Abstract
Background
Invasion-biology is largely based on non-experimental observation of larger organisms. Here, we apply an experimental approach to the subject. By using microbial-based microcosm-experiments, invasion-biology can be placed on firmer experimental, and hence, less anecdotal ground. A better understanding of the mechanisms that govern invasion-success of bacteria in soil communities will provide knowledge on the factors that hinder successful establishment of bacteria artificially inoculated into soil, e.g. for remediation purposes. Further, it will yield valuable information on general principles of invasion biology in other domains of life.
Methodology/Principal Findings
Here, we studied invasion and establishment success of GFP-tagged Pseudomonas fluorescens DSM 50090 in laboratory microcosms during a 42-day period. We used soil heating to create a disturbance gradient, and hypothesized that increased disturbance would facilitate invasion; our experiments confirmed this hypothesis. We suggest that the key factors associated with the heating disturbance that explain the enhanced invasion success are increased carbon substrate availability and reduced diversity, and thus, competition- and predation-release. In a second experiment we therefore separated the effects of increased carbon availability and decreased diversity. Here, we demonstrated that the effect of the indigenous soil community on bacterial invasion was stronger than that of resource availability. In particular, introduced bacteria established better in a long term perspective at lower diversity and predation pressure.
Conclusion
We propose increased use of microbial systems, for experimental study of invasion scenarios. They offer a simple and cost-efficient way to study and understand biological invasion. Consequently such systems can help us to better predict the mechanisms controlling changes in stability of communities and ecosystems. This is becoming increasingly relevant since anthropogenic disturbance causes increasing global change, which promotes invasion. Moreover, a thorough understanding of factors controlling invasion and establishment of artificially amended micro-organisms will mean a major step forward for soil-remediation microbiology.
Introduction
The concept of biological invasion plays an essential role in the study of biodiversity maintenance and ecosystem services [1]–[3]. Thus, a better understanding of the factors that control invasion can assist in the management of habitats, if the susceptibility to unwanted species can be regulated. Current hypotheses on mechanisms behind biological invasions mainly stem from macro-ecology, which have emphasized the importance of disturbance [1], species diversity [4], community composition [5], and resource availability [6]. Hence, invasion theory is largely based on observation and not on experiments. Disturbance is recognized as one of the most important factors to promote biological invasions because it can, concomitantly, alter community composition, diversity, as well as resource availability [6]–[9]. This has been demonstrated by several empirical studies [5], [6], [10], [11].
Hence, experimental disturbance studies provide a way to explore the integrated effects of resource and community changes. Most studies on impacts of disturbance on ecosystem stability, including community resistance to invasions, have focused exclusively on macro-organisms [12]. Lately, however, the stability of soil microbial communities and their functioning to disturbance has attracted more attention [8], [13]. Invasion is considered a serious environmental issue in macro-ecology. Oppositely, the failure of invasion and establishment of artificially amended micro-organisms (e.g. for soil-remediation purposes) is considered a serious issue in applied microbial ecology. Besides, microbial communities in soil are strongly related to above-ground functioning including invasion of plants [14]. Still, only few studies have explored the effects of disturbance on microbial invasion [12].
Disturbance can directly increase soil organic matter availability by exposing new substrate by breaking soil aggregates and by killing members of the native community [15], [16]. Further it can affect soil community diversity and structure [16]–[18]. Van Elsas et al [11] manipulated soil community complexity via progressively increasing disturbance intensity using fumigation. They introduced non-indigenous bacteria 60 days after disturbance to avoid confounding effects of temporary resource enrichment; hence they also excluded the possibility to see invasion success in a strongly disturbed community.
The use of soil microcosms offers a simple, fast and cost-efficient approach to perform experimental studies of the mechanisms behind biological invasions. To our knowledge, no study has previously explored the concomitant contributions of community diversity changes, resource availability and their interaction on invasion in microbial systems. Therefore, we conducted two sequential microcosm-experiments where we investigated the effect of disturbance exemplified by heating on the ability of a bacterium (Pseudomonas fluorescens DSM 50090) to colonize and establish in soil. In experiment 1, we simply examined the effect of heating. In experiment 2 we separated the effects of carbon release and community disturbance. We hypothesized that: 1) Microbial invasion success will increase with disturbance intensity, due to the combined effects of resource enrichment and recipient community disturbance and, 2) The relative importance of community disturbance compared to resource enrichment will increase with time following disturbance, as ephemeral resources are depleted, and the recipient system recovers. We found experimental evidence that confirmed both hypotheses.
