Abstract
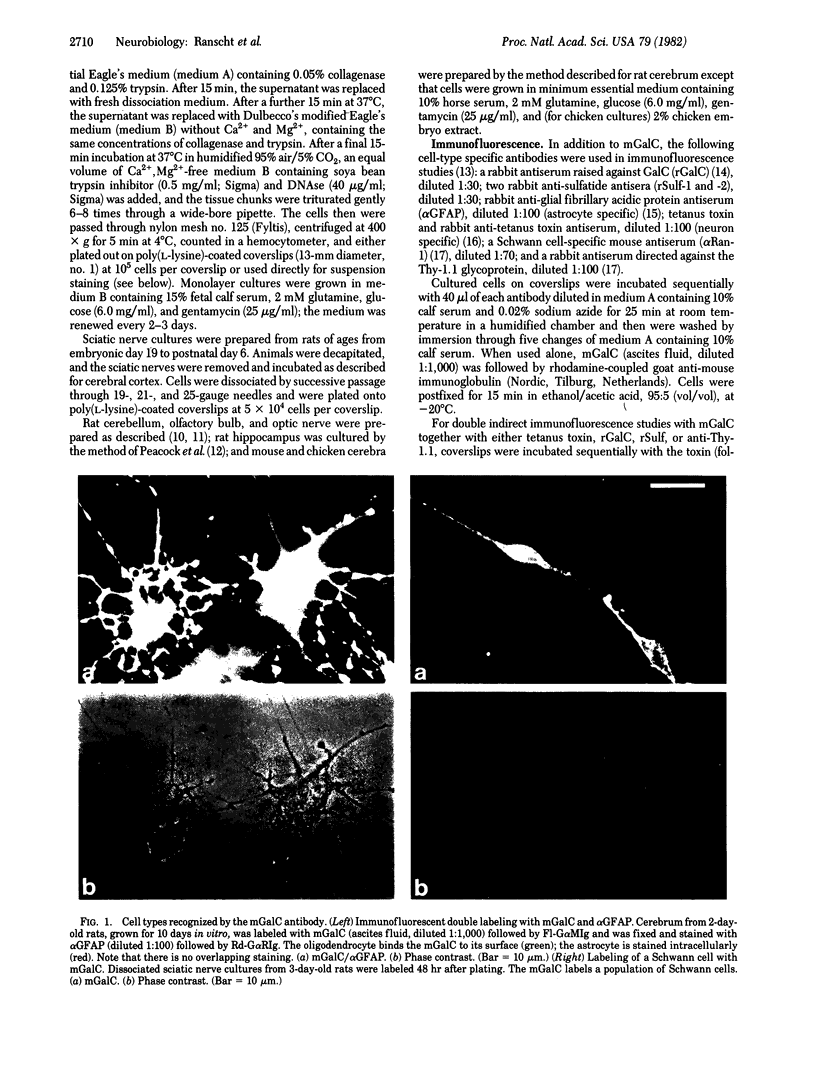
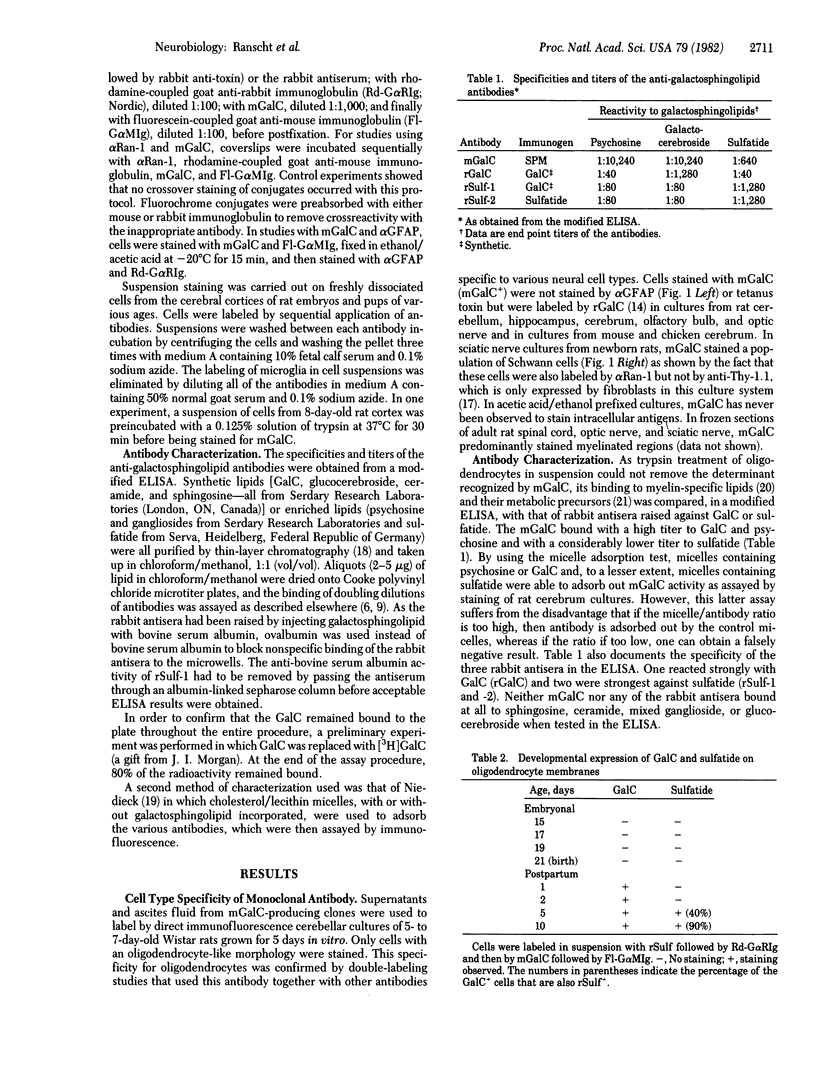
We have generated a hybridoma cell line secreting a monoclonal antibody that specifically binds to the surfaces of oligodendrocytes and Schwann cells, the cells involved in myelin formation in the central and peripheral nervous systems, respectively. Binding studies using purified sphingolipids showed that this antibody reacts strongly with galactocerebroside (GalC), the major galactosphingolipid of myelin. The antibody was used in conjunction with rabbit antisera against sulfatide, the sulfated form of GalC, to examine the developmental appearance of these lipids on the surfaces of oligodendrocytes and Schwann cells. In addition, the loss of GalC and sulfatide from freshly dissociated Schwann cells was compared. These studied showed that GalC is expressed on the cell surface prior to sulfatide on both of these cell types in vivo and in vitro. Conversely, dissociated Schwann cells lose their cell surface sulfatide more rapidly than they lose their surface GalC under nonmyelinating conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Bartlett P. P., Raff M. C. Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Dev Biol. 1981 Apr 30;83(2):301–310. doi: 10.1016/0012-1606(81)90476-0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., Radin N. S. Cerebroside galactosidase: a method for determination and a comparison with other lysosomal enzymes in developing rat brain. J Neurochem. 1969 Apr;16(4):501–511. doi: 10.1111/j.1471-4159.1969.tb06849.x. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. A surface antigenic marker for rat Schwann cells. Nature. 1977 Mar 24;266(5600):364–366. doi: 10.1038/266364a0. [DOI] [PubMed] [Google Scholar]
- Cohen J., Dutton G. R., Wilkin G. P., Wilson J. E., Balázs R. A preparation of viable cell perikarya from developing rat cerebellum with preservation of a high degree of morphological integrity. J Neurochem. 1974 Oct;23(4):899–901. doi: 10.1111/j.1471-4159.1974.tb04425.x. [DOI] [PubMed] [Google Scholar]
- Cohen J., Selvendran S. Y. A neuronal cell-surface antigen is found in the CNS but not in peripheral neurones. Nature. 1981 Jun 4;291(5814):421–423. doi: 10.1038/291421a0. [DOI] [PubMed] [Google Scholar]
