Abstract
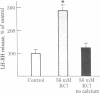
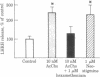
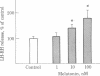
We have examined the release of radioimmunoassayable luteinizing hormone-releasing hormone (LH-RH) from fragments of rat medial basal hypothalamus. These fragments were cultured overnight in medium containing serum and then preincubated in groups of three for 10 min in medium resembling cerebrospinal fluid in its electrolyte constituents and containing bacitracin. This was followed by 30-min incubation periods during which some of the hypothalami were exposed to test substances. Potassium depolarization, effected by the addition of 56 mM potassium chloride to the incubation medium, caused a marked stimulation in LH-RH release, but only in the presence of calcium. Acetylcholine at 10 nM and the parasympathomimetic anticholinesterase agent neostigmine at 1 microM markedly stimulated LH-RH release. Hexamethonium, a nicotinic antagonist, at 1 microM abolished the acetylcholine-induced increment in LH-RH release. Melatonin, a pineal indolamine, caused significant stimulation of LH-RH release at a concentration as low as 10 nM. Bacitracin (21 microM) was employed in all these experiments. It had no effect on LH-RH release but did prevent the degradation of LH-RH in this system. We conclude that acetylcholine and melatonin are capable of inducing LH-RH release from the rat medial basal hypothalamus. These actions may account for some of the progonadotropic properties previously ascribed to these agents.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti W. L., Lozdziejsky R., Sala M. A., Monti J. M., Griñ E. Blockade of ovulation after atropine implants in the lateral hypothalamus of the rat. Experientia. 1969 Nov 15;25(11):1158–1159. doi: 10.1007/BF01900248. [DOI] [PubMed] [Google Scholar]
- Bennett G. W., Edwardson J. A., Holland D., Jeffcoate S. L., White N. Release of immunoreactive luteinising hormone-releasing hormone and thyrotrophin-releasing hormone from hypothalamus. Nature. 1975 Sep 25;257(5524):323–325. doi: 10.1038/257323a0. [DOI] [PubMed] [Google Scholar]
- Bigdeli H., Snyder P. J. Gonadotropin releasing hormone release from the rat hypothalamus: dependence on membrane depolarization and calcium influx. Endocrinology. 1978 Jul;103(1):281–286. doi: 10.1210/endo-103-1-281. [DOI] [PubMed] [Google Scholar]
- CHU E. W., WURTMAN R. J., AXELROD J. AN INHIBITORY EFFECT OF MELATONIN ON THE ESTROUS PHASE OF THE ESTROUS CYCLE OF THE RODENT. Endocrinology. 1964 Aug;75:238–242. doi: 10.1210/endo-75-2-238. [DOI] [PubMed] [Google Scholar]
- Copeland K. C., Aubert M. L., Rivier J., Sizonenko P. C. Luteinizing hormone-releasing hormone: sequential versus conformational specificity of antiluteinizing hormone-releasing hormone sera. Endocrinology. 1979 May;104(5):1504–1512. doi: 10.1210/endo-104-5-1504. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. Stimulation of uptake of calcium-45 in the adrenal gland by acetylcholine. Nature. 1961 Dec 30;192:1299–1299. doi: 10.1038/1921299a0. [DOI] [PubMed] [Google Scholar]
- Fiske V. M., MacDonald G. J., Bigelow L. L., Altschule M. D. Estrogen and progesterone secretion in monkeys (Macaca fascicularis) following melatonin or Altschule's pineal extrac. Fertil Steril. 1975 Jun;26(6):613–616. doi: 10.1016/s0015-0282(16)41181-7. [DOI] [PubMed] [Google Scholar]
- Fraschini F., Mess B., Martini L. Pineal gland, melatonin and the control of luteinizing hormone secretion. Endocrinology. 1968 May;82(5):919–924. doi: 10.1210/endo-82-5-919. [DOI] [PubMed] [Google Scholar]
