Abstract
We have determined high resolution crystal structures of a CDK2/CyclinA transition-state complex bound to ADP, substrate peptide and MgF3−. Compared to previous structures of active CDK2, the catalytic subunit of the kinase adopts a more closed conformation around the active site and now allows observation of a second Mg2+ ion in the active site. Coupled with a strong [Mg2+] effect on in vitro kinase activity, the structures suggest that the transient binding of the second Mg2+ ion is necessary to achieve maximum rate-enhancement of the chemical reaction and Mg2+ concentration could represent an important regulator of CDK2 activity in vivo. Molecular dynamics simulations illustrate how the simultaneous binding of substrate peptide, ATP and two Mg2+ ions is able to induce a more rigid and closed organization of the active site that functions to orient the phosphates, stabilize the buildup of negative charge, and shield the subsequently activated γ-phosphate from solvent.
Introduction
Protein kinases represent one of the fundamental components of cell signaling pathways in all organisms. CDK2 is a mammalian Ser/Thr kinase that plays a critical role in controlling the progression from G1 to S phase of the cell cycle(Morgan, 1997). CDK2 is functionally homologous to the well-studied cdc28a S.cerevisae protein. Consistent with its important role in influencing progression through the cell cycle, the activity of the enzyme is subject to many levels of regulation. Misregulation of CDK2 activity, for example through mutation, may contribute to the development of human cancers(Greenman et al., 2007; Malumbres and Barbacid, 2007) and CDK2 represents a potential therapeutic target(Malumbres and Barbacid, 2009). The isolated 34 kDa catalytic subunit of CDK2 exhibits relatively negligible catalytic activity and the cellular concentration remains constant through the cell cycle. Maximum protein kinase activity is not obtained until the catalytic subunit is bound by an allosteric Cyclin protein(Radzio-Andzelm et al., 1995) and the catalytic domain has been phosphorylated on Thr-160, located within the kinase “activation loop” motif(Stevenson et al., 2002). The binding of Cyclin and the phosphorylation of Thr-160 have both been shown to stabilize large-scale conformational changes in the catalytic domain that function both to increase affinity for substrate (reduce KM) as well as enhance the catalytic efficiency of the reaction (increase kcat)(Brown et al., 1999b; Pavletich, 1999). In addition to allosterically up-regulating catalytic activity upon binding to the catalytic domain, the Cyclins are also able to assist in the recruitment of specific protein substrates by binding to recruitment motifs. CDK2 is negatively regulated by the binding of a number of inhibitory proteins such as the p21Cip and p27KIP families, as well as by the phosphorylation of Tyr-15 and Thr-14 within the catalytic subunit. Multiple crystal structures are available for a number of the functional states of CDK2(Pavletich, 1999).
Because they carry out very similar chemistries, it has been suggested that the majority of protein kinases may be subject to functional restrictions that require them to adopt very similar conformations when they are in their catalytically competent state. Hence it could be the differences between their catalytically down-regulated structures that might be responsible for much of their functional diversity(Huse and Kuriyan, 2002). While there is growing evidence to support this hypothesis, direct structural and experimental data on active kinases caught in the act of catalysis has been difficult to obtain. Many enzymes catalyze similar reactions that liberate the γ-phosphate from a nucleotide tri-phosphate (NTP) but details such as the nature of the stabilizing protein side-chains or the number of catalytic metals are not always conserved. Given the large number of protein kinases and the diverse signaling pathways they take part in, we can not assume that they all operate using identical chemistries. For example, while many protein kinases are believed to require two divalent metal ions for optimum catalysis (Adams, 2001), it was recently established that at least one protein kinase, CASK, is only active in the complete absence of any divalent ions(Mukherjee et al.). There is structural evidence that many kinases may only utilize a single divalent ion. The potential difference in the number of Mg2+ ions utilized by different kinases is especially important in the context of looking at the entire ensemble of over 500 protein kinases in the human genome(Manning et al., 2002) as identifying any differences in the details of how individual enzymes catalyze the reaction could provide important insights into the origins of functional and regulatory diversity among kinases. This information could also contribute to our understanding of how activating mutations result in misregulation of specific kinases and could even be helpful in the rational design of specific protein kinase inhibitors. Kinase specific differences in the function of the highly conserved “DFG” motif, which is essential for coordinating active site Mg2+ ions, are believed to be a critical determinant of the specificity profile of the clinically successful kinase inhibitor Gleevec as well as other DFG-out or “type-II” kinase inhibitors(Liu and Gray, 2006).
The most detailed model for the transition-state conformation of a protein kinase is the AlF3 transition state (TS) mimic of Protein Kinase A (PKA) (Madhusudan et al., 2002). This 2.0Å structure highlights a significant point of distinction between PKA and CDK2: all published structures of CDK2 bound to nucleotides identify at most a single Mg2+ ion in the active site, while the PKA TS structure clearly contains two catalytic Mg2+ ions that are in direct coordination with the reactive phosphates. Many NTP enzymes utilize a single metal ion and a one Mg2+ mechanism for CDK2 is certainly reasonable. A structure of active CDK2 bound to nitrate (NO3−), a potential TS mimic, has been determined, showing a single Mg2+ ion in the active site(Cook et al., 2002), and a single Mg2+ mechanism for CDK2 has been proposed and shown to be energetically feasible via quantum mechanical simulations(Vivo et al., 2007). Given the flexible nature of protein kinases, it is likely that a number of different protein conformations and ligand/activator states are sampled along the reaction cycle and that different structures may indeed represent different conformations within the same ensemble of transiently occupied states. Extensive studies of PKA support the notion that the role of Mg2+ ions at different stages of the catalytic cycle is dynamic and rather complex, with one of the two Mg2+ ions referred to as “essential” and the second as “inhibitory” (Shaffer and Adams, 1999b). It is still uncertain, therefore, whether CDK2 utilizes a single Mg2+ ion for the phosphoryl-transfer step and is truly different from the two Mg2+ (2Mg) mechanism observed in PKA or whether a 2Mg CDK2 state has just not yet been observed.
A related and also elusive question in the mechanism of protein kinases is the possible role that protein dynamics may play in both the catalytic mechanism as well as the regulation of catalytic activity. Structures of a number of protein kinases have revealed how flexible the catalytic domain can be, especially in facilitating the disassembly of the catalytically competent active site to adopt a down-regulated state. It has been proposed that kinases, like other enzymes, may have evolved to channel or funnel specific protein motions in order to assist the reactants in sampling and progressing through the high-energy transition state of the reaction(Henzler-Wildman et al., 2007; Kamerlin and Warshel, 2009) but this idea is difficult to prove(Kamerlin and Warshel, 2009; Li et al.). Once again, the challenges associated with obtaining experimental and structural data on protein kinases at each step of the catalytic reaction has made it difficult to fully understand the functional roles of protein dynamics and its differences within the divergent protein kinases.
To better understand the detailed mechanism of the CDK2 kinase reaction, we have determined new crystal structures of an active Phosho-CDK2/CyclinA complex bound to ADP, a substrate peptide, and MgF3−, a mimic for the γ-phosphate of ATP in the TS. We believe this complex provides an informative model of the structure of the enzyme during the phosphoryl-transfer step, one of the key rate-limiting steps of the reaction. We have carried out a series of molecular dynamics (MD) simulations to investigate the possible roles of protein dynamics in the catalytic mechanism of CDK2. Based on these results, we propose that the transient binding of a second activating Mg2+ ion to the active site of CDK2 helps facilitate the phosphoryl-transfer step by simultaneously closing down the active site by expelling water, electrostatically stabilizing the reactants, and reducing the conformational flexibility of the ATP.
Results
Overall structure of the complex
The architecture of the phosphorylated P-Thr160 CDK2/CyclinA complex (pCDK2/CyclinA) bound to ADP/MgF3−/peptide is shown in Figure 1a. We have determined two independent but very similar structures of the complex, solved at slightly different pHs. The overall conformation of the complex in the transition state structures is similar to previously published structures of P-Thr160 CDK2 bound to CyclinA, all crystallized in different buffer conditions and different space groups. The overall Cα RMSD to an ATP-γS bound CDK2/Cyclin A complex is 1.1Å (Russo et al., 1996), and Cα RMSD to an AMPPNP/peptide CDK2/Cyclin A complex is 0.7Å (Brown et al., 1999a).
Figure 1. Structure of pCDK2/CyclinA-ADP/MgF3−/peptide complex and details of the active site.
A) Overview of the complex. Catalytic subunit is grey, CyclinA is yellow. B) Close-up of the protein active site (region highlighted in panel a) showing the ADP, Mg ions, MgF3-, and the peptide substrate (green). The C-terminal region of a second copy of the substrate peptide (PKTPKKAKKL) was determined to have bound to the “RXL” substrate binding groove on CyclinA and is also shown in green. The N-terminal region of this peptide shown in grey is largely disordered and was modeled at 0 occupancy. C) fo-fc difference map (5σ) from an averaged kicked omit map where the MgF3− was omitted from the model. D–F) Three views of the 2fo-fc electron density (blue=1.25σ magenta=3.5σ) at the active site. Red spheres are waters, green spheres are Mg2+. See also Supplementary Figure 1.
A close-up view of the pCDK2/CyclinA active site is shown in Figure 1b. Each structure contains two copies of the CDK2/CyclinA complex and we can clearly visualize the ADP, the 10-residue substrate peptide, and a trigonal-planar MgF3− ion positioned between the ADP β-phosphate and the Thr γ-O atom of the substrate peptide in each of them. A 2Fo-Fc electron density map of the reactants is shown in Figure 1d-f, including distinct density for the two Mg2+ ions we observe. Each Mg2+ is in direct van der Waals contact with one of the fluorine atoms of the MgF3− phosphate mimic as well as five additional electronegative oxygen atoms at distances of 1.9–2.5Å, described in more detail below.