Results
First experiment: effect of heating
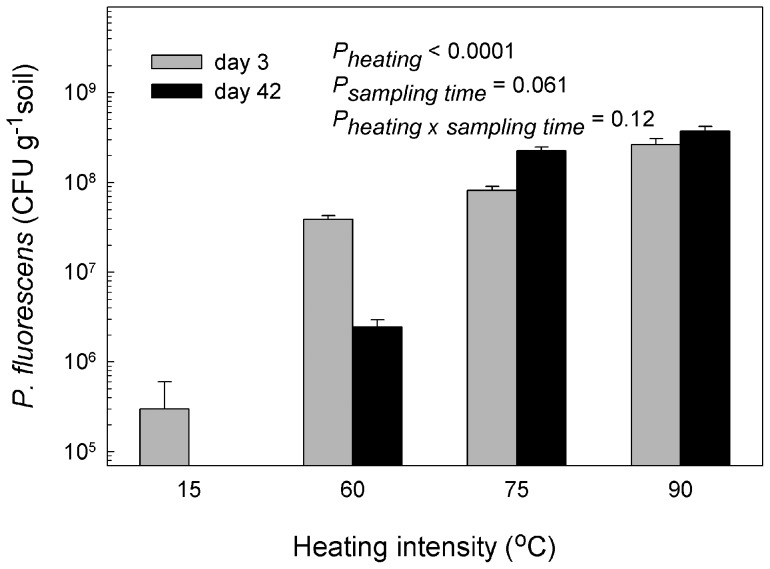
We exposed soil microcosms to four different heating intensities (15°C, 60°C, 75°C or 90°C) for 24 h. These heating intensities were chosen on the basis of a preliminary experiment including six temperatures (15°C, 30°C, 45°C, 60°C, 75°C and 90°C), which showed that heating to below 60°C had no severe impact on soil biodiversity. Subsequently, we introduced the non-indigenous Pseudomonas fluorescens DSM 50090 into the systems. After 3 days the number of P. fluorescens DSM 50090 correlated positively with increasing heating intensity (Figure 1). This trend persisted after 42 days, but whereas P. fluorescens DSM 50090 decreased in numbers for the 15 and 60°C heating-treatments from the first to the second sampling, in the 75°C and 90°C treatments, P. fluorescens DSM 50090 increased by 178% and 28%, respectively, between the two samplings.
Figure 1. Heating facilitated invasion of Pseudomonas fluorescens DSM 50090.
Soil microcosms were exposed to four different heating intensities (15°C, 60°C, 75°C or 90°C) for 24 h and subsequently amended with P. fluorescens DSM 50090. Culturable P. fluorescens were recovered from sampling of soil microcosms to reveal its ability to invade (sampling at day 3) and establish (sampling at day 42) in the systems. We used a two-way ANOVA with sampling time and heating intensity as quantitative variables to test the results. Data were log transformed prior to analysis to equalize variances. P-values <0.15 are shown.
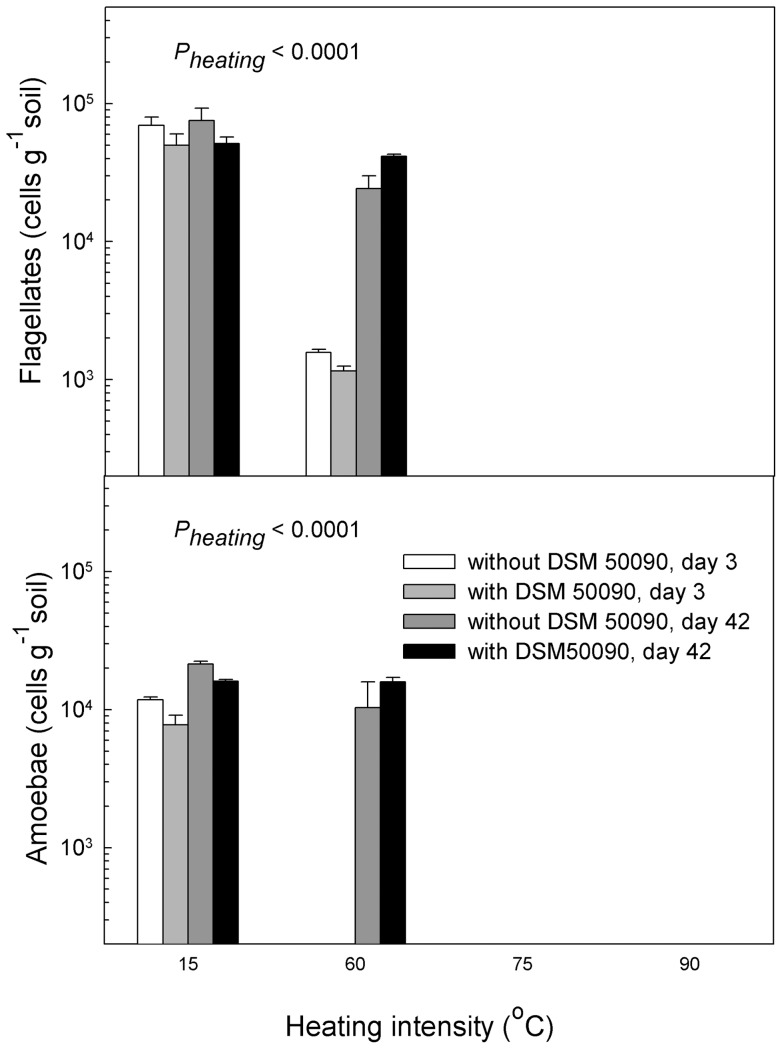
In all heated treatments (>15°C) the numbers of protozoa (flagellates and amoebae) were strongly reduced at the first sampling (Figure 2). In the 60°C-microcosms, protozoa recovered to a great extent at the second sampling, whereas the 75°C and 90°C treatments remained completely devoid of protozoa.
Figure 2. Heating decreased protozoan numbers.
Soil microcosms were exposed to four different heating intensities (15°C, 60°C, 75°C or 90°C) for 24 h and subsequently amended with either P. fluorescens DSM 50090 or just with phosphate buffer. Culturable protozoa (heterotrophic flagellates: upper panel, naked amoebae: lower panel) were measured after 3 and 42 days. Heating above 60°C completely eliminated the protozoa, whereas amendment with P. fluorescens had no effect. We used a three-way ANOVA with sampling time and heating intensity as quantitative variables and Pseudomonas fluorescens DSM 50090 as qualitative variable to test the results. Data were log transformed prior to analysis to equalize variances. P-values <0.05 are shown.
