Abstract
Purpose
To prospectively evaluate the association between hippocampal dose and long-term neurocognitive function (NCF) impairment for benign or low-grade adult brain tumors treated with fractionated stereotactic radiotherapy (FSRT).
Methods and Materials
Adult patients with benign or low-grade adult brain tumors were treated with FSRT per institutional practice. No attempt was made to spare the hippocampus. NCF testing was conducted at baseline and 18 months follow-up, on a prospective clinical trial. Regression-based standardized z scores were calculated by using similar healthy control individuals evaluated at the same test–retest interval. NCF impairment was defined as a z score ≤ −1.5. After delineation of the bilateral hippocampi according to the Radiation Therapy Oncology Group contouring atlas, dose–volume histograms were generated for the left and right hippocampi and for the composite pair. Biologically equivalent doses in 2-Gy fractions (EQD2) assuming an α/β ratio of 2 Gy were computed. Fisher’s exact test and binary logistic regression were used for univariate and multivariate analyses, respectively. Dos–eresponse data were fit to a nonlinear model.
Results
Of 29 patients enrolled in this trial, 18 completed both baseline and 18-month NCF testing. An EQD2 to 40% of the bilateral hippocampi >7.3 Gy was associated with impairment in Wechsler Memory Scale-III Word List (WMS-WL) delayed recall (odds ratio [OR] 19.3; p = 0.043). The association between WMS-WL delayed recall and EQD2 to 100% of the bilateral hippocampi >0.0 Gy trended to significance (OR 14.8; p = 0.068).
Conclusion
EQD2 to 40% of the bilateral hippocampi greater than 7.3 Gy is associated with long-term impairment in list-learning delayed recall after FSRT for benign or low-grade adult brain tumors. Given that modern intensity-modulated radiotherapy techniques can reduce the dose to the bilateral hippocampi below this dosimetric threshold, patients should be enrolled in ongoing prospective trials of hippocampal sparing during cranial irradiation to confirm these preliminary results.
Keywords: Neurocognitive function, Hippocampus, Conformal radiotherapy, Radiation Therapy Oncology Group 0933, Brain tumors
Introduction
Although memory impairment has been a well-documented toxicity of cranial irradiation, a pathophysiologic explanation remains ill defined. One plausible hypothesis, focused on the role of neurogenic stem cells located in the subgranular layer of the hippocampal dentate gyrus, has been proposed on the basis of preclinical data demonstrating that this stem cell compartment is exquisitely sensitive to therapeutic doses of cranial irradiation (1). Preclinical models have demonstrated loss of hippocampal-dependent functions of spatial learning and memory, as tested by water maze tests, as a consequence of hippocampal irradiation (2, 3). However, to date, there is a paucity of clinical data to suggest that radiation dose to the hippocampus leads to long-term memory effects. We sought to address this hypothesis by conducting a prospective observational study of adult patients with benign or low-grade brain tumors treated with fractionated stereotactic radiotherapy (FSRT) and correlating hippocampal dose–volume histogram (DVH) data with long-term neurocognitive function (NCF) impairment.
Methods and Materials
Patient population
Adult patients with diagnoses of pathologically confirmed or clinically suspected benign or low-grade intracranial neoplasms were enrolled in this prospective protocol, approved by the University of Wisconsin Health Sciences Institutional Review Board. Eligible patients were >18 years of age, with no history of prior chemotherapy or radiotherapy. If biopsy or therapeutic resection was performed, patients were enrolled at least 1 week after biopsy and at least 4 weeks after resection.