MgF3− transition-state mimic
A number of different chemical species have been employed along with an NDP to mimic the γ-phosphate TS in NTP reactions, including AlF3, AlF4−, BeF3, NO3−, and vanadate. Different species work best with different enzymes. Here we use MgF3− as a TS mimic of the γ-phosphate of ATP. The titration of MgF3− into the CDK2 kinase reaction demonstrates that MgF3− is able to inhibit the CDK2 kinase reaction in vitro (Supplemental Figure 1). The TS crystals were prepared by soaking apo crystals of the pCDK2/CyclinA complex in buffer containing ADP, Mg2+, NaF, and substrate peptide. To verify presence of MgF3− in the crystal, Figure 1a shows an Fo-Fc “average kicked” omit map calculated in Phenix (Adams et al.) after removing the MgF3− from the model. A series of studies have suggested that MgF3− can function as a superior transition state mimic for certain phosphoryl transfer enzymes because the −1 charge of MgF3− more closely resembles the true PO3− intermediate compared with neutral-charge species such as AlF3 (Baxter et al., 2008; Baxter et al., 2006; Graham et al., 2002). This hypothesis is supported by the observation that AlF3 is sometimes converted to the square-planar AlF4− form when used as a TS mimic. The trigonal-planar MgF3− in the CDK2 TS structures is coordinated by a number of positively charged protein atoms as well as metal ions, suggesting that reproducing the −1 charge of the true PO3− metaphosphate anion intermediate(Westheimer, 987) is important.
Glycine-rich loop
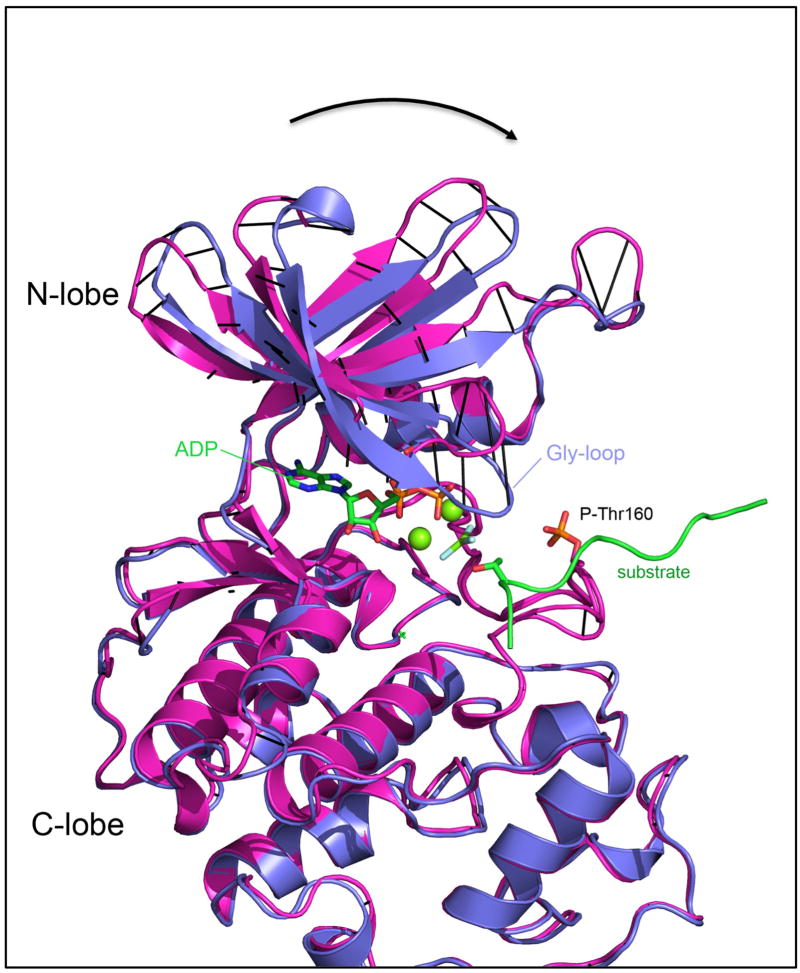
The conformation of the glycine-rich loop (residues 10–18) that functions somewhat like a lid on top of the ATP phosphates is quite different from what has been observed in all previously published structures of the pCDK2/CyclinA complex. As shown in Figure 2, the N-lobe of the kinase is rotated clockwise in the direction of the nucleotide phosphates relative to 1JST.pdb, a structure of ATP γ-S bound pCDK2/CyclinA with a single Mn2+ ion, such that the TS conformation significantly reduces the volume of the nucleotide binding pocket that sandwiches the phosphates. While there is considerable variation in the N-lobe position between the two conformations, Lys-33, which interacts with both an ATP α-phosphate oxygen and Glu-51 in α-helix-C, is nearly structurally invariant between the two conformations and appears almost to function as a fixed pivot point for the N-lobe rotation and closure.
Figure 2. Motion of the N-lobe induced upon binding of peptide substrate and the second Mg2+ ion.
A) Magenta is 1JST.pdb: the pCDK2/CyclinA complex bound to ATP and 1 Mn2+ ion. Blue: the transition state structure of pCDK2/CyclinA bound to ADP/MgF3−/2*Mg2+/peptide substrate. The structures were aligned on alpha-Carbons (Cα) of the whole complex using PyMOL(DeLano, 2010) with black lines drawn to indicate equivalent Cα atom positions.
We compare the conformation of the CDK2 Gly-rich loop TS structure with four published structures of pCDK2/CyclinA bound to ATP analogues in Figure 3. The range of Gly-rich loop conformations illustrates the flexibility of this substructure. The conformations of the nucleotide phosphates in these structures, especially the β and γ-phosphates, appear to mirror this flexibility. Despite the range of conformations observed for the ATP phosphates and the local flexibility of the Gly-rich loop, the positioning of the flexible Gly-rich loop in our TS structure (indicated in grey in Figure 3b) is a notable outlier. The conformation of the Gly-rich loop in the TS has closed down in such a way that the backbone amides make direct interactions with both the ADP β-phosphate and the MgF3− γ-phosphate mimic. The Gly-rich loop region of the protein is far from any crystallographic neighbors in the TS structure so it is not likely to be a consequence of crystal packing.
Figure 3. A comparison of the active site region of CDK2/CyclinA bound to ATP analogues and CDK2/CyclinA with the transition-state complex.
A) a superimposition of three independent crystal structures of the CDK2/Cyclin complex showing the different conformations of the nucleotide and Glycine-rich loop (pdb entries: 1fin, 1jst, 2hcc). Residues in the kinase activation loop (145–168) are colored magenta. B) Superposition of 1QMZ.pdb, pCDK2/CyclinA bound to AMPPNP/1Mg ion/substrate peptide (blue), with the transition-state structure (grey).
This closed conformation of the TS Gly-rich loop is similar to the one observed for the inactive state of the CDK2 catalytic domain (1HCK.pdb)(Schulze-Gahmen et al., 1996). While the conformation of the unphosphorylated activation loop in the inactive CDK2 monomer inhibits substrate peptide from binding via steric hindrance, we believe that the similar Gly-rich loop conformation attests to the thermodynamic stability of the closed conformation of the Gly-rich loop for CDK2.
The structure of the CDK2 TS Gly-rich loop is also strikingly similar to the structure of the TS mimic structure of PKA bound to ADP/AlF3 and a high-affinity PKI-derived substrate peptide(1L3R.pdb, Figure 4). Although the TS active site conformations are very similar, CDK2 is different from PKA as it appears to undergo both structural changes and an overall ordering of the active site when transitioning from the ATP-bound state to the TS conformation while PKA was described to undergo only very slight structural changes (Madhusudan et al., 2002). However, it should be noted that recent results and discussion point to the possibility that the PKA Gly-rich loop might also behave in a more dynamic way and undergo open to closed motions similar to what we see in CDK2 (Masterson et al., 2010; Zimmermann et al., 2008) when not interacting with high-affinity peptide inhibitors (Zimmermann et al., 2008).
Figure 4. Comparison of the CDK2 TS complex with PKA.
A, B) Superimposition of the transition-state mimic complexes of CDK2 and PKA (1l3r.pdb). CDK2 is blue, CyclinA is grey, PKA is orange.
Two Magnesium ions
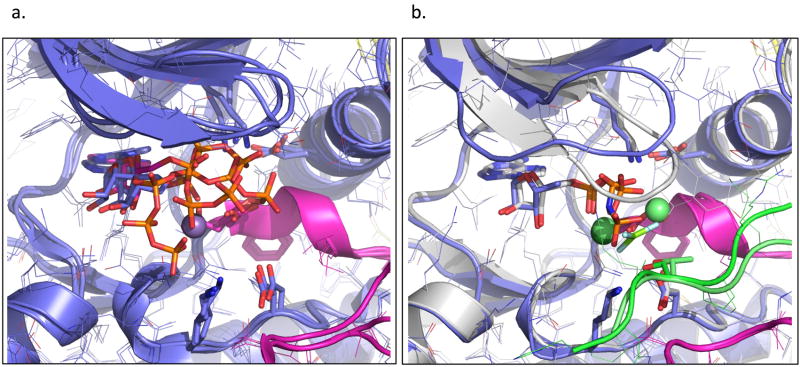
To the best of our knowledge, these are the first pCDK2/CyclinA crystal structures grown in the presence of Mg2+, as Mg2+ was observed to inhibit the growth of other CDK2/CyclinA crystal forms(Jeffrey et al., 1995). The TS crystals were grown in the presence of 20mM Mg2+ and were then transferred and soaked in a solution containing a more physiologically relevant 10mM MgCl2 prior to mounting. This might have resulted in our ability to identify two Mg2+ ions in the TS structure, which was not previously reported for other CDK2 structures. A series of views of the active-site of the TS structure including the two Mg2+ ions are shown in Figure 5. The ions have been labeled MgI and MgII, consistent with the nomenclature used in other protein kinases(Adams, 2001). Although MgI has been described as the “essential” catalytic Mg2+ in PKA and MgII as “inhibitory” (Cook et al., 1982; Shaffer and Adams, 1999b), MgII is the only Mg2+ that has been observed in previously published structures of pCDK2/CyclinA and the MgI site has always been unoccupied. In the CDK2 TS structure MgII is observed to be in a classic hexa-coordinate octahedral geometry with distances between Mg2+ and ligands ranging between 1.8Å and 2.5Å (Figure 5d and g). It is coordinated by a single water molecule, the ADP α and β phosphate oxygens, one of the MgF3− fluorine atoms, Asn132 γ–O, and one oxygen from Asp145 of the conserved kinase DFG motif. The second Mg2+ ion we observe (Figure 5d and f) is positioned in precisely the same position as MgI in the PKA TS. Unlike MgII, MgI is not in an ideal octahedral Mg2+ coordination geometry. This is largely due to the fact that it is positioned at the bisector of the two Asp145 (of the DFG motif) oxygens. The 2.1 and 2.3Å distances to each of these oxygens results in a roughly 60 degree angle (Figure 5e), rather than the 90 degree angle found in the ideal octahedral coordination geometry. The other coordinating groups include two waters (2.2, 2.5Å), an ADP β-phosphate oxygen (1.9Å), and one MgF3− fluorine (2.2Å). Although the geometry is not a perfect octahedron, this “bidentate” Mg2+ coordination has been determined to be the most stable conformation for Asp or Glu:Mg interactions in certain cases(Dudev and Lim) and we believe the distances to the coordinating oxygens are too short for this to be either a water or a monovalent Na+ ion. It is possible that the imperfect geometry could result in MgI being less thermodynamically stable compared to MgII, which could partially explain why MgI has not been observed in other pCDK2/CyclinA structures. The 2Mg TS structure also places a slight strain on the backbone of the DFG motif residues.