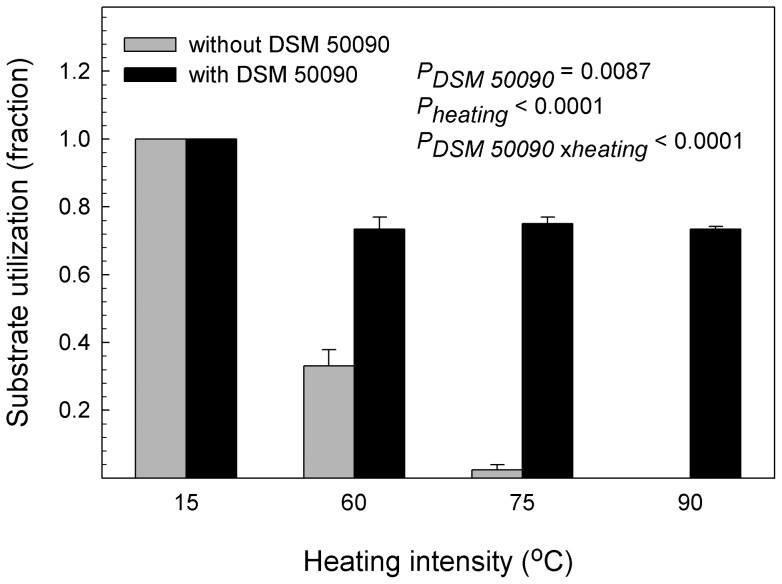
Functional diversity of indigenous micro-organisms, measured on day 42 as fraction of positive wells in Biolog EcoPlates (Figure 3), declined sharply with increasing heating intensity. In the 15°C-treatment, all substrate types in the EcoPlates were utilized, followed by 33%, 2% and 0% for the 60°C, 75°C and 90°C treatments, respectively. Surprisingly, amendment with P. fluorescens DSM 50090 alone could provide 75 % of the functional diversity in the Biolog EcoPlates.
Figure 3. Microbial functional diversity decreased with heating; P. fluorescens alone provides 75 % of the diversity.
Soil microcosms were exposed to four different heating intensities (15°C, 60°C, 75°C or 90°C) for 24 h and subsequently amended with either P. fluorescens DSM 50090 or just with phosphate buffer and incubated for 42 days. Microbial diversity was measured as the fraction of substrates metabolised in Biolog EcoPlates with soil from the microcosms. We used a two-way ANOVA with heating intensity as quantitative variables and P. fluorescens as qualitative variable to test the results., data were Arcsine-square root transformed to equalize variances. P-values <0.05 are shown.
Indigenous CFU-numbers also declined with increasing heating intensity, in systems without P. fluorescens DSM 50090. At the first sampling, only 52% and 32% of the number in the 15°C-treatment (2.2×107 CFUs g−1) were left at 60°C and 75°C, respectively (Data not shown). At the second sampling, indigenous bacterial CFU numbers in the 60°C treatment made up 31% of the 15°C-treatment (Data not shown). No indigenous bacteria were detected in the 90°C microcosms at any of the samplings.
Second experiment: separation of carbon and community change effects
In the second experiment, we separated the heat treatment effects on community disturbance and carbon release. We did so by leaching the microcosms after heating as to remove soluble carbon. Subsequently, we then amended all leached heating treatments with carbon amounts corresponding to amounts produced at the four heating treatments. Amendment with P. fluorescens DSM 50090, incubation, CO2 measurement and destructive sampling were as in Experiment I.
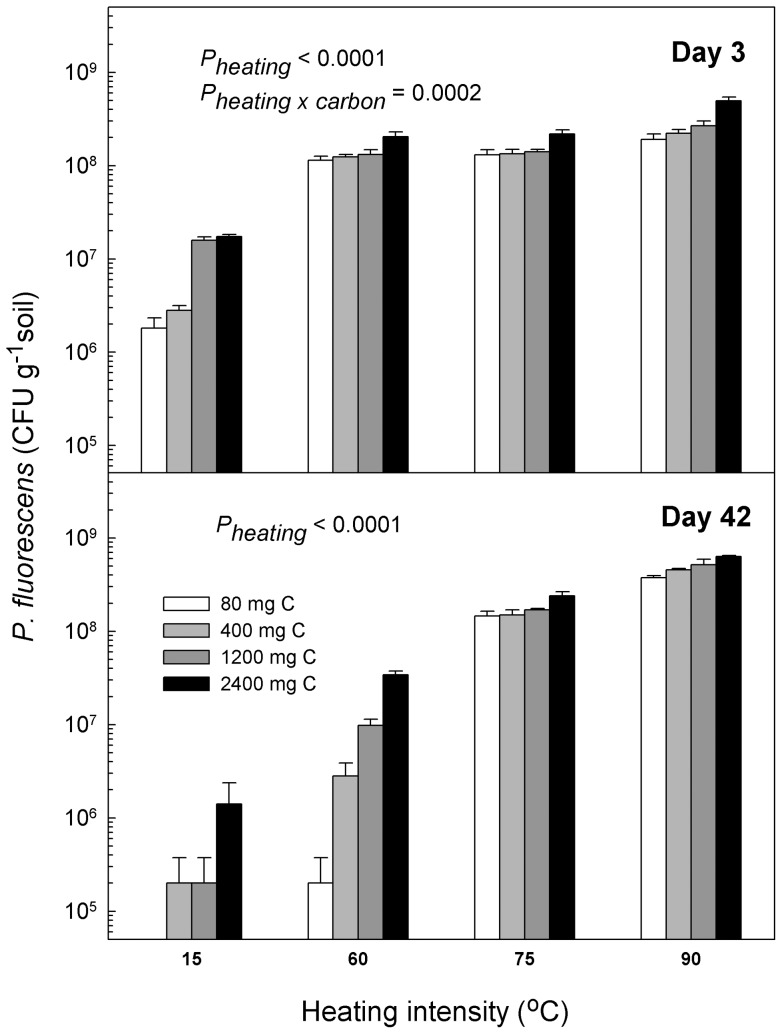
A three-way analysis of variance (ANOVA) (heating × sampling time × carbon amendment) revealed that heating significantly facilitated invasion of P. fluorescens DSM 50090 (Pheating = 0.03), whereas the effect of carbon amendment depended on sampling time (Pcarbon × sampling time = 0.046). To analyze this further, we separated the data sets for day 3 and day 42 and performed two separate two-way ANOVAs (heating × carbon amendment) for the two samplings (Figure 4). Here we found