] [Google Scholar]
- 2.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of Chemical Carcinogenesis by Vitamin-a and Its Synthetic Analogs (Retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 3.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 4.Wahlang B, Pawar YB, Bansal AK. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm. 2011;77:275–282. doi: 10.1016/j.ejpb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 6.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Klitsch M. Hormonal Implants - the Next Wave of Contraceptives. Fam Plann Perspect. 1983;15:239. [PubMed] [Google Scholar]
- 9.Fulzele SV, Satturwar PM, Dorle AK. Study of the biodegradation and in vivo biocompatibility of novel biomaterials. Eur J Pharm Sci. 2003;20:53–61. doi: 10.1016/s0928-0987(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 10.Dickers KJ, Huatan H, Cameron RE. Polyglycolide-based blends for drug delivery: A differential scanning calorimetry study of the melting behavior. J Appl Polym Sci. 2003;89:2937–2939. [Google Scholar]
- 11.Bansal SS, Vadhanam MV, Gupta RC. Development and in vitro-in vivo evaluation of polymeric implants for continuous systemic delivery of curcumin. Pharm Res. 2011;28:1121–1130. doi: 10.1007/s11095-011-0375-z. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RC. 32P-postlabeling for detection of DNA adducts. In: Pfeifer GP, editor. Technologies for Detection of DNA Damage and Mutations. Plenum press; New York: 1996. pp. 45–61. [Google Scholar]
- 13.Gupta RC, Bansal SS, Aqil F, Jeyabalan J, Cao P, Kauser H, Russell GK, Ravoori S, Munagala R, Vadhanam MV. Controlled-release systemic delivery – a new concept in cancer chemoprevention. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs209. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyabalan J, Vadhanam MV, Ravoori S, Gupta RC. Sustained overexpression of CYP1A1 and 1B1 and steady accumulation of DNA adducts by low-dose, continuous exposure to benzo[a]pyrene by polymeric implants. Chem Res Toxicol. 2011;24:1937–1943. doi: 10.1021/tx2002788. [DOI] [PubMed] [Google Scholar]
- 15.Ravoori S, Vadhanam MV, Sahoo S, Srinivasan C, Gupta RC. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int J Oncol. 2007;31:113–120. [PubMed] [Google Scholar]
- 16.Gupta RC. 32P-postlabelling analysis of bulky aromatic adducts. IARC scientific publications; 1993. pp. 11–23. [PubMed] [Google Scholar]
- 17.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 18.Arif JM, Gairola CG, Glauert HP, Kelloff GJ, Lubet RA, Gupta RC. Inhibition of cigarette smoke-related lipophilic DNA adducts in rat tissues by dietary oltipraz. Carcinogenesis. 1998;19:1515–1517. doi: 10.1093/carcin/19.8.1515. [DOI] [PubMed] [Google Scholar]
- 19.Moon R, Rao K, Detrisac C, Kelloff G, Steele V, Doody L. Chemoprevention of respiratory-tract neoplasia in the hamster by oltipraz, alone and in combination. Int J Oncol. 1994;4:661–667. doi: 10.3892/ijo.4.3.661. [DOI] [PubMed] [Google Scholar]
- 20.Sun HF, Mei L, Song CX, Cui XM, Wang PY. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735–1740. doi: 10.1016/j.biomaterials.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Arif JM, Gupta RC. Microsome-mediated bioactivation of dibenzo[a,l]pyrene and identification of DNA adducts by 32P-postlabeling. Carcinogenesis. 1997;18:1999–2007. doi: 10.1093/carcin/18.10.1999. [DOI] [PubMed] [Google Scholar]
- 22.Ireson CR, Jones DJL, Orr S, Coughtrie MWH, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidem Biomar. 2002;11:105–111. [PubMed] [Google Scholar]
- 23.Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, Chiang B, Hui J, Mahanian M, Baghaee A, Hong P, Cashman J. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 25.Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, Tighiouart M, Vertino PM, Harvey RD, Garcia A, Marcus AI. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–2755. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 26.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol. 2009;9:614–619. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]