Fractionated stereotactic radiotherapy
All patients were treated with FSRT by use of an optically guided intracranial radiotherapy system (4, 5). Treatment-planning computed tomography of the head was obtained with the patient in the supine position with custom bite-plate-fiducial-array-complex in place and subsequently fused to a three-dimensional spoiled gradient magnetic resonance imaging scan of the brain with gadolinium contrast medium and appropriate T2/FLAIR sequences. Gross and clinical target volumes were delineated per contemporary clinical practice. Planning target volume was generated by use of a 2-mm margin. Radiotherapy doses and fraction sizes were chosen on the basis of current clinical practice. All low-grade gliomas, meningiomas, and pituitary adenomas were treated to a dose of 50.4 to 54 Gy in 28 to 30 fractions of 1.8 Gy per fraction. All vestibular schwannomas were treated to a dose of either 20 Gy in five fractions of 4 Gy per fraction or 50.4 Gy in 28 fractions of 1.8 Gy per fraction. During treatment planning, no attempt was made to conformally avoid the hippocampus.
DVH analysis of hippocampus
After completion of FSRT, the bilateral hippocampi were delineated by use of a published contouring protocol currently in use in an ongoing cooperative-group Phase II trial of hippocampal avoidance during whole-brain radiotherapy (WBRT) for brain metastasis (Radiation Therapy Oncology Group [RTOG] 0933) (1, 6). This contouring protocol focuses on delineating the dentate gyrus and cornu ammonus, where neural progenitor cells important for memory-related function are believed to be anatomically clustered. This delineation was performed after completion of FSRT to ensure that no inadvertent effort at sparing the hippocampus would be undertaken during treatment planning. DVHs were generated for the left and right hippocampi individually and for the composite bilateral hippocampi. Doses were converted to biologically equivalent doses in 2-Gy fractions (EQD2) assuming an α/β ratio of 2 Gy. For this analysis, EQD2 to deciles (D10% to D100%), and the maximum EQD2 (Dmax), of individual and combined hippocampal volumes, were determined and tabulated.
Neurocognitive testing
All study participants underwent a battery of neurocognitive function (NCF) tests assessing estimated premorbid and current intelligence, language, visual perception, memory, executive function, and processing speed (Table 1).
Table 1.
Neurocognitive test battery administered at baseline and 18 months follow-up to all 18 experimental patients and 6 healthy control individuals
| Neurocognitive Function Test | Measure |
|---|---|
| Estimated premorbid function | |
| NART | Estimated premorbid intelligence |
| Intelligence | |
| WAIS Full Scale Intelligence Quotient | Adult intelligence |
| Language | |
| Boston Naming Test | Visual confrontation naming |
| Token Test | Language comprehension |
| Visual perception | |
| Judgment of Line Orientation | Visuospatial orientation |
| Facial Recognition Test | Discrimination of unfamiliar faces |
| Hooper Visual Organization Test | Visual integration |
| Memory | |
| Wechsler Memory Scale-III Word Lists (immediate and delayed recall) | Verbal memory |
| WMS-III Faces (immediate and delayed recall) | Visual memory |
| WMS-III Spatial Span and Letter-Number Sequencing | Working memory |
| Executive function | |
| Trail Making Test | Mental flexibility |
| Processing speed | |
| Wechsler Adult Intelligence Scale Digit Symbol and Symbol Search Tests | Cognitive and mental-motor speed |
| Stroop Test | Elemental cognitive processing speed |
Abbreviations: NART Z National Adult Reading Test; WAIS Z Wechsler Adult Intelligence Test; WMS Z Wechsler Memory Scale.
The test battery was 60 to 90 minutes long and was conducted at baseline (before FSRT was started) and at 18 months follow-up. A cohort of similarly aged healthy control individuals was also enrolled and administered the NCF test battery at the same test–retest interval. These control individuals were either biologic relatives (brother or sisters) or nonbiologic but sociologically similar relatives (spouses) of the patients. The baseline and retest scores of control individuals were used to derive predicted retest scores, controlling for known sources of error variance in test–retest paradigms (eg, regression to the mean, effects of age and education on retest performance). In this fashion, the nature and degree to which experimental patients’ test–retest performance was affected could be determined with precision.