that heating significantly increased abundance of P. fluorescens DSM 50090 at both samplings (Pheating <0.0001). But whereas, 0n day 3, carbon amendment amplified the effect of heating significantly (Pcarbon × heating = 0.0002), this effect was not significant at day 42, although (Pcarbon × heating = 0.15). Thus, carbon amendment affected P. fluorescens DSM 50090 much stronger at day 3 than at day 42 (Figure 4).
Figure 4. Heating facilitates invasion of Pseudomonas fluorescens DSM 50090.
We studied invasion and establishment of the bacterium P. fluorescens DSM 50090 in soil microcosms exposed to different heating intensities (15°C, 60°C, 75°C or 90°C) for 24 h and subsequently amended with the bacterium. After heating we washed the microcosms to remove soluble carbon, and to each heating treatment we subsequently amended soluble carbon corresponding to the amount produced from the four heating treatments to separate the effects of heating on community structure and on release of soluble carbon. Heating permanently facilitated P. fluorescens DSM 50090, whereas the effect of carbon was transient. We performed two separate two-way ANOVA's (heating intensity × carbon amendment) for the two samplings. P-values refer to these two-way ANOVA's performed on the two different depicted situations. Data were log transformed to equalize variances.
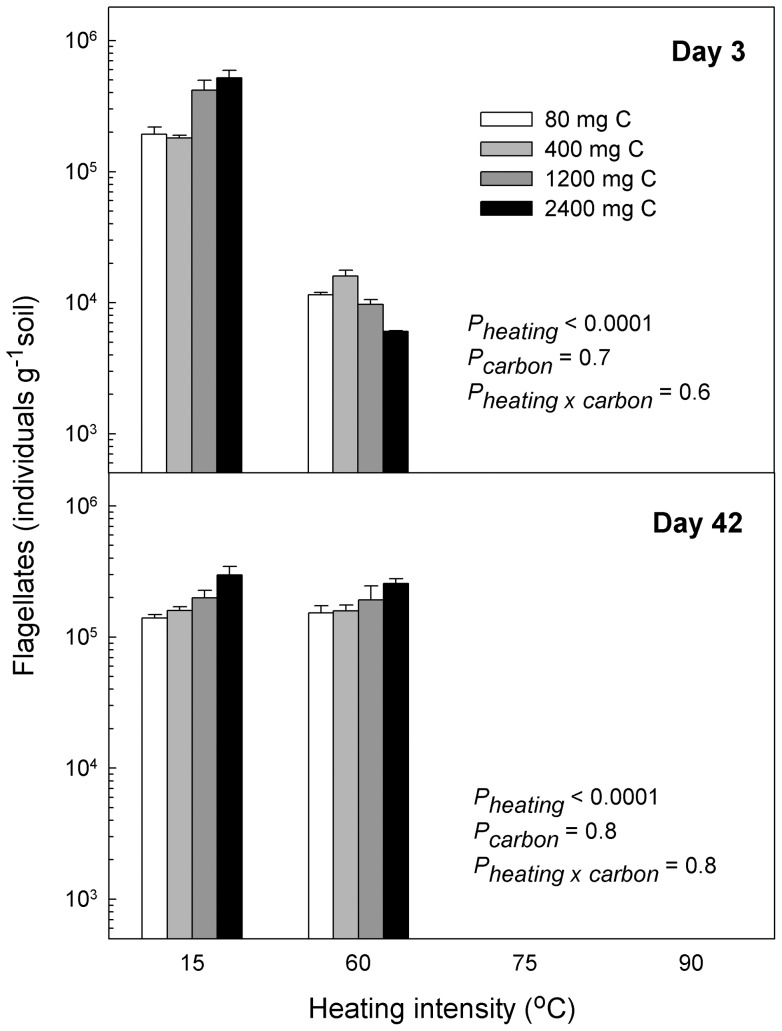
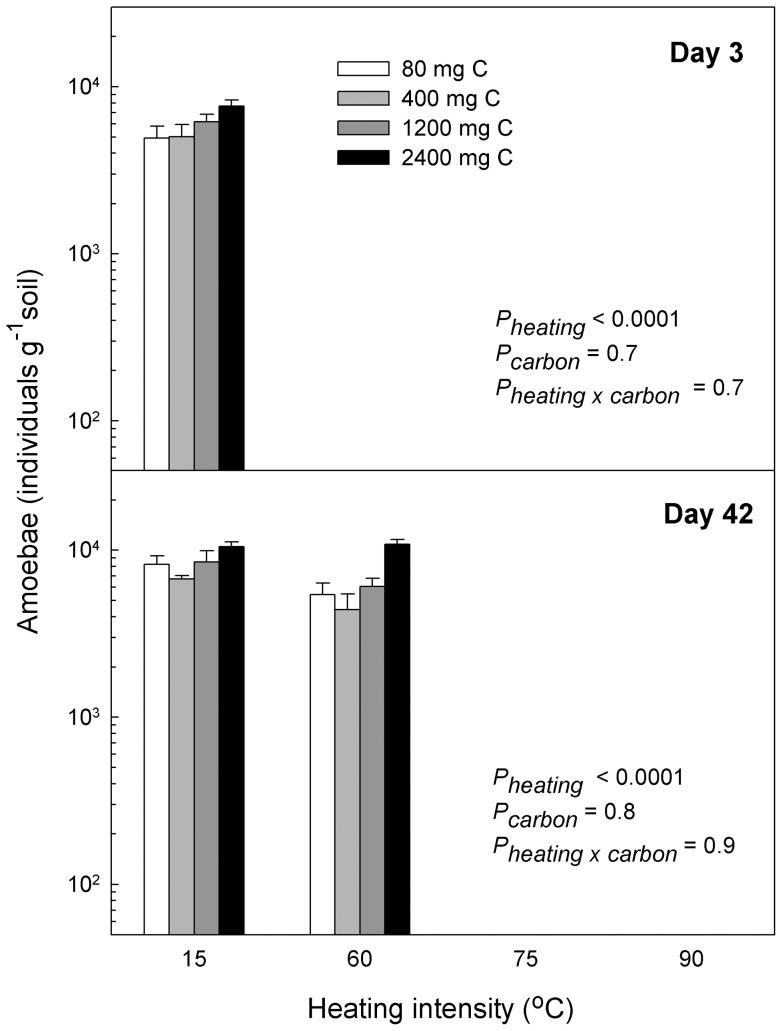
Protozoan numbers were only slightly affected by carbon amendment, though the treatments with highest carbon amendment generally had the highest numbers of flagellates and amoebae (Figures 5, 6). As in Experiment I, numbers of protozoa (especially amoebae) in the 60°C-heating treatment increased strongly between samplings (Figures 5, 6).
Figure 5. Heating decreased flagellate numbers.
We studied survival of cultivable heterotrophic flagellates in soil microcosms exposed to different heating intensities (15°C, 60°C, 75°C or 90°C) for 24 h. After heating we washed the microcosms to remove soluble carbon, and to each heating treatment we subsequently amended soluble carbon corresponding to that produced from the four heating treatments to separate the effects of heating on community structure and on release of soluble carbon. Heating to 75°C or 90°C permanently eliminated heterotrophic flagellates, whereas they recovered at 60°C heating. Carbon amendment had no significant effect on the heterotrophic flagellates. P-values refer to two-way ANOVA's performed on the two different situations: data were log transformed to equalize variances; all P-values shown.
Figure 6. Heating decreased numbers of amoebae.