- Dorfman S. H., Fry J. M., Silberberg D. H. Antiserum induced myelination inhibition in vitro without complement. Brain Res. 1979 Nov 9;177(1):105–114. doi: 10.1016/0006-8993(79)90921-1. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Matus A. I. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta. 1974 Aug 9;356(3):276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Wendon L. M., Black P., Stolkin C., Bray D. Tetanus toxin: a cell surface marker for neurones in culture. Brain Res. 1978 Jun 9;148(1):251–259. doi: 10.1016/0006-8993(78)90399-2. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Winter J., Abney E. R., Pruss R. M., Gavrilovic J., Raff M. C. Myelin-specific proteins and glycolipids in rat Schwann cells and oligodendrocytes in culture. J Cell Biol. 1980 Mar;84(3):483–494. doi: 10.1083/jcb.84.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P., Braun P. Biosynthesis and metabolic degradation of sphingolipids not containing sialic acid. J Lipid Res. 1972 May;13(3):293–310. [PubMed] [Google Scholar]
- Niedieck B. On a glycolipid hapten of myelin. Prog Allergy. 1975;18:353–422. [PubMed] [Google Scholar]
- Norton W. T., Autilio L. A. The lipid composition of purified bovine brain myelin. J Neurochem. 1966 Apr;13(4):213–222. doi: 10.1111/j.1471-4159.1966.tb06794.x. [DOI] [PubMed] [Google Scholar]
- Peacock J. H., Rush D. F., Mathers L. H. Morphology of dissociated hippocampal cultures from fetal mice. Brain Res. 1979 Jun 22;169(2):231–246. doi: 10.1016/0006-8993(79)91027-8. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Schachner M., Kim S. K., Zehnle R. Developmental expression in central and peripheral nervous system of oligodendrocyte cell surface antigens (O antigens) recognized by monoclonal antibodies. Dev Biol. 1981 Apr 30;83(2):328–338. doi: 10.1016/0012-1606(81)90478-4. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Tennekoon G. I., Kishimoto Y., Singh I., Nonaka G., Bourre J. M. The differentiation of oligodendrocytes in the rat optic nerve. Dev Biol. 1980 Sep;79(1):149–158. doi: 10.1016/0012-1606(80)90080-9. [DOI] [PubMed] [Google Scholar]