Figure 5. Coordination of the reactants and Mg2+ activators in the CDK2 active site. MgI has not been seen in previous CDK2 nucleotide structures.
A) Overview showing MgI and MgII (green) at 50% VdW contact radius. B) closeup highlighting the phosphate and substrate Thr interactions: α-phosphate: Lys33,MgII; β-phosphate: Gly16-NH,MgI,MgII; γ-phosphate(MgF3-):MgI,MgII,Thr14-NH; substrate Thr: Asp127,Lys129. Amides from the Gly-rich loop contact both the β and γ-phosphates. Asp127 and Lys129 participate in hydrogen bonds with the reactive substrate Thr. C) schematic of ATP phosphate interactions. D) Coordination environment of the two Mg ions. E) coordination of Asp145 in the DFG motif F) MgI is coordinated by both Asp145 (DFG) oxygens, two waters, the β-phosphate, and the γ phosphate mimic. G) MgII is coordinated by Asn132, the α and β-phosphates, a water and the γ phosphate mimic.
Mg2+ dependence of the reaction
To evaluate the functional relevance of the two Mg2+ ions, we have carried out in vitro kinase assays to measure the reaction velocity as a function of Mg2+ concentration(Figure 6). The non-hyperbolic shape of the curve indicates a potentially complex behavior. While activity is barely detectable at less than 1–2mM [Mg2+]total, the reaction velocity begins to be slightly inhibited by [Mg2+] greater than about 5–7mM. This type of curve has been observed for other kinases(Liu et al., 2010; Sun and Budde, 1997; Waas and Dalby, 2003), and a number of models have been proposed to explain the behavior. All models invoke a second essential activating Mg2+ to explain the sharp stimulatory [Mg2+] effect at 0–5mM [Mg2+]total. Because the stability constant of the first Mg2+ to ATP equates to a KD in the low micromolar range(O’Sullivan and Smithers, 1979), the 800μM ATP4− in this reaction should become saturated with a single Mg2+ at concentrations only about 50–100μM higher than what is needed for 1:1 stochiometry, i.e. at less than 1mM total Mg2+. So, consistent with earlier crystal structures, ATP*1Mg is the species that would be available to bind to the protein at 1mM Mg2+. The fact that we observe almost no activity at this concentration of Mg2+ indicates that an additional Mg2+ ion is needed at some step of the reaction. Details of these models will be discussed in the next section.
Figure 6. Mg2+ dependence of the reaction.
Activity of the pCDK2/CyclinA complex as a function of Mg2+ concentration. The dashed line indicates 1mM [Mg2+] where the 800μM ATP is expected to be saturated with 1 bound Mg2+. A) Assay using γ-P32 labelled ATP and peptide substrate. B) Coupled kinase assay using histone H1 substrate. Both assays were performed with saturating substrate concentrations.
Positioning and activation/stabilization of the nucleophile
Two different basic reaction mechanisms have been proposed for the majority of protein kinases(Adams, 2001; Cook et al., 2002; Vivo et al., 2007). They differ primarily in the mechanism of the substrate OH group activation. In the first reaction scheme, the substrate OH (a threonine side-chain in this structure) is deprotonated, most likely by a residue acting as a general base, to activate and stabilize the electronegative substrate oxygen that acts a nucleophile to attack the closely positioned γ-phosphate. The primary difference in the second reaction scheme, sometimes referred to as “substrate catalysis”, is that the phosphate transfer is initiated via a simultaneous transfer of the substrate OH proton to one of the ATP γ-phosphate oxygens rather than a protein sidechain. Therefore, the key difference is in the mechanism of deprotonation. The present structure places the Thr oxygen 2.3Å from Asp127 and 2.6Å from the closest MgF3− fluorine, thus within a H-bond distance from either of these potential proton acceptors (Figure 5). To distinguish between the two schemes, it may be important to note that Lys129 is positioned roughly 3.2Å from the phosphate-mimic F closest to the substrate Thr and 3.0Å from the substrate Thr oxygen. This orientation, along with the additional cation:MgF3− interactions described below, would significantly decrease the likelihood that a γ-phosphate oxygen could accept a proton from the Thr-OH in its current environment. It would, however, allow Lys129 to help stabilize the buildup of charge on the (unprotonated) γ-phosphate and ensure its correct alignment with the Thr-O− as it is being transferred. If this is what is occurring, Asp127 is optimally positioned to function either as a general base to enhance the substrate’s reactivity as a nucleophile, or to function as a proton trap or shuttle (Valiev et al., 2003) functioning to align and accept the proton from the substrate Thr via the first reaction scheme(Zhou and Adams, 1997).
In both schemes the destabilization of the dissociating β-γ phosphate bond can be accelerated by the action of one or two divalent metal ions, possibly assisted and/or substituted by additional cationic groups, that stabilize the buildup of negative charge on the β and γ-phosphates via direct interaction with the phosphate oxygens. If the γ-phosphate is subject to nucleophilic attack while it is still closely associated with the β-phosphate and proceeds through a penta-coordinated intermediate, the reaction is classified as “associative”. Alternatively, if the γ-phosphate largely breaks the connection to the β-phosphate and forms a trigonal-planar anionic intermediate before nucleophilic attack, it is classified as a dissociative reaction. These are two extremes of the reaction intermediate and most phosphoryl transfer reactions probably exist somewhere in between(Mildvan, 1997).
The geometry of the CDK2 TS structure more closely resembles a dissociative transition state but it is not 100% dissociative. In monomer A the distances of the γ-phosphate mimic to both the ADP leaving group and Thr nucleophile are 2.75 and 2.5Å. In the two extreme reaction mechanisms, the ideal O-P TS distance in a 100% associative reaction is 1.73Å while the fully dissociative O-P distance is 3.3Å(Mildvan, 1997). Once formed, the final O-P phosphate covalent bond is roughly 1.6Å. Based on the total O-P-O distances alone, the TS is roughly 57% dissociative: 100% associative=3.46Å < TS crystal=5.25Å < 100% dissociative=6.6Å. While we cannot rule it out from the current structure, the generation of a purely penta-coordinated associative intermediate would require significant additional motion in the active site to simultaneously bring the ADP and peptide Thr oxygen close enough to one another to form the associative penta-coordinated γ-phosphate bridge. What is also evident from this structure is that further protein and reactant motion is not necessarily required for the phosphoryl transfer to occur as the total Oleaving-Pγ- Onucleophile distance is already considerably less than what is observed in a purely dissociative reaction.
Protein dynamics of CDK2
In order to clarify the role of Mg2+ ions in the CDK2 active site and better understand the structural consequences of having either one or two Mg2+ ions present, we have carried out a series of explicit-solvent molecular dynamics (MD) simulations of both the transition-state complex and the ATP bound complex coordinated to either one or two Mg2+ ions.
TS-mimic simulations were carried out with a deprotonated Thr on the substrate peptide (the proton was transferred to Asp127), as this state is more stable in the simulations and may be what is present in the crystal. A 50 nanosecond (ns) simulation of the ADP/MgF3−/2Mg2+/peptide complex demonstrates the stability of the crystallized conformation. The backbone atom RMSD of the simulation structures fluctuates at 1.70 +/− 0.17Å from the TS crystal structure and the RMSD of the average atomic structure computed over the entire MD trajectory relative to the crystal structure is 0.98Å. As shown in the top time-series panel in Figure 7, the Gly-rich loop remains in the closed conformation and the Gly-rich loop amides maintain one or more direct contacts with the β phosphate and/or MgF3− throughout the entire 50ns trajectory.
Figure 7. Structures and analysis from four MD simulations of the pCDK2/CyclinA complex.
The simulations predict that the binding of the second Mg ion in the active site (MgI position) stabilizes the down conformation of the Gly-rich loop, expels waters from around the nucleotide phosphates, and reduces the conformational flexibility of the phosphates. A–C) three snapshots (orange) from a simulation of pCDK2/CyclinA bound to ATP and 2Mg ions beginning from1QMZ.pdb (pCDK2/CyclinA/AMPPNP/1Mg/peptide). The crystal structure of the transition state is superimposed in grey. A second Mg was placed in the MgI site at the start of the simulation. The Gly-rich loop converts to the closed conformation after ~18ns. D) a superposition of 1ns interval snapshots from the last 30ns of the trajectory, after the Gly-rich loop has adopted the closed conformation. E) 1ns interval snapshots from a 50ns simulation also starting from 1QMZ.pdb, but with only 1 Mg bound in the active site (MgII position). The top and middle time series graphs quantify the Gly-rich loop proximity to the ATP phosphates by measuring the distance from the ATP or ADP β phosphate to the closest amide group in the Gly-rich loop. The origins of the structures shown in panels a–c and e are indicated with circles. top) simulations of the transition state mimic (grey) and the 2Mg*ATP pCDK2/CyclinA complex. middle) two simulations of pCDK2/CyclinA bound to 1Mg and ATP. The corresponding distances measured in 3 crystal strutures are indicated by horizontal lines. Bottom graph) A measurement of the number of waters less than 4Å away from the ATP/ADP b-phosphate during the indicated trajectories (1ns window average with 1 standard deviation). F,G) Snapshots from the 2Mg (F) and 1Mg (G) trajectories showing the waters less than 4Å away from the ATP phosphates. See also Supplementary Figure 2.