We studied survival of cultivable amoebae in soil microcosms exposed to different heating intensities (15°C, 60°C, 75°C or 90°C) for 24 h. After heating we washed the microcosms to remove soluble carbon, and to each heating treatment we subsequently amended soluble carbon corresponding to that produced from the four heating treatments to separate the effects of heating on community structure and on release of soluble carbon. Heating to 75°C or 90°C permanently eliminated amoebae whereas they recovered at 60°C heating. Carbon amendment had no significant effect on the amoebae. P-values refer to two-way ANOVA's performed on the two different situations: data were log transformed to equalize variances; all P-values shown.
Discussion
First experiment: Disturbance facilitates colonization
As we anticipated, increased heating intensity caused increased system disturbance; indicated by reduced bacterial and protozoan numbers and by reduced functional diversity. In line with our hypotheses, we also observed that heat treatment facilitated invasion by P. fluorescens DSM 50090. Elton [4] hypothesized that high biodiversity impeded invasive organisms; a view which is shared by [11] and [19] for microbial communities. The decreased functional diversity at increased disturbance agrees with the conception that disturbance may reduce competition by decreasing indigenous diversity and biomass [20]. Predation is another factor, which impedes intrusion of foreign bacteria [21]; in line with this, we observed that protozoan numbers were inversely related to successful invasion of P. fluorescens DSM 50090.
The easily decomposable carbon released during heating may likewise facilitate invasion of P. fluorescens DSM 50090. Community resistance may be more important than avoiding initial invasion in reducing establishment of invaders [22]. Thus, we suggest that the combination of resource limitation and the successional recovery of the disturbed community towards a more mature stage will result in increasing relative importance of biotic resistance. We observed that under low or intermediate disturbance (i.e. 15°C and 60°C heating), P. fluorescens DSM 50090 declined from day 3 to day 42. In the early successional stages, the fast growing P. fluorescens DSM 50090 will benefit from both carbon produced during heating and from low competition and predation [23]. However, with time, resources will be depleted concomitantly with recovery of predators and competitors. At the more intense heating, (i.e. 75°C and 90°C), where the indigenous populations of competitors and predators are permanently reduced, P. fluorescens DSM 50090 can persist. To further explore the situation, we separated the heating effects on the indigenous community from the heating effects on the carbon release in the second experiment.