- Gallardo E., Ramirez V. D. A method for the superfusion of rat hypothalami: secretion of luteinizing hormone-releasing hormone. Proc Soc Exp Biol Med. 1977 May;155(1):79–84. doi: 10.3181/00379727-155-39749. [DOI] [PubMed] [Google Scholar]
- Green A. R., Koslow S. H., Costa E. Identification and quantitation of a new indolealkylamine in rat hypothalamus. Brain Res. 1973 Mar 15;51:371–374. doi: 10.1016/0006-8993(73)90392-2. [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O., Tokuyama T., Seno S. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 1974 Feb 25;249(4):1311–1313. [PubMed] [Google Scholar]
- Hirooka Y., Hollander C. S., Suzuki S., Ferdinand P., Juan S. I. Somatostatin inhibits release of thyrotropin releasing factor from organ cultures of rat hypothalamus. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4509–4513. doi: 10.1073/pnas.75.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. T., Hillhouse E. W., Burden J. L. Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. J Endocrinol. 1977 Jun;73(3):405–417. doi: 10.1677/joe.0.0730405. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical localization of cholinesterases in the central nervous system of the rat. J Comp Neurol. 1954 Feb;100(1):211–235. doi: 10.1002/cne.901000108. [DOI] [PubMed] [Google Scholar]
- Kamberi I. A. The role of brain monoamines and pineal indoles in the secretion of gonadotrophins and gonadotrophin-releasing factors. Prog Brain Res. 1973;39:261–280. doi: 10.1016/S0079-6123(08)64083-2. [DOI] [PubMed] [Google Scholar]
- Kao L. W., Weisz J. Release of gonadotrophin-releasing hormone (Gn-RH) from isolated, perifused medial-basal hypothalamus by melatonin. Endocrinology. 1977 Jun;100(6):1723–1726. doi: 10.1210/endo-100-6-1723. [DOI] [PubMed] [Google Scholar]
- Koslow S. H., Racagni G., Costa E. Mass fragmentographic measurement of norepinephrine dopamine, serotonin and acetylcholine in seven discrete nuclei of the rat tel-diencephalon. Neuropharmacology. 1974 Dec;13(12):1123–1130. doi: 10.1016/0028-3908(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Krnjević K. Central cholinergic pathways. Fed Proc. 1969 Jan-Feb;28(1):113–120. [PubMed] [Google Scholar]
- Libertun C., Timiras P. S., Kragt C. L. Sexual differences in the hypothalamic cholinergic system before and after puberty: inductory effect of testosterone. Neuroendocrinology. 1973;12(2):73–85. doi: 10.1159/000122157. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- McKelvy J. F., LeBlanc P., Laudes C., Perrie S., Grimm-Jorgensen Y., Kordon C. The use of bacitracin as an inhibitor of the degradation of thyrotropin releasing factor and luteinizing hormone releasing factor. Biochem Biophys Res Commun. 1976 Nov 22;73(2):507–515. doi: 10.1016/0006-291x(76)90736-1. [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A., Ojeda S. R., McCann S. M. Catecholaminergic modulation of luteinizing hormone-releasing hormone release by median eminence terminals in vitro. Endocrinology. 1979 Jun;104(6):1749–1757. doi: 10.1210/endo-104-6-1749. [DOI] [PubMed] [Google Scholar]
- Nett T. M., Akbar A. M., Niswender G. D., Hedlund M. T., White W. F. A radioimmunoassay for gonadotropin-releasing hormone (Gn-RH) in serum. J Clin Endocrinol Metab. 1973 May;36(5):880–885. doi: 10.1210/jcem-36-5-880. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Negro-Vilar A., McCann S. M. Release of prostaglandin Es by hypothalamic tissue: evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology. 1979 Mar;104(3):617–624. doi: 10.1210/endo-104-3-617. [DOI] [PubMed] [Google Scholar]