Statistical analysis
The experimental and control individuals were compared by use of chi-squared statistics for categoric factors and the F statistic from analysis of variance for continuous factors. Descriptive statistics were generated for patient characteristics and NCF measures. Baseline scores for control individuals were regressed on retest scores with age, years of education, and sex as possible predictors for each cognitive measure. The resulting equations were then used to derive predicted retest scores for the experimental patients undergoing FSRT. The differences between predicted and observed retest scores for the experimental patients were transformed into standardized Z-scores using the equation:
| (1) |
NCF impairment was defined as a z-score negative change of at least −1.5 per standard clinical practice (7). For each hippocampal dosimetric parameter, patients were dichotomized into two groups around the median dose. Consistent with our hypothesis that hippocampal dosimetry is associated with NCF loss, a one-tailed Fisher’s exact test was used to compare high-dose vs. low-dose groups in terms of NCF impairment. In addition, dose–response data with NCF impairment as a binary outcome variable was fitted to a nonlinear model. In modeling the dose–response curve, control individuals were plotted at the origin because they did not receive irradiation and did not experience neurocognitive impairment over the study period. For the high-dose and low-dose groups, the percentage of patients with NCF impairment was plotted at the midpoint of each dose interval. These data points were then used to fit the dose–response curve model. A two-tailed Wald test was used to test the statistical significance of the model. For statistically significant dose–response relationships, estimates of , the hippocampal EQD2 leading to a 50% probability of NCF impairment, and m, the slope of the dose–response curve in the standard Lyman normal-tissue complication probability (NTCP) model without volume effect, were derived.
| (2) |
where,
For values of and m, a 95% confidence contour was derived by use of the model comparison method (via the F test) for the best-fit values. From this confidence contour, the 95% confidence interval values were estimated. With age being used as a categoric variable (≥50 vs. >50 years old), binary logistic regression was used to determine the independent effects of hippocampal dosimetric parameters on NCF impairment.
A p value <0.05 was considered statistically significant. Nonlinear modeling of the dose–response curve was carried out with the use of R (www.r-project.org). Remaining statistical analyses were performed by use of SPSS Statistics 19 (SPSS Inc., Chicago, IL).
Results
In this prospective study, 29 experimental patients and 12 healthy control individuals were enrolled and underwent baseline NCF testing. However, statistical analysis was limited to the 18 experimental patients and 6 healthy control individuals who also completed NCF testing at the 18-month follow-up. Comparison of patient characteristics between experimental patients and control individuals revealed no significant differences (Table 2). In addition, no significant differences were observed between patients who were and were not compliant with NCF testing at the 18-month follow-up (data not shown). Of the 9 patients with vestibular schwannoma, 5 were treated to a dose of 20 Gy in 4-Gy fractions, and 4 were treated to 50.4 Gy in 1.8-Gy fractions. Of the 4 patients with low-grade glioma, 2 were treated to 50.4 Gy, and 2 were treated to 54.0 Gy, in 1.8-Gy fractions. Of the 3 patients with meningioma, 2 were treated to 50.4 Gy and 1 was treated to 54 Gy, in 1.8-Gy fractions. Both patients with pituitary adenoma were treated to 50.4 Gy in 1.8-Gy fractions.
Table 2.
Comparison of experimental patients and healthy control individual
| Characteristic | Experimental cohort (n = 18) | Healthy control cohort (n = 6) | p value |
|---|---|---|---|
| Age | Median 56 Range 19–82 |
Median 54 Range 32–72 |
0.935 |
| Sex | Male: 10 (55.6%) Female: 8 (44.4%) |
Male: 3 (50.0%) Female: 3 (50.0%) |
0.590 |
| Years of education | Mean 16.0 Range 12–20 |
Mean 14.3 Range 12–18 |
0.155 |
| Dominant handedness | Right: 17 (94.4%) Left: 1 (5.6%) |
Right: 5 (83.3%) Left: 1 (16.7%) |
0.394 |
| Tumor type | Vestibular schwannoma: 9 (50.0%) Meningioma: 3 (16.7%) Low-grade glioma: 4 (22.2%) Pituitary adenoma: 2 (11.1%) |
N/A | |
| Planning target volume (cc) | Median 10.1 Range 1.2–316.7 |
Chi-squared statistics was used for categoric factors, and analysis of variance was used for continuous factors.