Second experiment: Separation of resource amendment and diversity reduction effects
We observed that resource amendment as well as community-diversity-reduction facilitated invasion of P. fluorescens DSM 50090 in our microcosms. Recently, the relative importance of resource availability and community diversity in determining the susceptibility of communities to invasion has become an issue. It has been suggested that resource availability is a primary factor in determining susceptibility to invasion [6], [24], [25]. Other researchers have though concluded that community diversity played the key role in resistance to invasion regardless of resource availability [26], [27]. In our experiments, diversity reduction was the more important factor for facilitation of invasion of P. fluorescens DSM 50090. Even though enhanced resource availability enhanced invasion success, it could not counterbalance the resistance in the systems with relatively higher community diversity.
Conclusion
Most studies on the relation between disturbance, resource availability, and community structure on one hand, and biological invasion on the other, have been performed in a macro-ecological context, mostly in plant communities, and only a small effort has been done to understand the importance of these factors in determining the invasion potential in microbial systems [12]. Here, we provided experimental evidence that the factors and mechanisms that govern invasion in microbial systems are much the same as in communities with macro-organisms. Increasing disturbance led to increasing invasion success of P. fluorescens DSM 50090, which could be attributed to increased resource availability as well as predation and competition release. Our second experiment suggested that effects on community structure were more essential for invasion success than increased resource availability.
We notice that our study may have practical implications for both micro- and macro-biology. In soil-remediation microbiology, it is a major issue that microorganisms artificially amended to soil mostly cannot survive due to competition and predation. The present results suggest that it is possible to facilitate survival of artificially amended soil organisms by accompanying their introduction with addition of organic substances or with soil fumigation. Extrapolation of the results to a macroscopic context, e.g. a plant community, would suggest that a temporary nutrient stress induces a reversible invasion, whereas a permanent habitat diversity-loss would make inversion irreversible. Hence, it could be hypothesized that habitat fragmentation, which permanently reduces habitat diversity, facilitates invasive species more than a transient fertiliser stress.
We suggest using microbial systems for experimental study of invasion scenarios, firstly they offer a simple and cost-efficient way to study and understand the mechanisms controlling biological invasion and consequently help us to better predict changes in stability of communities and ecosystems in response to anthropogenic disturbance and global change. Secondly, a thorough understanding of factors controlling invasion and establishment of artificially amended micro-organisms will mean a major step forward for soil-remediation microbiology.
Materials and Methods
Experimental preparations
We collected a surface silt loam soil (0–15 cm) from permanent grassland (Löddeköpinge, Sweden). The location is not privately-owned or protected in any way. No specific permits were required for the described field studies; the field studies did not involve endangered or protected species. The soil samples were homogenized and sieved (<2 mm) to remove large particles. Due to the high organic content (ca. 8%) in the soil, we mixed soil with quartz sand (1∶2) before use. The water holding capacity of soil-sand mixture was about 40%.
Microcosms were now prepared from 15.0 g (dry weight) of the soil-sand mixture in 117 ml serum bottles, each microcosm was further supplied with 3.0 ml distilled water to obtain a water content of 60% of water holding capacity. Bottles were covered with aluminium foil and incubated at 15°C for eight days to decompose easily available soil carbon. The bottles were then sealed with a rubber septum and heated to 15°C, 30°C, 45°C, 60°C, 75°C and 90°C for 24 h. After heating, bottles were cooled and opened in a fume-hood for 2 h to remove CO2 released during heating.
Three bottles from each heating treatment were now amended with 3.0 ml soil inoculum (15 g fresh soil shaken with 150 ml sterile water for 1 h, and three replicate bottles from each heating treatment with 3.0 ml sterile water. These bottles were incubated at 15°C for 4 weeks. Accumulated CO2 was measured daily at the beginning to weekly at the end of incubation. We found that in the absence of soil inoculum, CO2 emission was only significantly affected in microcosms exposed to 60°C, 75°C and 90°C. Hence, we concluded that soil diversity was not severely affected after heating to 30°C or 45°C. Therefore 15°C, 60°C, 75°C and 90°C were chosen for the final experiments.
First experiment: effect of heating
Prior to the experiments, a GFP-tagged Pseudomonas fluorescens DSM 50090 [28] was grown on Tryptic Soy Broth (0.3 gl−1, BactoTM, Becton, Dickinson and Company, New Jersey, USA) until mid-exponential growth phase (24 h), washed and re-suspended in phosphate buffer (Modified Neff's Amoeba Saline [29]), and centrifuged (5000 g, 10 min) three times. Microcosms were now heat treated (15°C, 60°C, 75°C, 90°C) and aerated for 2 h as above. For each of the four heating treatments,, we amended eight replicate microcosms with 3.0 ml sterile water containing 106 cells g soil−1 GFP-tagged P. fluorescens DSM 50090 and eight microcosms with 3.0 ml sterile water without bacteria. Microcosms were incubated at 15°C in the dark. Destructive samplings of four microcosms of each type were conducted twice, i.e. 3 and 42 days after experimental setup.