- Orts R. J., Kocan K. M., Wilson I. B. Inhibitory action of melatonin on a pineal antigonadotropin. Life Sci. 1975 Sep 15;17(6):845–850. doi: 10.1016/0024-3205(75)90434-8. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Lynch H. J., Wurtman R. J. Melatonin in rat pineal, plasma, and urine: 24-hour rhythmicity and effect of chlorpromazine. Endocrinology. 1976 Jun;98(6):1418–1424. doi: 10.1210/endo-98-6-1418. [DOI] [PubMed] [Google Scholar]
- Parvizi N., Ellendorff F. Norepinephrine and luteinizing hormone secretion: intrahypothalamic and intraventricular microinjections of norepinephrine. Brain Res. 1978 Jun 16;148(2):521–525. doi: 10.1016/0006-8993(78)90741-2. [DOI] [PubMed] [Google Scholar]
- Peat F., Kinson G. A. Testicular steroidogeneis in vitro in the rat in response to blinding, pinealectomy and to the addition of melatonin. Steroids. 1971 Feb;17(2):251–264. doi: 10.1016/s0039-128x(71)80128-9. [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Blask D. E., Vaughan M. K. A counter antigonadotrophic effect of melatonin in male rats. Neuroendocrinology. 1975;19(1):72–80. doi: 10.1159/000122427. [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Vaughan M. K., Vaughan G. M. Melatonin action on the time course of compensatory ovarian hypertrophy in the Swiss-Webster mouse. Int J Fertil. 1972;17(1):59–62. [PubMed] [Google Scholar]
- Reiter R. J., Vaughn M. K., Blask D. E., Johnson L. Y. Melatonin: its inhibition of pineal antigonadotrophic activity in male hamsters. Science. 1974 Sep 27;185(4157):1169–1171. doi: 10.1126/science.185.4157.1169. [DOI] [PubMed] [Google Scholar]
- Richardson S. B., Hollander C. S., D'Eletto R., Greenleaf P. W., Thaw C. Acetylcholine inhibits the release of somatostatin from rat hypothalamus in vitro. Endocrinology. 1980 Jul;107(1):122–129. doi: 10.1210/endo-107-1-122. [DOI] [PubMed] [Google Scholar]
- Richardson S. B., Hollander C. S., Prasad J. A., Hirooka Y. Somatostatin release from rat hypothalamus in vitro: effects of melatonin and serotonin. Endocrinology. 1981 Aug;109(2):602–606. doi: 10.1210/endo-109-2-602. [DOI] [PubMed] [Google Scholar]
- Roche J. F., Foster D. L., Karsch F. J., Dziuk P. J. Effect of castration and infusion of melatonin on levels of luteinizing hormone in sera and pituitaries of ewes. Endocrinology. 1970 Dec;87(6):1205–1210. doi: 10.1210/endo-87-6-1205. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Frazier G. R. Statistical analysis of radioligand assay data. Methods Enzymol. 1975;37:3–22. doi: 10.1016/s0076-6879(75)37003-1. [DOI] [PubMed] [Google Scholar]
- Rotsztejn W. H., Charli J. L., Pattou E., Kordon C. Stimulation by dopamine of luteinizing hormone-releasing hormone (LHRH) release from the mediobasal hypothalamus in male rats. Endocrinology. 1977 Nov;101(5):1475–1483. doi: 10.1210/endo-101-5-1475. [DOI] [PubMed] [Google Scholar]
- Rotsztejn W. H., Drouva S. V., Pattou E., Kordon C. Met-enkephalin inhibits in vitro dopamine-induced LHRH release from mediobasal hypothalamus of male rats. Nature. 1978 Jul 20;274(5668):281–282. doi: 10.1038/274281a0. [DOI] [PubMed] [Google Scholar]
- Simonovic I., Motta M., Martini L. Acetylcholine and the release of the follicle-stimulating hormone-releasing factor. Endocrinology. 1974 Nov;95(5):1373–1379. doi: 10.1210/endo-95-5-1373. [DOI] [PubMed] [Google Scholar]
- Weinberg U., D'Eletto R. D., Weitzman E. D., Erlich S., Hollander C. S. Circulating melatonin in man: episodic secretion throughout the light-dark cycle. J Clin Endocrinol Metab. 1979 Jan;48(1):114–118. doi: 10.1210/jcem-48-1-114. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Mieno M., Shimizu T., Yamashita E. Inhibition by melatonin of the pituitary response to luteinizing hormone releasing hormone in vivo. J Endocrinol. 1978 Mar;76(3):487–491. doi: 10.1677/joe.0.0760487. [DOI] [PubMed] [Google Scholar]