At the 18-month follow-up, all patients remained free of disease progression. NCF impairment was noted in 8.3% of patients for Trail Making Test A, 25.0% for Trail Making Test B, 4.2% for Wechsler Adult Intelligence Scale (WAIS)-III Digit Symbol Test, 0% for WAIS-III Symbol Search Test, 4.2% for Wechsler Memory Scale (WMS)-III Faces Immediate Recall Test, 33.3% for WMS-III Letter-Number Sequencing Test, 33.3% for WMS-III Spatial Span Test, 4.2% for WMS-III Faces Delayed Recall Test, 12.5% for WMS-III Word Lists (WMS-WL) Immediate Recall Test, 29.2% for WMS-WL Delayed Recall Tests, 12.5% for Letter/Semantic Fluency Test, 0% for Benton Judgment of Line Orientation Test, 12.5% for Boston Naming Test, 4.2% for Facial Recognition Test, 0% for Token Test, 12.5% for National Adult Reading Test, 20.8% for Full-Scale Intelligence Quotient Test, and 16.7 % for Hooper Visual Organization Test (HVOT).
Predictive relationships between impairment in each NCF measure and hippocampal dosimetric parameters were studied in detail for each hippocampus individually and for the bilateral hippocampi as a composite structure. Univariate analysis demonstrated statistical significance for the following associations:
Impairment in WMS-WL Delayed Recall and D40% >7.3 Gy of the bilateral hippocampi (p = 0.025) (Table 3)
Impairment in WMS-WL Delayed Recall and D100% >0.0 Gy (p = 0.047) of the bilateral hippocampi (Table 3)
Impairment in HVOT and Dmax >15.0 Gy of the left hippocampus (p = 0.041) (Supplementary Table)
Impairment in HVOT and D30% >6.2 Gy of the left hippocampus (p = 0.041) (Supplementary Table)
Table 3.
Association between hippocampal dosimetry and impairment in Wechsler Memory Scale-III Word Lists Delayed Recall at 18 months
| Dosimetry | Dosimetric cut point |
No impairment |
Impairment* |
p value |
|---|---|---|---|---|
| Bilateral hippocampi | ||||
| Maximum | ≤24.7 Gy | 66.7% | 33.3% | 0.500 |
| >24.7 Gy | 55.6% | 44.4% | ||
| D30% | ≤8.2 Gy | 77.8% | 22.2% | 0.167 |
| >8.2 Gy | 44.4% | 55.6% | ||
| D40% | ≤7.3 Gy | 88.9% | 11.1% | 0.025 |
| >7.3 Gy | 33.3% | 66.7% | ||
| D50% | ≤3.8 Gy | 66.7% | 33.3% | 0.500 |
| >3.8 Gy | 55.6% | 44.4% | ||
| D80% | ≤0.5 Gy | 55.6% | 44.4% | 0.500 |
| >0.5 Gy | 66.7% | 33.3% | ||
| D100% | ≤0.0 Gy | 76.9% | 23.1% | 0.047 |
| >0.0 Gy | 20.0% | 80.0% | ||
| Left hippocampus | ||||
| Maximum | ≤15.0 Gy | 55.6% | 44.4% | 0.500 |
| >15.0 Gy | 66.7% | 33.3% | ||
| D30% | ≤6.2 Gy | 66.7% | 33.3% | 0.500 |
| >6.2 Gy | 55.6% | 44.4% | ||
| D40% | ≤5.9 Gy | 77.8% | 22.2% | 0.167 |
| >5.9 Gy | 44.4% | 55.6% | ||
| D50% | ≤5.7 Gy | 77.8% | 22.2% | 0.167 |