Sampling was done by adding 60 ml sterile phosphate buffer (Modified Neff's Amoeba Saline, [29]) to each microcosm and shaking them on an end-to-end shaker (180 rpm) for 30 min. The resulting suspension was used for the following procedures: Culturable bacteria were scored on agar-plates as in [30]. Fluorescent colonies of P. fluorescens DSM 50090 were counted on a UV transilluminator in a dark room. Protozoa were enumerated as in [31] using a 0.1 gl−1 Tryptic Soy Broth medium. Functional diversity was measured in EcoPlates (Biolog, California, USA) at the second sampling by further diluting the soil suspensions prepared above to 2.5×10−2. Aliquots of 125 μl of this suspension were added to each well in the EcoPlates, and the plates were incubated in the dark at 15°C for 21 days. The EcoPlates were read at 0 h and once every 24 h for 21 days using a Biorad Benchmark Microplate Reader (plates read at 595 nm; Amphotech Ltd., Massachusetts, USA). An absorbance above 0.5 was regarded as positive. The readings for 0 h were subtracted from all subsequent readings to correct for potential colour development due to particles added. The readings for day 21 were used to estimate the functional diversity, defined as the number of carbon sources utilized.
Second experiment: separation of carbon and community change effects
We obtained water-extractable organic carbon (WEOC) from heat treated microcosms, as prepared above, by adding 45 ml double distilled water and shaking for 30 min on an end to end shaker (120 rpm.). After sedimentation the liquid was centrifuged (5000 g, 10 min) and filtered (0.8 µm filter). The concentration of organic C in the filtrates was determined by dry combustion using an automated total C analyzer (TOC-5050, Shimadzu Corp., Tokyo, Japan).
To measure biodegradability of WEOC produced by the four heating treatments, we prepared three replicate bottles from 20.0 gram sterile sand, 20.0 ml WEOC from each treatment, and added 1.0 ml soil inoculum suspension (15 g fresh soil shaken with 150 ml water for 1 h, three replicates). Bottles were placed on an end-to end-shaker (150 rpm) at 20°C. CO2 accumulated in the bottles were continuously measured until the CO2 level was constant after two weeks. We found that the biodegradability of the WEOC for the four temperatures were: 15°C: 29 (1.9)%, 60°C: 32 (2.0)%, 75°C: 53 (2.0)%, and 90°C: 55 (2.6)%; numbers in brackets are standard errors. We only used WEOC obtained from 90°C treatment in the following experiment. By doing this, we minimised the risk of contamination of microcosms from WEOC, as most organisms in the 90°C treatment were killed.
To produce microcosms depleted of WEOC, we added 60 ml sterile water to bottles with soil exposed to each of the four heating levels, shook them (180 rpm, 30 min), centrifuged (5000 g, 10 min) and discarded the supernatant, this procedure was repeated twice. The third supernatant contained the same low WEOC concentration for all heating treatments. Remaining water was evaporated by leaving all bottles in a fume-hood for 24 h. The resulting WEOC depleted microcosms were slightly lower in CFU number (TSA, 0.3 gl−1) and functional diversity (indicated by Biolog EcoPlates) but did not differ significantly from unwashed microcosms in these values.
We now set up a factorial experiment. We used four levels of soil heating; i.e. the WEOC depleted microcosms described above, and four levels of resource carbon enrichment, i.e. WEOC from the 90°C treatment diluted to concentrations corresponding to that released from soil under the four heating treatments (i.e. 80, 400, 1200 and 2400 mg C kg−1 soil, for the 15°C, 60°C, 75°C and 90°C treatments, respectively). For each of these 16 treatment combinations, we set up six replicate microcosms, all amended with P. fluorescens DSM 50090. Amendment with P. fluorescens DSM 50090, incubation, CO2 measurement, and destructive sampling were as in Experiment I.
Statistical methods
We used SAS Enterprise Guide 4.1 interface to the SAS 9.1 package (Statistical Analysis System Institute, 2002–2003) to perform two or three way ANOVAs. When needed, data were transformed to equalize variances. We consider P-values <0.05 as significant and have thus reported these; to increase clarity larger P-values are reported in some cases.
Funding Statement
ML was fully sponsored by the National Foundation of Sciences in China (No. 30900210), the Chinese-Danish Government Exchange Scholarship, and the PADA (Priority Academic Program Development of Jiangsu Higher Education Institutions). FE received funding from The Danish Strategic Research Council (Det Strategiske Forskningsråd, DSF, 2104-08-0012; MIRESOWA). FE and RR received funding from the Danish Environmental Agency (MST: 667-00081, GENEPEASE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crawley MJ (1987) What makes a community invasible? In: Gray AJ, Crawley MJ, Edwards PJ, Editors. Colonization, Succession, and Stability. London: Blackwell Scientific publications. 429–453.
- 2. Levine JE (2000) Species diversity and biological invasions: relating local process to community pattern. Science 288: 852–854. [DOI] [PubMed] [Google Scholar]
- 3. Vitousek PM, D'Antonio CM, Loop LD, Rejmánek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. New Zeal J Ecol 21: 1–16. [Google Scholar]
- 4.Elton CS (1958) The ecology of invasions by animals and plants. London: Methuen 181 p.
