Abstract
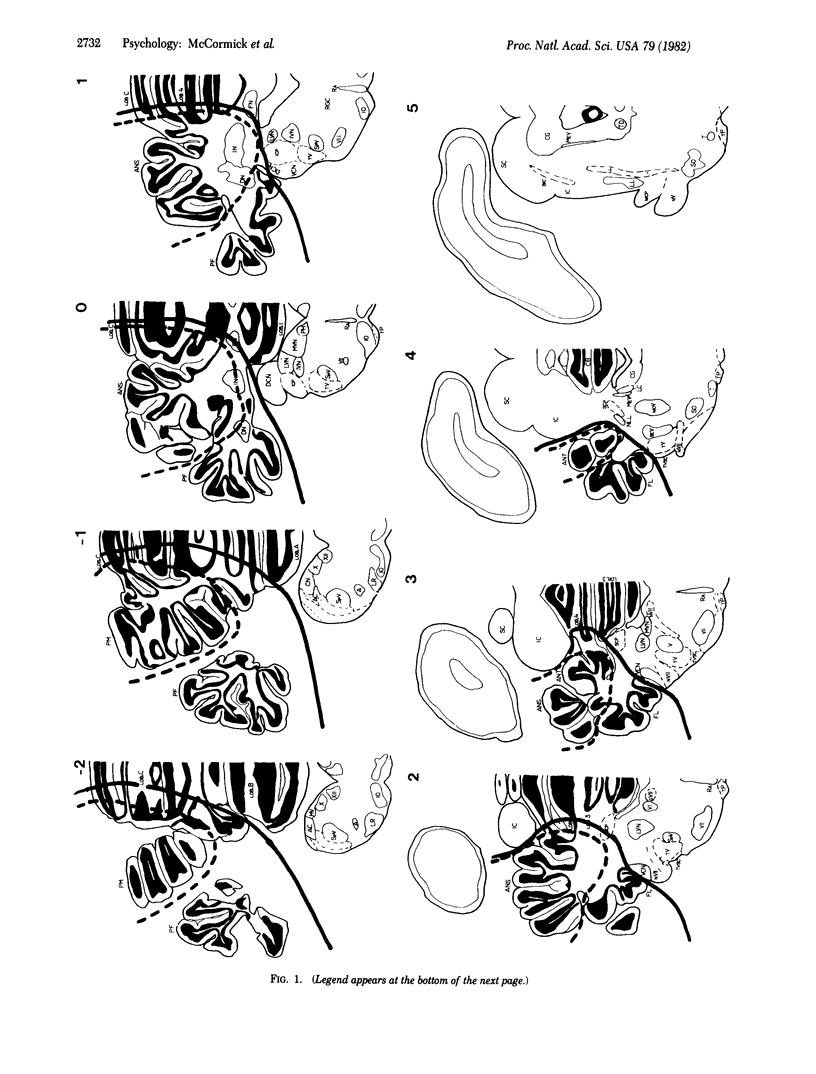
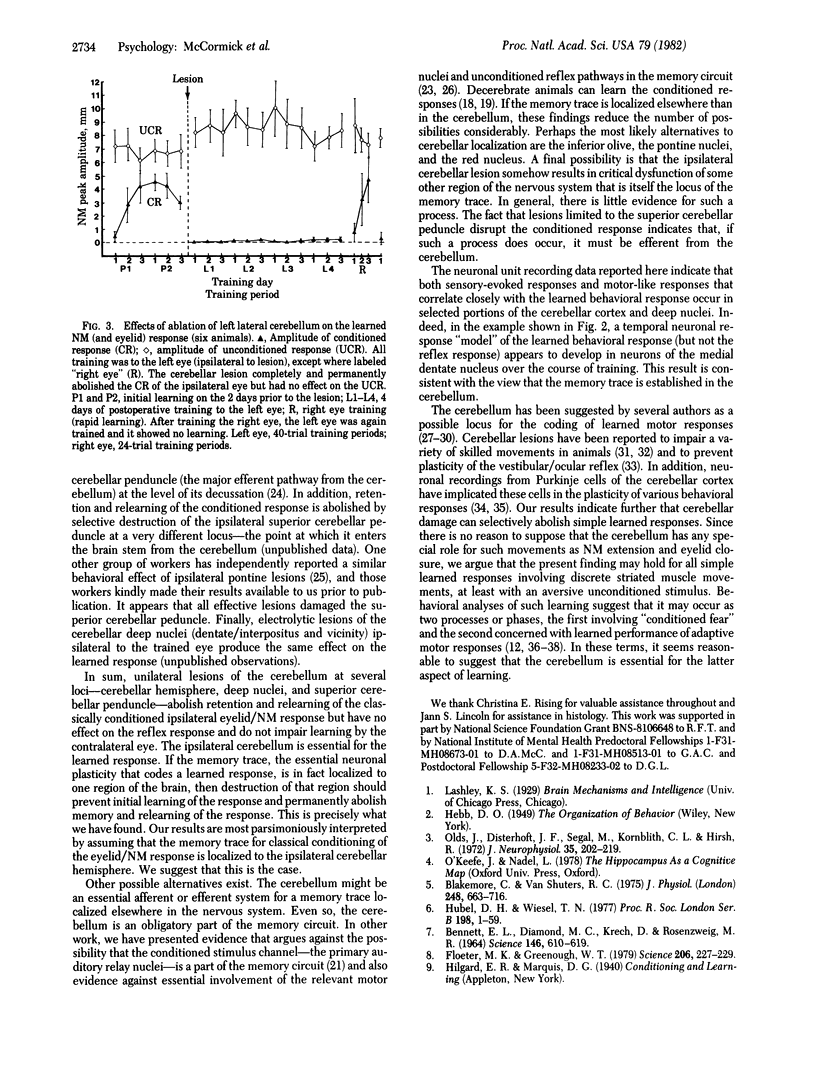
Electrophysiological recording of neuronal unit activity during paired training trials from various regions of the ipsilateral cerebellum in rabbits well trained in the classically conditioned eyelid/nictitating membrane response have revealed both stimulus-evoked responses and responses that form an amplitude/temporal model of the learned behavioral response. Ablation of the ipsilateral, lateral cerebellum completely and permanently abolished the behavioral conditioned response in well-trained animals but had no effect at all on the unconditioned reflex response. In marked contrast, conditioned responses were easily trained in the eye contralateral to the cerebellar lesion. We suggest that at least part of the essential neuronal plasticity that codes the learned response may be localized to the cerebellum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT E. L., DIAMOND M. C., KRECH D., ROSENZWEIG M. R. CHEMICAL AND ANATOMICAL PLASTICITY BRAIN. Science. 1964 Oct 30;146(3644):610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Thompson R. F. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response. I. The hippocampus. Brain Res. 1978 Apr 28;145(2):323–346. doi: 10.1016/0006-8993(78)90866-1. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks V. B., Kozlovskaya I. B., Atkin A., Horvath F. E., Uno M. Effects of cooling dentate nucleus on tracking-task performance in monkeys. J Neurophysiol. 1973 Nov;36(6):974–995. doi: 10.1152/jn.1973.36.6.974. [DOI] [PubMed] [Google Scholar]
- Cegavske C. F., Patterson M. M., Thompson R. F. Neuronal unit activity in the abducens nucleus during classical conditioning of the nictitating membrane response in the rabbit (Oryctolagus cuniculus). J Comp Physiol Psychol. 1979 Aug;93(4):595–609. doi: 10.1037/h0077601. [DOI] [PubMed] [Google Scholar]