| >5.7 Gy | 44.4% | 55.6% | ||
| D80% | ≤4.7 Gy | 77.8% | 22.2% | 0.167 |
| >4.7 Gy | 44.4% | 55.6% | ||
| D100% | ≤0.0 Gy | 44.4% | 55.6% | 0.167 |
| >0.0 Gy | 77.8% | 22.2% | ||
| Right hippocampus | ||||
| Maximum | ≤12.5 Gy | 55.6% | 44.4% | 0.500 |
| >12.5 Gy | 66.7% | 33.3% | ||
| D30% | ≤6.4 Gy | 66.7% | 33.3% | 0.500 |
| >6.4 Gy | 55.6% | 44.4% | ||
| D40% | ≤5.8 Gy | 66.7% | 33.3% | 0.500 |
| >5.8 Gy | 55.6% | 44.4% | ||
| D50% | ≤4.7 Gy | 66.7% | 33.3% | 0.500 |
| >4.7 Gy | 55.6% | 44.4% | ||
| D80% | ≤1.1 Gy | 77.8% | 22.2% | 0.167 |
| >1.1 Gy | 44.4% | 55.6% | ||
| D100% | ≤0.0 Gy | 57.1% | 42.9% | 0.485 |
| >0.0 Gy | 75.0% | 25.0% | ||
Abbreviations: D30% = equivalent dose in 2-Gy fractions (EQD2) assuming α/β=2 Gy to 30% of the structure volume; D40%=EQD2 to 40% of the structure volume; D50% = EQD2 to 50% of the structure volume; D80% = EQD2 to 80% of the structure volume; D100% = EQD2 to 100% of the structure volume; Dmax = maximum EQD2 to the structure volume.
Impairment defined by use of regression-based z scores standardized to similar healthy control individuals evaluated at the same test–retest interval. Impairment defined as z score ≤−1.5.
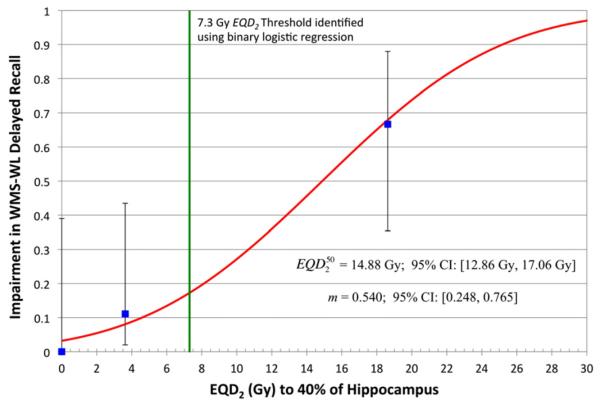
Remaining associations were not significant (selected data shown in Table 3 and Supplementary Table). Probit modeling of the relationship between D40% of the bilateral hippocampi and impairment in WMS-WL Delayed Recall was statistically significant (p < 0.01) and demonstrated an of 14.9 Gy (95% confidence interval [CI],12.9–17.1 Gy) and a value of m of 0.540 (95% CI, 0.248–0.765) (Fig. 1).
Fig. 1.
Dose–response relationship between equivalent dose in 2-Gy fractions (assuming α/β = 2 Gy) (EQD2) to 40% of the bilateral hippocampi (D40%) and impairment in Wechsler Memory Scale-III Word Lists (WMS-WL) Delayed Recall at 18 months. Experimental patients were dichotomized into two groups around the median dose (7.3 Gy), and the proportion of patients with impairment in WMS-WL Delayed Recall at 18 months was plotted (blue square) with 95% confidence intervals (95% CI) at the midpoint of each dose interval. Control individuals were included and plotted at the origin. D40% to the bilateral hippocampi leading to a 50% probability of WMS-WL Delayed Recall impairment () was estimated to be 14.88 Gy (95% CI, 12.86–17.06 Gy). The slope (m) of the dose–response curve in the standard Lyman normal-tissue complication probability NTCP model without volume effect was estimated to be 0.540 (95% CI, 0.248–0.765). The dose–response model was statistically significant (p < 0.01) on two-tailed Wald test.