- 5. Kneitel JM, Perrault D (2006) Disturbance-induced changes in community composition increase species invasion success. Community Ecol 7: 245–252. [Google Scholar]
- 6. Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88: 528–534. [Google Scholar]
- 7. Eschtruth AK, Battles JJ (2009) Assessing the relative importance of disturbance, herbivory, diversity, and propagule pressure in exotic plant invasion. Ecol Monogr 79: 265–280. [Google Scholar]
- 8. Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion-implications for conservations. Conserv Biol 6: 324–337. [Google Scholar]
- 9. Otfinowski R, Kenkel N (2010) Covariance between disturbance and soil resources dictates the invasibility of northern fescue prairies. Biol Invasions 12: 1349–1361. [Google Scholar]
- 10. Schooler SS, Cook T, Prichard G, Yeates AG (2010) Disturbance-mediated competition: the interacting roles of inundation regime and mechanical and herbicidal control in determining native and invasive plant abundance. Biol Invasions 12: 3289–3298. [Google Scholar]
- 11. van Elsas JD, Hill P, Chronakova A, Grekova M, Topalova Y, et al. (2007) Survival of genetically marked Escherichia coli O157: H7 in soil as affected by soil microbial community shifts. ISME J 1: 204–214. [DOI] [PubMed] [Google Scholar]
- 12. Litchman E (2010) Invisible invaders: non-pathogenic invasive microbes in aquatic and terrestrial ecosystems. Ecol Lett 13: 1560–1572. [DOI] [PubMed] [Google Scholar]
- 13. Fleming GM, Diffendorfer JE, Zedler PH (2009) The relative importance of disturbance and exotic-plant abundance in California coastal sage scrub. Ecol Appl 19: 2210–2227. [DOI] [PubMed] [Google Scholar]
- 14. Wolters V, Silver WL, Bignell DE, Coleman DC, Lavelle P, et al. (2000) Effects of global changes on above- and belowground biodiversity in terrestrial ecosystems: Implications for ecosystem functioning. BioScience 50: 1089–1098. [Google Scholar]
- 15. Chantigny MH, Curtin D, Beare MH, Greenfield LG (2010) Influence of temperature on water-extractable organic matter and ammonium production in mineral soils. Soil Sci Soc Am J 74: 517–524. [Google Scholar]
- 16. Griffiths BS, Kuan HL, Ritz K, Glover LA, McCaig AE, et al. (2004) The relationship between microbial community structure and functional stability, tested experimentally in an upland pasture soil. Microbial Ecol 47: 104–113. [DOI] [PubMed] [Google Scholar]
- 17. Adair EC, Burke IC, Lauenroth WK (2008) Contrasting effects of resource availability and plant mortality on plant community invasion by Bromus tectorum L. Plant Soil. 304: 103–115. [Google Scholar]
- 18. Davis MA, Pelsor M (2001) Experimental support for a resource-based mechanistic model of invasibility. Ecol Lett 4: 421–428. [Google Scholar]
- 19. Matos A, Kerkhof L, Garland JL (2005) Effects of microbial community diversity on the survival of Pseudomonas aeruginosa in the wheat rhizosphere. Microbial Ecol 49: 257–264. [DOI] [PubMed] [Google Scholar]
- 20. Clark GF, Johnston EL (2009) Propagule pressure and disturbance interact to overcome biotic resistance of marine invertebrate communities. Oikos 118: 1679–1686. [Google Scholar]
- 21. Ekelund F, Rønn R (1994) Notes on protozoa in agricultural soil, with emphasis on heterotrophic flagellates and naked amoebae and their ecology. FEMS Microbiol Rev 15: 321–353. [DOI] [PubMed] [Google Scholar]
- 22. Hooper DU, Dukes JS (2010) Functional composition controls invasion success in a California serpentine grassland. J Ecol 98: 764–777. [Google Scholar]
- 23. Wardle DA, Yeates GW (1993) The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: evidence from decomposer food-webs. Oecologia 93: 303–306. [DOI] [PubMed] [Google Scholar]
- 24. Fridley JD (2002) Resource availability dominates and alters the relationship between species diversity and ecosystem productivity in experimental plant communities. Oecologia 132: 271–277. [DOI] [PubMed] [Google Scholar]
- 25. Romanuk TN, Kolasa J (2005) Resource limitation, biodiversity, and competitive effects interact to determine the invasibility of rock pool microcosms. Biol Invasions 7: 711–722. [Google Scholar]
- 26. Maron J, Marler M (2007) Native plant diversity resists invasion at both low and high resource levels. Ecology 88: 2651–2661. [DOI] [PubMed] [Google Scholar]
- 27. Roscher C, Bessler H, Oelmann Y, Engels C, Wilcke W, et al. (2009) Resources, recruitment limitation and invader species identity determine pattern of spontaneous invasion in experimental grasslands. J Ecol 97: 32–47. [Google Scholar]
- 28. Pedersen AL, Nybroe O, Winding A, Ekelund F, Bjørnlund L (2009) Bacterial feeders, the nematode Caenorhabditis elegans and the flagellate Cercomonas longicauda, have different effects on outcome of competition among the Pseudomonas biocontrol strains CHA0 and DSS73. Microb Ecol 57: 501–509. [DOI] [PubMed] [Google Scholar]
- 29.Page FC (1988) A New Key to Freshwater and Soil Amoebae. Ambleside: Freshwater Biological Association, Scientific Publication.
- 30. Thirup L, Ekelund F, Johnsen K, Jacobsen CS (2000) Population dynamics of the fast-growing sub-populations of Pseudomonas and total bacteria, and their protozoan grazers, revealed by fenpropimorph treatment. Soil Biol. Biochem. 32: 1615–1623. [Google Scholar]
- 31. Rønn R, Ekelund F, Christensen S (1995) Optimizing soil extract and broth media for MPN-enumeration of naked amoebae and heterotrophic flagellates in soil. Pedobiologia 39: 10–19. [Google Scholar]