We have found that the substrate peptide can be quite flexible and somewhat weakly bound in simulations of the ATP bound complex and, to minimize simulation sampling limitations, here we report results from ATP simulations carried out without a substrate peptide. The instability of the substrate peptide in the simulations is consistent with the somewhat weak apparent affinity of this peptide (KM=120μM at 150mM KCl, supplementary Figure 2). Full-length protein substrates are typically much better substrates for CDK2, due to stabilizing interactions located outside of the active site, for example the substrate binding groove found in the cyclin A subunit(Brown et al., 1999a).
Gly-rich loop conformations
Figure 7d and e show superimpositions of 50 snapshots from one of the 2Mg simulations and one of the 1Mg ATP simulations. These simulations were both started from the same AMPPNP*1Mg/peptide structure (1QMZ.pdb) but the second Mg2+ ion was modeled in the MgI site for the 2Mg simulation. Data is also shown from an additional 1Mg simulation started from a different ATP structure (1JST.pdb). While both the 2Mg and 1Mg simulations are very stable overall (backbone RMSD=2.0 and 1.6Å relative to the 1QMZ.pdb starting structure, respectively), we find that the presence of the second Mg2+ ion results in two notable changes to the CDK2 active site: 1) a spontaneous transition to the closed conformation of the Gly-rich loop seen in the TS structure, 2) considerably less motion of the ATP phosphates. Considerable motion is observed in the Gly-rich loop in the beginning of all 3 simulations but roughly 18ns into the 2Mg simulation the Gly-rich loop closes down and remains in that conformation for the duration of the 50ns trajectory. Once closed, the Gly-rich loop backbone amides make direct contacts with both the ATP β and γ phosphates (Figure 7c). This closed conformation (shown in Figure 7c and d) is virtually identical to the conformation observed in the TS crystal structure (superimposed in grey).
The closed conformation of the Gly-rich loop is only sampled once a layer of water molecules located between the Gly-rich loop and the ATP phosphates has been expelled. The number of water molecules found less than 4Å away from the β phosphate is shown in the bottom time-series plot in Figure 7. The closing down of the Gly-rich loop, seen at ~18ns in the top time series plot, is concurrent with the discreet drop in the number of waters directly contacting the phosphates indicated in the bottom panel. Once the layer of phosphate-solvating waters has been expelled, the number of waters interacting with the phosphates remains exactly the same as is observed for the TS-mimic simulation. The analysis in the Figure 7 time-series panels thus indicates that direct ATP phosphate:water interactions are replaced by direct interactions with Gly-rich loop backbone amides when the Gly-rich loop adopts the closed TS-like conformation.
We have also observed that the Gly-rich loop spontaneously transitions in the opposite direction, from closed to open, when simulations are started with the closed (TS) conformation but with only a single Mg2+ bound to the ATP. The simulations as a whole clearly support a model where the Gly-rich loop exchanges between closed and open conformations and that the population of the open-closed equilibrium is strongly influenced by the binding of the second Mg2+ at the MgI site. All of these simulations have been repeated after randomizing ion positions within the solvent. In total, 4 out of 5 2Mg simulations started from the open Gly-rich loop conformation transitioned to a closed conformation within 50ns and 3 out of 5 1Mg simulations started from the closed Gly-rich loop conformation transitioned to an open conformation during the same time period. This Gly-rich loop equilibrium is also supported by the TS crystal structures. We have been able to build one of the four CDK2 TS Gly-rich loops reported here into two 50% occupancy closed and open conformations and the occupancy of the MgI site in this subunit is somewhat less well defined.
Reduced motion in the active site
In addition to influencing the closing of the Gly-rich loop, the simulations also indicate that the binding of the second Mg2+ ion notably reduces the magnitude of ns timescale fluctuations for the ATP phosphates, Gly-rich loop, and the active-site region of the protein. This is evident both in the structural superimpositions shown in Figure 7d and e and in the analysis shown in Figure 8a-c, depicting backbone protein flexibility by drawing ellipsoids describing the extents of 50% positional occupancy of the protein Cα, nucleotide, and Mg2+ ions over the 20–50ns time period of each simulation. The results show that there is a hierarchy of ns timescale flexibility at the protein active site that is correlated with both substrate and activator Mg2+ binding. The most flexible state is the ATP*1Mg complex, followed by the ATP*2Mg complex, and the least flexible is the 2Mg ADP/TS/peptide complex.
Figure 8. Extents of protein backbone fluctuations in 3 MD trajectories.
Anisotropic thermal ellipsoids are displayed to encompass 50% atomic positional probabilities for the Cα atoms, computed relative to the average atom positions over the 20–50ns time period of each simulation. Simulations are the same as in Figure 7: A) pCDK2/CyclinA with ATP and 1Mg, B) pCDK2/CyclinA with ATP and 2Mg, C) pCDK2/CyclinA/ADP/2Mg/MgF3−/substrate peptide. D) The top graph indicates the extent of fluctuation for each residue by plotting the log10 of the 50% probability Cα ellipsoid volumes. The bottom plot shows the difference between the fluctuations (log10 ellipsoid volumes) in the TS simulation and the 1Mg (green) and 2Mg (yellow) simulations. Shaded regions indicate residues within 5.0Å of the nucleotide. G indicates the Gly-rich loop, D is the DFG region; A,R,P indicate the Adenosine, ribose and phosphate atoms in the ATP.
The multiple ATP phosphate conformations the CDK2 active site is able to accommodate (Figure 3a and b) probably reflects an equilibrium of states which exist prior to the phosphoryl-transfer event. Although we do not observe interconversion between the precise crystal-structure conformations within the current simulation time periods, we suggest that the differences in the ns timescale dynamics between the ATP*1Mg and ATP*2Mg states are reporting on the relative flexibilities of these two states. By replacing diffuse water interactions with explicit Gly-rich loop amide and direct Mg2+ interactions, the ATP phosphates become more tightly integrated into an extensive scaffold of ionic interactions within the protein active site. What follows is that the binding of the second Mg2+ ion not only reduces the magnitude of the ns-timescale fluctuations of the ATP, but the additional ionic interactions it enables may also limit the permitted conformations of the ATP phosphates.
Taken together, this model and the available structural data suggest that the binding of the second activating Mg ion must be an ordered, possibly sequential event that results in numerous changes to the active site that contribute to phosphoryl transfer when it occurs after ATP*1Mg binding during the catalytic cycle. In addition to directly coordinating the ATP β-phosphate, the binding of the second (MgI) ion results in the ATP simultaneously becoming more ordered, more shielded from solvent and participating in additional ionic interactions with the protein. Simulations of the ATP*1Mg state containing only a single Mg at the MgII site suggest that the MgI site would certainly be accessible to ions from outside the protein as we consistently observe transient localization of monovalent Na+ ions from the simulation bulk solvent into the vacant electronegative MgI site. Free Mg2+ ions were not present in the simulation bulk solvent. Simulations starting with a single Mg at the MgI site are less stable and thus consistent with the crystallographic evidence that the MgII site is the first to become populated when ATP*1Mg binds.
Catalytic mechanism
The CDK2 TS structure illustrates how CDK2 achieves two general mechanisms that enzymes can employ to accelerate chemical reactions: 1) correctly positioning the reactants in the optimum orientation, 2) electrostatically stabilizing the high-energy transition state and/or product leaving groups. The structural and dynamic changes of the CDK TS relative to reactant complexes supports a model where the enzyme only optimally performs both of these rate-enhancing mechanisms once all the necessary ligands are bound in the active site. Notably, if either the second activating Mg2+ ion (MgI) or the peptide substrate is missing from the active site, the Gly-rich loop is predominantly in the up conformation and the position of the β and γ phosphates are more delocalized and flexible. As all of the reactants bind, the complex network of ionic interactions is completed, the active site becomes less flexible, and the restricted flexibility may force the phosphates to converge upon the TS geometry.
Positioning the phosphates and holding them still
Further evidence that optimal phosphate alignment appears to be correlated to the binding of the second Mg2+ ion is the observation that each of the 1Mg crystal structures and MD simulations lacks one or more of the direct phosphate interactions that are observed in the TS structure shown in Figure 5b and c. This is in addition to missing the direct MgI:phosphate interactions. In particular, the β-phosphate is held in place by direct interactions with MgII:O3B, MgI:O2B, and Gly16(NH):O1B and the MD simulations find that not a single one of these three interactions is maintained in the 1Mg bound state. The γ-phosphate mimic is held in place by interactions with MgII:O3G, MgI:O2G, and Thr14(NH):O1G. The only one of these γ-phosphate interactions observed in the 1Mg state is MgII:O2G. Although the MD finds that the position of the γ-phosphate may not be as sensitive to the loss of the stabilizing interactions in the 1Mg state as the β-phosphate is, the orientation of the β-γ phosphate linkage that is cleaved in the reaction is still non-optimal in the 1Mg state as a result of the flexible β-phosphate position.