- Dufossé M., Ito M., Jastreboff P. J., Miyashita Y. A neuronal correlate in rabbit's cerebellum to adaptive modification of the vestibulo-ocular reflex. Brain Res. 1978 Jul 21;150(3):611–616. doi: 10.1016/0006-8993(78)90825-9. [DOI] [PubMed] [Google Scholar]
- Floeter M. K., Greenough W. T. Cerebellar plasticity: modification of Purkinje cell structure by differential rearing in monkeys. Science. 1979 Oct 12;206(4415):227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- Gilbert P. F., Thach W. T. Purkinje cell activity during motor learning. Brain Res. 1977 Jun 10;128(2):309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Ito M. Neurophysiological aspects of the cerebellar motor control system. Int J Neurol. 1970;7(2):162–176. [PubMed] [Google Scholar]
- Ito M., Shiida T., Yagi N., Yamamoto M. Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res. 1974 Jan 4;65(1):170–174. doi: 10.1016/0006-8993(74)90344-8. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman R. J., Buchwald J. S., Villablanca J. R. Classical conditioning with auditory discrimination of the eye blink in decerebrate cats. Science. 1977 Apr 29;196(4289):551–553. doi: 10.1126/science.850800. [DOI] [PubMed] [Google Scholar]
- Oakley D. A., Russell I. S. Neocortical lesions and Pavlovian conditioning. Physiol Behav. 1972 May;8(5):915–926. doi: 10.1016/0031-9384(72)90305-8. [DOI] [PubMed] [Google Scholar]
- Olds J., Disterhoft J. F., Segal M., Kornblith C. L., Hirsh R. Learning centers of rat brain mapped by measuring latencies of conditioned unit responses. J Neurophysiol. 1972 Mar;35(2):202–219. doi: 10.1152/jn.1972.35.2.202. [DOI] [PubMed] [Google Scholar]
- Rescorla R. A., Solomon R. L. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967 May;74(3):151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Solomon P. R., Moore J. W. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol. 1975 Dec;89(10):1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]