With adjustment for age, binary logistic regression analysis of risk of impairment in WMS-III Word Lists Delayed Recall Test demonstrated a significant association with D40% to the bilateral hippocampi >7.3 Gy (odds ratio [OR] 19.3; p = 0.043) and an association that trended to significance with D100% to the bilateral hippocampi >0.0 Gy (OR 14.8; p = 0.068) (Table 4). Associations between HVOT impairment and maximum dose or D30% to the left hippocampus were nonsignificant (p = 0.999 for both dosimetric parameters). In addition, the association between risk of impairment in WMS-III Word Lists Delayed Recall Test and D40% to the bilateral hippocampi >7.3 Gy remained significant when adjustment was made for both age and planning target volume (p = 0.013).
Table 4.
Binary logistic regression analysis for risk of impairment in Wechsler Memory Scale-III Word Lists Delayed Recall at 18 months
| Variable | Odds ratio |
95% CI | p value |
|---|---|---|---|
| Age, y (≤50 vs. >50) | 1.5 | 0.1–20.9 | 0.774 |
| D40% of hippocampus >7.3 Gy | 19.3 | 1.1–338.0 | 0.043 |
| Age, y (≤50 vs. >50) | 1.2 | 0.1–15.8 | 0.876 |
| D100% of hippocampus>0.0 Gy | 14.8 | 0.8–266.2 | 0.068 |
Abbreviations: D40% = equivalent dose in 2-Gy fractions (EQD2) assuming α/β = 2 Gy to 40% of the structure volume; D100% = EQD2 to 100% of the structure volume; CI = confidence interval.
Discussion
In all adult mammals, including humans, new hippocampal granule cells are generated from mitotically active neural stem cells, which are located in the subgranular zone of the dentate gyrus and which migrate into the granular cell layer (8, 9). Preclinical evidence has associated neurogenesis within the dentate gyrus with normal cognitive function (10). Cranial irradiation in rat models has been observed to induce apoptosis of these precursor cells and to alter their differentiation toward a gliogenic fate, resulting in a significant reduction in hippocampal neurogenesis (11) and associated cognitive impairment (2). Thus, we hypothesize that radiation dose to the hippocampus plays a role, at least in part, in the pathophysiologic process of radiation-induced cognitive impairment.
To explore this hypothesis, we conducted a prospective controlled observational study of adult patients with benign or low-grade brain tumors treated with FSRT and observed a dose—response relationship between radiation dose to the hippocampal dentate gyrus and long-term memory impairment. Specifically, equivalent dose in 2-Gy fractions (EQD2) (assuming α/β = 2 Gy) to 40% of the bilateral hippocampi greater than 7.3 Gy is associated with long-term impairment in list-learning delayed verbal recall, as measured by the Wechsler Memory Scale-III Word Lists delayed recall test. In addition, fitting data to a nonlinear model using the standard Lyman NTCP model without volume effect demonstrates a significant dose–response relationship with an to 40% of the bilateral hippocampi of 14.9 Gy and a slope of m = 0.540. The asymmetric 95% confidence intervals for (12.9–17.1 Gy) and m (0.248–0.765) indicate that our estimates of these values are defined within a limited range. Similar results have been reported by Hsiao and colleagues, who observed a correlation between mean dose to the temporal lobes during intensity-modulated radiotherapy for nasopharyngeal carcinoma and subsequent decline in short-term memory and other cognitive domains (12). In addition, Jalali et al. observed a significant correlation between IQ decline and dose to the left temporal lobe (13). The summation of these clinical observations, therefore, provides a rationale for exploring the hypothesis that conformal avoidance of the hippocampus using intensity-modulated radiotherapy (IMRT) may spare patients some of the cognitive sequelae of cranial irradiation.