In addition to being affected by the binding of MgI, the conformation of the β-phosphate may also be sensitive to the presence of substrate peptide. In the simulation of CDK2 bound to ATP*2Mg, but without peptide, both MgI and the Gly-rich loop amides make stabilizing interactions with O1B and O2B of the β phosphate but the MgII:O3B interaction that was present in the beginning of these simulations is not preserved. O3B is the β-γ bridging oxygen that is dissociated from the γ-phosphate during the reaction. The MgII:O3B interaction is observed in 1QMZ.pdb, a structure with AMPPNP/1Mg and peptide (where the O3B is replaced with a nitrogen). Thus, although the ns timescale dynamics of the phosphates are greatly reduced when both MgI and MgII are present, the notable loss of the O3B:MgII interaction in the 2Mg simulations without substrate results in a β-γ phosphate geometry that is quite different from the TS orientation. Our hypothesis is thus that the precise β-γ phosphate coordination and positioning is sensitive to the presence of both the second Mg2+ ion (MgI) and the protein substrate. This apparent linkage between β-phosphate positioning and both substrate binding and Mg2+ coordination could reduce the probability of a water substituting for the Ser/Thr OH as a nucleophile which would lead to ATP hydrolysis to ADP + Pi, a non-productive side reaction known to occur for CDK2 and many other activated protein kinases.
Discussion
Roles of the flexible Glycine-loop
The flexible Gly-rich loop that rests on top of the nucleotide phosphates is present in most protein kinases and is thus believed to be critical to the function of these enzymes. It is the site of numerous drug resistance mutations in Abl protein kinase(Shah et al., 2002). The protein kinase Gly-rich loop is homologous to the lid-like Gly-rich loop region found in many NTPase enzymes that is often referred to as the P-loop (for phosphate-binding loop). This and other similarities support the likelihood that many of the mechanisms used by NTPase enzymes to accelerate the reaction will be related, regardless of the exact details of the catalytic mechanism. For example, it has been shown that stabilization of the buildup of negative charge on the β-phosphate of the NDP leaving group can lead to a substantial rate increase for GTPase enzymes(Maegley et al.). This is accomplished in small GTPases by at least three mechanisms, all of which can function as points of regulation: 1) a coordinating Mg2+ ion, 2) a cationic side chain such as an Arg “finger” from a GAP, and 3) backbone amide groups from the Gly-rich “P-loop” motif.
We believe that the similar reaction catalyzed by the well-studied small G-proteins provides a useful model for understanding aspects of our current CDK2 structure. The presence of a second catalytic Mg2+ ion in CDK2 means that CDK2 also utilizes two cations to stabilize the β-phosphate, only replacing the Arg finger with a second Mg2+. The GTPases also help us understand the implications of multiple conformations of the Gly-rich loop in CDK2. One of the most potent oncogenic mutations in small GTPases is a Gly to Val mutation in the Gly-rich P-loop that renders the enzymes nearly unable to catalyze the hydrolysis of GTP to GDP, hence locking them in a perpetual GTP-bound signaling state(Chen et al., 2009; Maegley et al., 1996). This mutation functions by sterically limiting the ability of the P-loop amides to adopt the somewhat contorted conformation required to intimately interact with the GTP phosphates and stabilize the buildup of charge during the reaction. Our structure demonstrates how the Gly-rich loop in CDK2 carries out a similar role of electrostatically stabilizing the ATP phosphates during catalysis(Maegley et al., 1996). The positive electrostatic potential presented to the phosphates generated by the down conformation of the Gly-rich loop is depicted in Figure 9a and b.
Figure 9. Flexible Glycine-loop.
A,B: Two views of the electrostatic potential in the active site of CDK2 in the TS structure. The scale ranges from −30kT (red) to 0 (white) to +30kT (blue). Only the protein atoms are included in the calculation, but the ADP (stick), Mg ions (green spheres), and MgF3− (stick) are shown for reference. The view in (b) is rotated roughly perpendicular relative to (a) such that the viewpoint is from the perspective of the substrate Thr, along the Thr—MgF3−—ADP reaction vector. C.D: Closing of the Gly-rich loop expels solvent and encloses the phosphates. Two slices through a solvent accessible surface rendering of the active site.
The structural heterogeneity of the CDK2 Gly-rich loop across various structures suggests that the Gly residues fulfill several unique functions: 1) they must be flexible enough to accommodate the closed conformation of the loop and 2) additional flexibility may also be required to allow the Gly-rich loop to open, potentially facilitating nucleotide binding and release, 3) despite the lack of functional side-chains, the backbone amides of the Gly residues are flexible enough to adopt otherwise unfavorable backbone torsions required to provide optimal electrostatic stabilization of the transition-state. The protonated Thr14 side chain also contributes to the electrostatic stabilizing potential of the Gly-rich loop. The stabilizing capacity of this residue would be eliminated upon phosphorylation of Thr14 and/or Tyr15 by Myt1/Wee1, two inhibitory modifications of CDK2. Figure 9a and b also highlight the strong electronegative coordination environment surrounding both Mg2+ sites, testament to the likelihood of localizing divalent ions at both sites.
An additional function of the flexible Gly-rich loop in CDK2 is to exclude solvent surrounding the ATP phosphates. Two views of the tight channel formed around the ATP phosphates in the TS are shown in Figure 9c and d. As described above, this closed conformation is stabilized by additional ionic interactions between the phosphates and backbone amides in the Gly-rich loop and only appears to occur once the second Mg2+ and substrate peptide has become bound. The substrate/Mg2+ activator triggered closure may function to minimize undesirable ATP hydrolysis which occurs when ATP reacts with a water molecule instead of a substrate Ser/Thr. The exclusion of solvent should also result in an amplification of the electrostatic stabilizing effects of the Gly-rich loop amides by reducing the dielectric environment surrounding the phosphates.
DFG motif
The presence of the short Asp-Phe-Gly “DFG” motif is nearly invariant in protein kinases. Located just before the N-terminal end of the activation loop motif (residues 145–147 in CDK2), Asp145 is observed to directly coordinate both of the Mg2+ ions at the active site in the TS complex (Figure 5d–g). The transient occupancy of the MgI ion could have some interesting implications for the functions of this residue. The sidechain of Asp145 has been observed to exist in different conformations in some structures of CDK2 bound to nucleotides with one Mg2+, such as 1HCK.pdb. Returning again to GTPases as a model, Kannan(Kannan and Neuwald, 2005) pointed out that conformational changes of the homologous Asp residue in GTPases, sometimes induced by the binding of exchange factor proteins (GEFs), can function to destabilize a bound Mg2+ ion and facilitate nucleotide exchange. It will be interesting to determine if the observed Asp145 conformational changes play a role in the binding or release of MgI during the catalytic cycle. Wedged in between the two Asp145 oxygens and the ATP β and γ phosphates, the MgI site in CDK2 has an extremely negative electrostatic potential, even greater than the nucleotide-free potential shown in Figure 9c and d. We have found that when monovalent ions present in our MD simulations spontaneously localize at or near the MgI site, they do not have the same structural, dynamic, or electronic effects as a bound divalent Mg2+. A Propka(Bas et al., 2008) calculation of the 1Mg bound nucleotide state of CDK2 (1QMZ.pdb) suggest that the DFG Asp has a highly elevated pKa (>10.5) and it, or the nucleotide γ-phosphate, may predominantly exist in a protonated state at neutral pH. Because they could shift the equilibrium of MgI binding, both of these observations have important implications for salt and pH effects in the model where the MgI site is only transiently occupied by Mg2+. The DFG facilitated MgI equilibrium is also consistent with the results of simulations investigating the mechanism of ADP release from PKA which demonstrated that it would be extremely energetically unfavorable to remove the two Mg2+ bound ADP from the active site of PKA(Khavrutskii et al., 2009). Like the ordered binding of ATP*1Mg before MgI, the release of MgI may be required to precede the release of ADP.
We believe that this ADP/MgF3−/peptide complex represents an informative model for the transition state of the phosphoryl transfer reaction catalyzed by the pCDK2/CyclinA holoenzyme. This new model proposes that the formation of the catalytically competent active site conformation involves the creation of a transient network of ionic interactions between the protein, the ATP, two Mg2+ ions, and the substrate peptide. The active-site TS conformation is highly homologous to another structure of a protein kinase transition-state mimic, the ADP/AlF3/peptide complex of PKA. In order to achieve this active site conformation, the already activated CDK2 is required to undergo two critical conformational transitions compared with structures lacking any one of the active-site ligands: 1) a substantial closing down of the Gly-rich loop, partially stabilized by backbone amide interactions with the β and γ phosphates, and 2) a precise ordering of the ATP, particularly the β and γ phosphates. More globally, MD simulations suggest that the formation of the TS complex is concurrent with a large-scale dampening of protein and reactant motions in and around the active site. This is partially achieved via the stabilizing effect of replacing nearly every single water-ATP phosphate interaction with direct ionic interactions, particularly interactions with the second catalytic Mg2+ and the Gly-rich loop backbone amides, to effectively wedge the N and C-lobes of the kinase together to more tightly engage the dissociating ATP.
Combination of the new crystal structure of the CDK2 kinase described here, with the collection of previous structures and the MD simulations enables us to construct a more complete model of the dynamic conformational ensemble of CDK2. Before all substrates and two activating Mg2+ ions are bound, the active site of the “activated” state of CDK2 is still relatively flexible and the Gly-rich loop is primarily in an open conformation. The simultaneous occupancy of the ATP, peptide and two Mg2+ ion activators increases the ordering of the active site by forming a complex network of ionic interactions where the protein and Mg2+ activators engage every one of the ATP phosphate oxygens as well as the substrate sidechain nucleophile. This increased ordering increases the probability of aligning the ATP phosphates in the optimum alignment for catalysis, stabilizes the buildup of negative charge on the phosphate oxygens (destabilizing the β-γ phosphate linkage), and is correlated with the release of waters from around the phosphates to both enhance the electrostatic field effect of the protein and potentially limit the ATP hydrolysis side-reaction.
The inhibition of activity that we observe at high Mg2+ concentrations can be rationalized by three models: 1) a second Mg2+ slows the rate of ADP product release. 2) ATP bound to 2 Mg2+ in solution does not bind as well as ATP*1Mg, 3) Mg2+ has different relative effects on the affinities of ATP and ADP for the protein, resulting in product inhibition. We hope to investigate this phenomenon in more detail in future studies. One conclusion we can make from the observed data is that a single Mg2+ ion is not sufficient to achieve maximum reaction velocity.