Clinical implementation of hippocampal sparing, however, poses several important challenges. Recently, we demonstrated the ability of modern IMRT techniques to selectively spare the bilateral hippocampi of significant doses of radiation during WBRT (6). For a prescription dose of 30 Gy in 10 fractions to the whole brain, our techniques were able to reduce the EQD2 received by 40% of the bilateral hippocampi (D40%) to approximately 4.6 to 5.4 Gy by use of helical tomotherapy and to approximately 7.1 to 7.5 Gy by use of linear accelerator-based IMRT (Fig. 2). Importantly, these values do not significantly exceed the D40% threshold of 7.3 Gy and are considerably lower than the to 40% of the bilateral hippocampi of 14.9 Gy established by this prospective observational study, implying that modern IMRT techniques may achieve sufficient hippocampal sparing to potentially prevent radiation-induced memory impairment. We seek to explore this hypothesis through prospective trials of hippocampal sparing during cranial irradiation. One such trial, RTOG 0933, is a multi-institutional Phase II clinical trial of hippocampal avoidance during WBRT in patients with brain metastases. In addition to serving as a feasibility study, RTOG 0933 also seeks to make a statistical comparison of impairment in Hopkins Verbal Learning Test (HVLT)-delayed recall in patients receiving hippocampal avoidance during WBRT with a historical control of patients who received WBRT without hippocampal avoidance. At the time of manuscript submission, RTOG 0933 has been activated and is currently enrolling patients.
Fig. 2.
Dose–volume histogram for hippocampal avoidance during whole-brain radiotherapy using (A) helical tomotherapy and (B) linear accelerator–based LINAC-based IMRT. Modified from (6) to include equivalent dose in 2-Gy fractions (assuming α/β = 2 Gy) (EQD2) along the X axis. EQD2 to 40% (D40%) of the bilateral hippocampi is 4.6 to 5.4 Gy by use of helical tomotherapy and 7.1 to 7.5 Gy by use of LINAC-based IMRT. These values do not significantly exceed the D40% threshold associated with long-term memory impairment on this study. PTV = whole brain planned target volume.
The selective effects of cranial irradiation on list-learning delayed verbal recall have been described in previous clinical trials of cranial irradiation. These trials have used the Hopkins Verbal Learning Test-Revised, which is similar in construct to the Wechsler Memory Scale-III Word Lists test, inasmuch as both involve multiple trials of new learning of a 12-item word list followed by delayed free recall. For instance, Chang and colleagues conducted a single-institution Phase III trial of stereotactic radiosurgery (SRS) with or without WBRT in patients with one to three brain metastases. That study was halted because of an interim observation of a twofold increase in the mean posterior probability of impairment in HVLT-delayed recall (52% SRS + WBRT vs. 24% SRS alone) (14). RTOG 0214 was a Phase III trial of prophylactic cranial irradiation vs. observation in locally advanced non–small-cell lung cancer. Despite not reaching target accrual, this trial also demonstrated a significantly greater decline in HVLT-delayed recall in the prophylactic cranial irradiation arm at 3, 6, and 12 months follow-up (15).
High death rates and noncompliance with NCF testing at long-term follow-up have prevented these clinical trials from providing data on the long-term memory effects of cranial irradiation. Our study encountered similar challenges: compliance with 18-month follow-up NCF testing was 62% among experimental patients and 50% among healthy control individuals. An 18-month interval was selected in our study to avoid focus on short-term adverse cognitive changes; instead, we attempted to identify more long-standing and likely fixed cognitive declines. The complication of such a long test–retest interval is the potential inability to maintain compliance by the research participants. Importantly, baseline characteristics did not differ between patients who were compliant and those who were non-compliant with the 18-month NCF follow-up. However, because of the limited sample size, the results could not be adjusted for multiple testing of correlations and therefore should be considered preliminary at this point.
In addition, we unexpectedly observed impairment in working memory, as assessed by the Letter-Number Sequencing and Spatial Span tests, in a third of patients treated with FSRT. Douw and colleagues have similarly demonstrated long-term impairment in working memory after cranial irradiation for low-grade gliomas (16). Working memory, as originally conceived by Baddeley’s model, has been shown to be independent of the hippocampus and related temporal lobe structures (17). In that regard, the absence of correlation between impairment in working memory and hippocampal radiation dose in our study is not surprising. However, radiation dose to other regions of the brain such as the prefrontal cortex and striatum may play a role, and efforts are ongoing to explore these hypotheses (18).
Conclusion
Preclinical evidence suggests that the neurogenic stem cell compartment in the hippocampal dentate gyrus is central to radiation-induced cognitive impairment after cranial irradiation. In this prospective observational study of adult patients with benign or low-grade brain tumors treated with FSRT, we observed a dose relationship between EQD2 to the bilateral hippocampi and likelihood of long-term memory impairment. Specifically, we observed that a EQD2 to 40% of the bilateral hippocampi (D40%) greater than 7.3 Gy predicts for impairment in list-learning delayed verbal recall, assessed by the Wechsler Memory Scale-III Word Lists at the 18-month follow-up. Given the ability of modern IMRT techniques to spare the hippocampus of doses in excess of this dosimetric threshold during 30-Gy WBRT, we seek to explore this finding in ongoing and future clinical trials of hippocampal sparing during cranial irradiation.
Supplementary Material
Acknowledgments
Supported by grant R01-CA109656 (PI: W.A. Tomé) from the National Cancer Institute of the United States of America.
Footnotes
Conflict of interest: Minesh Mehta has or has had the following roles in the past 2 years (2010–2011): Consultant: Adnexus, Bayer, Merck, Roche, Tomotherapy; Stock options: Colby, Pharmacyclics, Procertus, Stemina Tomotherapy; Data Safety Monitoring Boards: Apogenix; Board of Directors: Pharmacyclics; Medical Advisory Boards: Colby, Stemina, Procertus; Speaker: Merck. The authors report no other conflict of interest.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 3.Madsen TM, Kristjansen PE, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 4.Tomé WA, Meeks SL, McNutt TR, et al. Optically guided intensity modulated radiotherapy. Radiother Oncol. 2001;61:33–44. doi: 10.1016/s0167-8140(01)00414-5. [DOI] [PubMed] [Google Scholar]
- 5.Tomé WA, Meeks SL, Buatti JM, et al. A high-precision system for conformal intracranial radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1137–1143. doi: 10.1016/s0360-3016(00)00502-2. [DOI] [PubMed] [Google Scholar]
- 6.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: A “how-to” technique using helical tomotherapy and linear accelerator—based intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermann BP, Seidenberg M, Schoenfeld J, et al. Empirical techniques for determining the reliability, magnitude, and pattern of neuropsychological change after epilepsy surgery. Epilepsia. 1996;37:942–950. doi: 10.1111/j.1528-1157.1996.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 8.Cameron HA, Woolley CS, McEwen BS, et al. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 10.Lemaire V, Koehl M, Le Moal M, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao KY, Yeh SA, Chang CC, et al. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: A prospective study. Int J Radiat Oncol Biol Phys. 2010;77:722–726. doi: 10.1016/j.ijrobp.2009.06.080. [DOI] [PubMed] [Google Scholar]
- 13.Jalali R, Mallick I, Dutta D, et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:974–979. doi: 10.1016/j.ijrobp.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 15.Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol. 2010;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009;8:810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 17.Shrager Y, Levy DA, Hopkins RO, et al. Working memory and the organization of brain systems. J Neurosci. 2008;28:4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller NG, Knight RT. The functional neuroanatomy of working memory: Contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.