We believe that the biochemical [Mg2+] data, along with the two Mg2+ ions identified in the TS crystal structure, are consistent with a model where maximum reaction velocity is achieved when sufficient Mg2+ ions are available to bind to both sites, MgI and MgII, but that the occupancy of the MgI site in particular is near equilibrium. It is likely that two Mg2+ ions are at least transiently necessary to stabilize the phosphoryl-transfer step but that the presence of two Mg2+ ions may also function to kinetically inhibit other steps in the reaction such as the release of the ADP product. This model accounts for both observations that saturating ATP with 1Mg results in barely observable turnover while even higher concentrations of Mg2+ inhibit the reaction. The fact that all previously determined crystal structures of active CDK2 contain a single divalent metal ion had led to much speculation that this kinase only utilizes a single divalent for catalysis. We believe that the current structure and in vitro data suggests that a second divalent ion is utilized at the active site, at least for the phosphoryl-transfer step of the catalytic cycle.
Although it is challenging to measure, there is evidence that intracellular Mg2+ ion concentrations vary as a function of the cell cycle(Walker, 1986) and that misregulation of intracellular Mg2+ levels may be associated with both cell transformation and increased proliferation(Sgambato et al., 1999). Cell-culture studies have identified a correlation between low intracellular Mg2+ levels and failure to progress through the G1-S phase transition(Killilea and Ames, 2008), the very checkpoint that is released by CDK2/Cyclin activity. This effect has previously been associated with increased production of the CDK2 inhibitor proteins P21Cip and P16INK(Sgambato et al., 1999) at low [Mg2+]. So, while changes in Mg2+ ion concentration would most likely have an effect on numerous cellular enzymes, it is possible that the Mg2+ dependence of the catalytic subunit of CDK2 enables CDK2 to function as one link between fluctuating local Mg2+ concentrations and progression through the cell cycle. ERK2 is anther protein kinase that has been shown to require two Mg2+ ions and has been proposed to potentially be regulated by physiological fluctuations in intracellular Mg2+ levels(Waas and Dalby, 2003).
Our results suggest that the chemistry carried out by CDK2 may indeed be much more similar to PKA than some had expected, while at the same time the regulatory mechanisms of these two kinases remain quite different. Data from PKA kinase suggests that the binding of substrates may be coupled to important entropic changes within that protein kinase (Li et al.). It has been determined that still uncharacterized slow conformational transitions may occur at two critical steps of the reaction cycle of PKA, 1) following ATP binding and preceding catalysis, and 2) after catalysis and preceding the release of the reaction products(Shaffer and Adams, 1999a). If we propose that the Gly-rich loop dynamics we observe in CDK2 also takes place in PKA and other kinases, perhaps it is the slow ordered binding of the second catalytic Mg2+ ion and accompanied open to closed transition of the Gly-rich loop that represents the slow conformational change identified to occur after ATP binding to PKA, once hypothesized to represent Mg2+ binding(Zhou and Adams, 1997). Similarly, perhaps the subsequent release of the second catalytic Mg represents the second slow conformational transition observed post-catalysis in PKA.
Recent studies of adenylate kinase (ADK) reported that the apo state of that enzyme transiently samples the closed substrate-bound conformation of the enzyme(Henzler-Wildman et al., 2007). It was proposed that the ADK enzyme has been structurally programmed through evolution to sample the transition-state conformation and because of this it can more efficiently catalyze the chemical reaction once the substrates do become bound. We believe that CDK2, which is not nearly as efficient an enzyme as ADK (with a roughly 40-fold lower kcat), may have evolved differently. As an integral signaling protein, CDK2 is under evolutionary pressure to be both catalytically efficient as well as robustly regulated. It is a molecular switch. Although it is similar to ADK in that conformational changes are observed upon substrate binding, CDK2 may be different in that it may work to minimize sampling of the TS conformation until it is completely activated. CDK2 appears to maintain relatively high conformational flexibility of the active site and substrates up until the enzyme is fully ready to react and this pre-catalytic protein flexibility may function to probabilistically keep the enzyme away from sampling the TS conformation until it is the appropriate time to react. While tolerating this flexibility may have the effect of slowing down the maximum possible rate of the reaction, it may also have evolved to minimize potentially damaging uncontrolled catalytic activity and to facilitate multiple points of regulation of the enzyme. The active site of the fully activated TS complex, including ATP, 2 Mg2+, and peptide substrate, appears to be much more rigid than it is when only ATP and a single Mg2+ is bound. Keeping the reactants rigidly aligned could represent the most efficient way to increase the probability of a successful nucleophilic attack on the activated γ-phosphate by the activated substrate oxygen, a low-probability event that requires the concerted occurrences of γ-phosphate dissociation and substrate oxygen deprotonation to activate or stabilize the nucleophile. If rigidity is a requirement for the reaction, any number of external influences such as Cyclin binding, Mg activator binding, substrate binding, CDK2 phosphorylation, assembly of a hydrophobic spine(Kornev et al., 2006), could readily influence catalysis by altering the flexibility of the otherwise flexible and intrinsically inefficient enzyme.
As a final note, we would like to suggest that we believe that each of the growing number of crystal structures of the CDK2 enzyme, when taken together, are providing us a much more complete picture of the different conformations that can be sampled by this flexible enzyme. We are grateful that so many different structures of this single enzyme have been made available in the protein databank(Berman et al., 2002).
Experimental Procedures
Detailed methods are included in the supplementary materials.
The pCDK2/CyclinA complex was generated by coexpression of 3 vectors containing CDK2, CyclinA and Cak1p in E.coli. Crystals were generated by growing crystals of the apo pCDK2/CyclinA complex using vapor diffusion with 22% w/v Poly(acrylic acid sodium salt) 5,100, 20mM MgCl2, 100mM HEPES and then soaking these crystals with the active site ligands prior to cryo-protection. X-ray diffraction data was collected at LS-CAT (APS). Initial models were generated with molecular replacement and refinement was carried out using PHENIX. Enzyme kinetics were carried out using either a coupled kinase reaction with Histone H1 substrate or a P32 labelled ATP assay using peptide substrate. All molecular dynamics simulations were carried out using the AMBER 10.0 package and FF99SB force-field.
Experimental methods
Protein expression and purification
Protein for the crystals was grown using a co-expression of three proteins in BL21 E. coli: Full-length CDK2 in a pCDF-Duet vector containing a TEV cleavable His-tag, residues 173–432 of mouse CyclinA in a modified pET24 vector containing an N-terminal His tag-Smt3 fusion, and GST-Cak1p in pGEX-2T (a gift of Mark Solomon, Yale University) to phosphorylate the CDK2. Cells were grown in 1L flasks of TB media containing 3 antibiotics and 5mM MgSO4 at 37 deg until OD600 ~1.4, when they were equilibrated to 18 deg C and protein expression was induced with 0.2mM IPTG. Expression continued for 20hrs.
Cells were harvested by centrifugation at 5000 × g, mixed with lysis buffer (100mM NaPO4, 500mM NaCl) and flash-frozen until needed. Cells were lysed using an Avestin homogenizer and 2U/ml Benzonase (Sigma). Purification began with a 5ml HisTrap HP Ni-chelating column (GE). Fractions were pooled, TEV and ULP1 proteases were added, and then dialyzed overnight to lower the salt and remove imidazole. Samples were passed through a second HisTrap column to remove the cleaved tags and tagged proteases, concentrated, and run on an S-200 size-exclusion column.
Crystallography
Crystals were grown in 96-well sitting-drop vapor diffusion trays at 22 deg C. 1ul of 12mg/ml pCDK2/CyclinA complex was mixed with an equal volume of 22% w/v Poly(acrylic acid sodium salt) 5,100, 20mM MgCl2, 100mM HEPES pH 8.0. Large plate crystals typically appeared after 1–6 days. Crystals were harvested and transferred to a new tray containing ~10ul of ligand-soaking/cryoprotecting solution of 27% w/v glycerol, 2% PEG 3350, 3.2mM ADP, 10mM MgCl2, 25mM NaF, 20mM peptide, 25mM HEPES pH 8.0 or pH 8.2. We also attempted to soak crystals with AlNH4 together with the NaF but we were not able to obtain comparable quality diffraction data and resolve the active site ligands. Data was collected at the 21-ID-F and D beamlines (LS-CAT) at the Advanced Photon Source (APS) at Argonne National Labs at 100 K and λ=0.97872 Å. Integration and processing were carried out using mosflm and SCALA, respectively.
An initial molecular replacement solution was obtained in the Phaser program (McCoy et al., 2007) using previously solved but unpublished structures of CDK2 and CyclinA as search models. To minimize potential model bias, residues 150–167 and 39–45 of CDK2, two potentially flexible regions, were not included in the search model. The search was carried out for the two proteins separately, locating two copies of the CDK2/CyclinA complex in the asymmetric unit. Refinement was mostly carried out using the Phenix program suite (Adams et al.), with some steps in Refmac (Winn et al., 2003). Briefly, the refinement began with rigid body refinement, followed by simulated annealing and successive rounds of Cartesian and temperature-factor minimization with manual model building in Coot (Emsley et al., 2010). NCS restraints were built up during successive rounds of refinement to eventually restrain the following chemically equivalent regions to one another: chains A and C (CDK2), chains B and D (CyclinA), chains J and K (substrate peptide). At the very end of the refinement, NCS restraints were also included for the ADPs. Distance and angle restraints were used for the Mg ions at some earlier stages of refinement but were removed in the final stages of refinement. TLS refinement was used for the protein chains, with groups calculated using the MD TLS server (Painter and Merritt, 2006). Connected density was identified in a region of the Cyclin protein previously identified to function as a substrate-recognition motif(Brown et al., 1999a). We built the c-terminal segment of the substrate peptide into this density, which turns out to match the “RXL” sequence recognized by the Cyclin motif. Density for the N-terminal portion of this peptide was visible but not well resolved so it was only included in the final model at 0 percent occupancy.
Enzyme kinetics
Magnesium dependence of the phosphoryl transfer reaction was carried out using two experiments. In the first, γ-P32 labeled ATP was used to measure the generation of P32 labeled peptide substrate. Experiments were carried out at 100mM Tris pH 8.0, 150mM KCl, increasing concentrations of MgCl2 and aliquots were taken out and quenched at 30sec intervals. Substrate peptide (sequence: PKTPKKAKKL) was isolated by binding to P80 filter paper, washed and radioactivity quantified using a scintillation counter.
In a second experiment, a coupled enzyme assay was used with histone H1, a protein substrate. Results were similar between the two assays. In this assay, kinase activity was monitored using a continuous spectrophotometric assay as described before(Barker et al., 1995). The consumption of ATP is coupled via the pyruvate kinase/lactic dehydrogenase (PK/LDH) enzyme pair to the oxidation of NADH which can be monitored through the decrease in absorption at 340 nm. Reactions contained 100 mM Tris pH 8.0, 150mM KCl, increasing concentrations of MgCl2, 0.8 mM ATP, 1 mM phosphoenolpyruvate, 0.6 mg/mL NADH, 75 U/mL pyruvate kinase, 105 U/mL lactate dehydrogenase and 0.1 mM Histone H1 (Sigma) substrate. 75 μL reactions were started through the addition of 25 nM kinase (final concentration) and the decrease in absorbance was monitored over 20 min at 30°C in a microtiter plate spectrophotometer (SpectraMax).
The inhibition of the pCDK2/CyclinA complex by MgF3- was measured using the coupled assay described above performed in a 96-well format. The experiment was carried out by titrating Fluorine ions as NaF into a reaction mixture containing a constant 10mM MgCl2. We must assume a weak equilibrium exists between the F− and Mg2+ that will generate a small amount of the inhibitory MgF3- species. The calculated KI for NaF is roughly 15mM so we believe that MgF3− is probably at least a sub-millimolar range inhibitor of the CDK2 kinase reaction.
Substrate KM experiments were carried out in 100mM Tris, pH 8.0, 150mM KCl, 10mM MgCl2.
Molecular Dynamics Simulations
All simulations were carried out using the FF99SB all-atom potential (Hornak et al., 2006) in the AMBER 10.0 simulation package (Milde-Langosch et al.). Crystal structures were prepared by taking the biological unit and associated ligands and ordered solvent molecules, protonating ionizable groups at expected pH 7.5 protonation states, immersing in a periodic water box extending 12Å beyond all protein atoms, neutralizing the total charge with monovalent ions, and then adding Na+/Cl− ion pairs randomly throughout the solvent to approximate 150mM salt. The ATP + 1 Mg simulations were started from pdb entries 1JST and 1QMZ, after removing the substrate peptide from 1QMZ. AMPPNP was replaced with ATP and Mn was replaced with Mg, when needed. The two Mg simulation started from pdb entry 1QMZ, with the second Mg (MgI) placed in the position observed for this ion in the TS structure. The final simulation began from the TS structure. Parameters for the MgF3− were generated using the AMBER RESP protocol (Cieplak et al., 1995; Cornell et al., 1995) using with in vacuo HF 6–31G* geometry optimization in Guassian02. We transferred the hydrogen from the substrate reactive Thr-OH onto Asp127 of CDK2. Parameters for phospho-Thr were obtained from(Homeyer et al., 2006) and ATP and ADP parameters originated from (Meagher et al., 2003). The systems were all energy minimized and gradually equilibrated to a temperature of 303 K using while slowly decreasing harmonic positional restraints on the protein over a period of 160 picoseconds. Production phases of the trajectories were carried out using constant Temperature and constant Pressure without restraints for total simulation times of 50ns.
The electrostatic surface potentials in Figure 9 were calculated using the AMBER all-atom FF99SB all-atom potential(Hornak et al., 2006) in the APBS Poisson-Boltzmann solver(Baker et al., 2001) including just the protein atoms (not the ligands) in 150mM added salt.
Supplementary Material
Supplementary Figure 1. related to Figure 1. Inhibition of kinase activity by NaF (MgF3−).
Data was measured using a coupled kinase assay which links oxidation of NADH to the regeneration of ATP from ADP produced by the kinase. Reactions were carried out at 30 °C using 12.5nM pCDK2/CyclinA and 100μM histone H1 as substrate. MgF3− is in equilibrium with Mg2+/F− in solution so MgF3− was generated by the addition of NaF to the reaction buffer containing 10mM MgCl2. Total NaF is indicated on the x-axis. Data was fit to a standard KM type equation. Values in parenthesis are standard error.
Supplementary Figure 2. related to Figure 7. pCDK2/CyclinA KM for peptide.
Data was measured using a coupled kinase assay which links oxidation of NADH to the regeneration of ATP from ADP produced by the kinase. Reactions were carried out at 30 °C using 12.5nM pCDK2/CyclinA and the same PKTPKKAKKL peptide used in the crystal structures.
Table 1.
Data collection and refinement statistics (molecular replacement)
| pCDK2/CyclinA/ADP/MgF3−/peptide (pH 8.0) | pCDK2/CyclinA/ADP/MgF3−/peptide (pH 8.25) | |
|---|---|---|
| Data collection | ||
| Space group | P21 | P21 |
| Cell dimensions | ||
| a, b, c (Å) | a= 70.69Å, b=163.91Å, c=73.28Å | a= 71.03Å, b=163.45Å, c=73.39Å |
| α β γ (°) | α=90.0°, β=107.38°, γ=90.0° | α=90.0°, β=107.08°, γ=90.0° |
| Resolution (Å) | 2.17 (2.29–2.17) * | 1.91 (2.01–1.91) * |
| Rsym | 13.2 (112) | 14.0 (114) |
| I/σI | 7.4 (1.5) | 8.6(1.5) |
| Completeness (%) | 100 (100) | 100 (100) |
| Redundancy | 3.9 (3.8) | 7.6 (7.5) |
| Refinement | ||
| Resolution (Å) | 37.8–2.17 | 39.75–1.91 |
| No. reflections | 323,456 (83,902 unique) | 935,485 (123,328 unique) |
| Rwork/Rfree | 17.38/20.71% | 19.02/21.42% |
| No. atoms | 10,089 | 10,041 |
| Protein | 9,476 | 9,348 |
| Ligand/ion | 33 | 33 |
| Water | 613 | 684 |
| B-factors | ||
| Protein | 54 | 42 |
| Ligand/ion | 66 | 46 |
| Water | 59 | 47 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.012Å | 0.008Å |
| Bond angles (°) | 1.3° | 1.1° |
Data was collected from a single crystal. * Values in parentheses are for highest-resolution shell.
Acknowledgments
D.M.J. was supported by Bioinformatics Training Program T32 GM070449-05 and Proteome Informatics of Cancer Training Program T32 CA140044-01. M.A.Y. was supported by Burroughs Wellcome career award at the scientific interface 1003999. We thank Bernard Rupp for advice during refinement and Peng Jiang for assistance with ATP P32 assays. We thank all of the support staff at the APS LS-CAT beamlines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chem Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SC, Kassel DB, Weigl D, Huang X, Luther MA, Knight WB. Characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochem. 1995;34:14843–14851. doi: 10.1021/bi00045a027. [DOI] [PubMed] [Google Scholar]
- Bas DC, Rogers DM, Jensen JH. Very fast prediction and rationalization of pKa values for protein-ligand complexes. Proteins. 2008;73:765–783. doi: 10.1002/prot.22102. [DOI] [PubMed] [Google Scholar]
- Baxter NJ, Blackburn GM, Marston JP, Hounslow AM, Cliff MJ, Bermel W, Williams NH, Hollfelder F, Wemmer DE, Waltho JP. Anionic charge is prioritized over geometry in aluminum and magnesium fluoride transition state analogs of phosphoryl transfer enzymes. J Am Chem Soc. 2008;130:3952–3958. doi: 10.1021/ja078000n. [DOI] [PubMed] [Google Scholar]
- Baxter NJ, Olguin LF, Golicnik M, Feng G, Hounslow AM, Bermel W, Blackburn GM, Hollfelder F, Waltho JP, Williams NH. A Trojan horse transition state analogue generated by MgF3- formation in an enzyme active site. Proc Natl Acad Sci U S A. 2006;103:14732–14737. doi: 10.1073/pnas.0604448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, Feng Z, Gilliland GL, Iype L, Jain S, et al. The Protein Data Bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- Brown NR, Noble ME, Endicott JA, Johnson LN. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat Cell Biol. 1999a;1:438–443. doi: 10.1038/15674. [DOI] [PubMed] [Google Scholar]
- Brown NR, Noble ME, Lawrie AM, Morris MC, Tunnah P, Divita G, Johnson LN, Endicott JA. Effects of phosphorylation of threonine 160 on cyclin-dependent kinase 2 structure and activity. J Biol Chem. 1999b;274:8746–8756. doi: 10.1074/jbc.274.13.8746. [DOI] [PubMed] [Google Scholar]
- Chen X, Mitsutake N, LaPerle K, Akeno N, Zanzonico P, Longo VA, Mitsutake S, Kimura ET, Geiger H, Santos E, et al. Endogenous expression of Hras(G12V) induces developmental defects and neoplasms with copy number imbalances of the oncogene. Proc Natl Acad Sci U S A. 2009;106:7979–7984. doi: 10.1073/pnas.0900343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieplak P, Cornell WD, Bayly C, Kollman PA. Application of the Multimolecule and Multiconformational RESP Methodology to Biopolymers: Charge Derivation for DNA, RNA and Proteins. J Comp Chem. 1995;16:1357–1377. [Google Scholar]
- Cook A, Lowe ED, Chrysina ED, Skamnaki VT, Oikonomakos NG, Johnson LN. Structural studies on phospho-CDK2/cyclin A bound to nitrate, a transition state analogue: implications for the protein kinase mechanism. Biochem. 2002;41:7301–7311. doi: 10.1021/bi0201724. [DOI] [PubMed] [Google Scholar]
- Cook PF, Neville ME, Jr, Vrana KE, Hartl FT, Roskoski R., Jr Adenosine cyclic 3′,5′-monophosphate dependent protein kinase: kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochem. 1982;21:5794–5799. doi: 10.1021/bi00266a011. [DOI] [PubMed] [Google Scholar]
- Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- DeLano W. The PyMOL Molecular Graphics System. Schrödinger, LLC; 2010. [Google Scholar]
- Dudev T, Lim C. Monodentate versus Bidentate Carboxylate Binding in Magnesium and Calcium Proteins: What Are the Basic Principles? J Phys Chem B. 2004;108:4546–4557. [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Lowe PN, Grime GW, Marsh M, Rittinger K, Smerdon SJ, Gamblin SJ, Eccleston JF. MgF(3)(-) as a transition state analog of phosphoryl transfer. Chem Biol. 2002;9:375–381. doi: 10.1016/s1074-5521(02)00112-6. [DOI] [PubMed] [Google Scholar]
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;44(6):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzler-Wildman KA, Thai V, Lei M, Ott M, Wolf-Watz M, Fenn T, Pozharski E, Wilson MA, Petsko GA, Karplus M, et al. Intrinsic motions along an enzymatic reaction trajectory. Nature. 2007;450:838–844. doi: 10.1038/nature06410. [DOI] [PubMed] [Google Scholar]
- Homeyer N, Horn AH, Lanig H, Sticht H. AMBER force-field parameters for phosphorylated amino acids in different protonation states: phosphoserine, phosphothreonine, phosphotyrosine, and phosphohistidine. J Mol Model. 2006;12:281–289. doi: 10.1007/s00894-005-0028-4. [DOI] [PubMed] [Google Scholar]
- Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of multiple amber force fields and development of improved protein backbone parameters. Proteins-Structure Function and Bioinformatics. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich NP. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- Kamerlin SC, Warshel A. Proteins. 2009. At the dawn of the 21st century: Is dynamics the missing link for understanding enzyme catalysis? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N, Neuwald AF. Did protein kinase regulatory mechanisms evolve through elaboration of a simple structural component? J Mol Biol. 2005;351:956–972. doi: 10.1016/j.jmb.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Khavrutskii IV, Grant B, Taylor SS, McCammon JA. A transition path ensemble study reveals a linchpin role for Mg(2+) during rate-limiting ADP release from protein kinase A. Biochem. 2009;48:11532–11545. doi: 10.1021/bi901475g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc Natl Acad Sci U S A. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornev AP, Haste NM, Taylor SS, Eyck LF. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci U S A. 2006;103:17783–17788. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Gangal M, Juliano C, Gorfain E, Taylor SS, Johnson DA. Evidence for an internal entropy contribution to phosphoryl transfer: a study of domain closure, backbone flexibility, and the catalytic cycle of cAMP-dependent protein kinase. J Mol Biol. 2002;315:459–469. doi: 10.1006/jmbi.2001.5256. [DOI] [PubMed] [Google Scholar]
- Liu M, Girma E, Glicksman MA, Stein RL. Kinetic mechanistic studies of Cdk5/p25-catalyzed H1P phosphorylation: metal effect and solvent kinetic isotope effect. Biochem. 2010;49:4921–4929. doi: 10.1021/bi100244j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol. 2006;2:358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
- Madhusudan Akamine P, Xuong NH, Taylor SS. Crystal structure of a transition state mimic of the catalytic subunit of cAMP-dependent protein kinase. Nat Struct Biol. 2002;9:273–277. doi: 10.1038/nsb780. [DOI] [PubMed] [Google Scholar]
- Maegley KA, Admiraal SJ, Herschlag D. Ras-catalyzed hydrolysis of GTP: a new perspective from model studies. Proc Natl Acad Sci U S A. 1996;93:8160–8166. doi: 10.1073/pnas.93.16.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle kinases in cancer. Current opinion in genetics & development. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Masterson LR, Cheng C, Yu T, Tonelli M, Kornev A, Taylor SS, Veglia G. Dynamics connect substrate recognition to catalysis in protein kinase A. Nat Chem Biol. 2010;6:821–828. doi: 10.1038/nchembio.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher KL, Redman LT, Carlson HA. Development of polyphosphate parameters for use with the AMBER force field. J Comput Chem. 2003;24:1016–1025. doi: 10.1002/jcc.10262. [DOI] [PubMed] [Google Scholar]
- Milde-Langosch K, Bamberger AM, Goemann C, Rossing E, Rieck G, Kelp B, Loning T. Expression of cell-cycle regulatory proteins in endometrial carcinomas: correlations with hormone receptor status and clinicopathologic parameters. Journal of cancer research and clinical oncology. 2001;127:537–544. doi: 10.1007/s004320100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildvan AS. Mechanisms of signaling and related enzymes. Proteins. 1997;29:401–416. [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Sudhof TC, Wahl MC. CASK functions as a Mg2+-independent neurexin kinase. Cell. 2008;133:328–339. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan WJ, Smithers GW. Stability constants for biologically important metal-ligand complexes. Methods Enzymol. 1979;63:294–336. doi: 10.1016/0076-6879(79)63014-8. [DOI] [PubMed] [Google Scholar]
- Painter J, Merritt EA. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr D Biol Crystallogr. 2006;62:439–450. doi: 10.1107/S0907444906005270. [DOI] [PubMed] [Google Scholar]
- Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol. 1999;287:821–828. doi: 10.1006/jmbi.1999.2640. [DOI] [PubMed] [Google Scholar]
- Radzio-Andzelm E, Lew J, Taylor S. Bound to activate: conformational consequences of cyclin binding to CDK2. Structure. 1995;3:1135–1141. doi: 10.1016/s0969-2126(01)00249-0. [DOI] [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Pavletich NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- Schulze-Gahmen U, De Bondt HL, Kim SH. High-resolution crystal structures of human cyclin-dependent kinase 2 with and without ATP: bound waters and natural ligand as guides for inhibitor design. J Med Chem. 1996;39:4540–4546. doi: 10.1021/jm960402a. [DOI] [PubMed] [Google Scholar]
- Sgambato A, Wolf FI, Faraglia B, Cittadini A. Magnesium depletion causes growth inhibition, reduced expression of cyclin D1, and increased expression of P27Kip1 in normal but not in transformed mammary epithelial cells. J Cell Physiol. 1999;180:245–254. doi: 10.1002/(SICI)1097-4652(199908)180:2<245::AID-JCP12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Shaffer J, Adams JA. An ATP-linked structural change in protein kinase A precedes phosphoryl transfer under physiological magnesium concentrations. Biochem. 1999a;38:5572–5581. doi: 10.1021/bi982768q. [DOI] [PubMed] [Google Scholar]
- Shaffer J, Adams JA. Detection of conformational changes along the kinetic pathway of protein kinase A using a catalytic trapping technique. Biochem. 1999b;38:12072–12079. doi: 10.1021/bi991109q. [DOI] [PubMed] [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Stevenson LM, Deal MS, Hagopian JC, Lew J. Activation mechanism of CDK2: role of cyclin binding versus phosphorylation. Biochem. 2002;41:8528–8534. doi: 10.1021/bi025812h. [DOI] [PubMed] [Google Scholar]
- Sun G, Budde RJ. Requirement for an additional divalent metal cation to activate protein tyrosine kinases. Biochem. 1997;36:2139–2146. doi: 10.1021/bi962291n. [DOI] [PubMed] [Google Scholar]
- Valiev M, Kawai R, Adams JA, Weare JH. The role of the putative catalytic base in the phosphoryl transfer reaction in a protein kinase: first-principles calculations. J Am Chem Soc. 2003;125:9926–9927. doi: 10.1021/ja029618u. [DOI] [PubMed] [Google Scholar]
- Vivo MD, Cavalli A, Carloni P, Recanatini M. Computational Study of the Phosphoryl Transfer Catalyzed by a Cyclin-Dependent Kinase. Chemistry - A European Journal. 2007;13:8437–8444. doi: 10.1002/chem.200700044. [DOI] [PubMed] [Google Scholar]
- Waas WF, Dalby KN. Physiological concentrations of divalent magnesium ion activate the serine/threonine specific protein kinase ERK2. Biochem. 2003;42:2960–2970. doi: 10.1021/bi027171w. [DOI] [PubMed] [Google Scholar]
- Walker GM. Magnesium and cell cycle control: an update. Magnesium. 1986;5:9–23. [PubMed] [Google Scholar]
- Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- Winn MD, Murshudov GN, Papiz MZ. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 2003;374:300–321. doi: 10.1016/S0076-6879(03)74014-2. [DOI] [PubMed] [Google Scholar]
- Zhou J, Adams JA. Is there a catalytic base in the active site of cAMP-dependent protein kinase? Biochem. 1997;36:2977–2984. doi: 10.1021/bi9619132. [DOI] [PubMed] [Google Scholar]
- Zimmermann B, Schweinsberg S, Drewianka S, Herberg FW. Effect of metal ions on high-affinity binding of pseudosubstrate inhibitors to PKA. Biochem J. 2008;413:93–101. doi: 10.1042/BJ20071665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. related to Figure 1. Inhibition of kinase activity by NaF (MgF3−).
Data was measured using a coupled kinase assay which links oxidation of NADH to the regeneration of ATP from ADP produced by the kinase. Reactions were carried out at 30 °C using 12.5nM pCDK2/CyclinA and 100μM histone H1 as substrate. MgF3− is in equilibrium with Mg2+/F− in solution so MgF3− was generated by the addition of NaF to the reaction buffer containing 10mM MgCl2. Total NaF is indicated on the x-axis. Data was fit to a standard KM type equation. Values in parenthesis are standard error.
Supplementary Figure 2. related to Figure 7. pCDK2/CyclinA KM for peptide.
Data was measured using a coupled kinase assay which links oxidation of NADH to the regeneration of ATP from ADP produced by the kinase. Reactions were carried out at 30 °C using 12.5nM pCDK2/CyclinA and the same PKTPKKAKKL peptide used in the crystal structures.