Abstract
Loss of the tumor suppressor Pdcd4 was reported for various tumor entities and proposed as a prognostic marker in tumorigenesis. We previously characterized decreased Pdcd4 protein stability in response to mitogenic stimuli, which resulted from p70S6K1-dependent protein phosphorylation, β-TrCP1-mediated ubiquitination, and proteasomal destruction. Following high-throughput screening of natural product extract libraries using a luciferase-based reporter assay to monitor phosphorylation-dependent proteasomal degradation of the tumor suppressor Pdcd4, we succeeded in showing that a crude extract from Eriophyllum lanatum stabilized Pdcd4 from TPA-induced degradation. Erioflorin was identified as the active component and inhibited not only degradation of the Pdcd4-luciferase-based reporter but also of endogenous Pdcd4 at low micromolar concentrations. Mechanistically, erioflorin interfered with the interaction between the E3-ubiquitin ligase β-TrCP1 and Pdcd4 in cell culture and in in vitro binding assays, consequently decreasing ubiquitination and degradation of Pdcd4. Interestingly, while erioflorin stabilized additional β-TrCP-targets (such as IκBα and β-catenin), it did not prevent the degradation of targets of other E3-ubiquitin ligases such as p21 (a Skp2-target) and HIF-1α (a pVHL-target), implying selectivity for β-TrCP. Moreover, erioflorin inhibited the tumor-associated activity of known Pdcd4- and IκBα-regulated αtranscription factors, that is, AP-1 and NF-κB, altered cell cycle progression and suppressed proliferation of various cancer cell lines. Our studies succeeded in identifying erioflorin as a novel Pdcd4 stabilizer that inhibits the interaction of Pdcd4 with the E3-ubiquitin ligase β-TrCP1. Inhibition of E3-ligase/target-protein interactions may offer the possibility to target degradation of specific proteins only as compared to general proteasome inhibition.
Introduction
Programmed cell death 4 (Pdcd4) is a novel tumor suppressor that inhibits translation rather than transcription. Specifically, Pdcd4 interferes with the activity of the eukaryotic initiation factor (eIF) 4A by displacing the scaffold protein eIF4G from its binding to the RNA helicase eIF4A [1]. As a consequence, Pdcd4 attenuates neoplastic transformation, AP-1 transactivation, intravasation, and invasion in vitro [2], [3]. In addition, Pdcd4-deficient mice were shown to be more susceptible to the two stage skin carcinogenesis model, whereas transgenic overexpression of Pdcd4 decreased papilloma incidence and multiplicity [4], [5]. In line, Pdcd4 is lost in various tumor entities such as lung, colon, breast, ovarian and pancreatic cancer [6], [7]. Interestingly, loss of Pdcd4 appears not to be attributable to mutational inactivation [8]. Instead, post-transcriptional regulatory mechanisms appear to control Pdcd4 expression in tumors. Specifically, in addition to miR-21-dependent repression of Pdcd4 expression [9], [10], increased proteasomal degradation was recently identified to determine Pdcd4 levels in response to mitogens and inflammatory tumor environments [5], [11], [12]. Mechanistically, Pdcd4 protein contains a p70S6K1 consensus phosphorylation sequence directly followed by the binding motif for the E3-ubiquitin ligase β-transducin repeat-containing protein (β-TrCP). Activation of p70S6K1 in response to mitogens such as the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) results in phosphorylation of Pdcd4, followed by binding of β-TrCP, polyubiquitination and subsequent proteasomal degradation [5], [11]. Overactivation of the PI3K-Akt-mTOR-p70S6K axis is common in many tumor types [13]. Consequently, interference with this signaling cascade is widely used for current tumor therapeutic regimens, e.g. mTOR inhibitors are in clinical use for the treatment of renal cell carcinomas and mantle-cell lymphomas [14].
The E3-ubiquitin ligase β-TrCP represents the substrate recognition subunit of the SCF (Skp1-Cul1-FBP) ligase complex that transfers ubiquitin molecules to label target-proteins for proteasomal degradation [15]. Despite the high diversity in reported β-TrCP-targets, including tumor suppressive (e.g. IκBα) and oncogenic factors (e.g. β-catenin), β-TrCP is considered an oncoprotein [16]. In line, cancer tissues are often associated with elevated β-TrCP levels [17]. Furthermore, β-TrCP-deficient cells were shown to be more sensitive to various anti-cancer drugs such as doxorubicin, tamoxifen and paclitaxel [18]. Targeting the ubiquitin proteasome system (UPS) for tumor therapeutic purposes was proven to be a promising approach by the introduction of the general proteasome inhibitor velcade (bortezomib) for the treatment of multiple myeloma [19]. As expected, the unselective inhibition of protein degradation causes adverse side effects, limiting the use of such an approach. Since substrate specificity of the UPS is achieved by the E3-ligases, this class of proteins offers a novel avenue for tumor therapies. To date, the only compounds targeting an E3-ubiquitin ligase in clinical trials are substances of the nutlin family that disrupt the Hdm2-p53 binding and, thus prevent Hdm2-induced p53 degradation [20]. Accordingly, attenuating the interaction of β-TrCP with its target-proteins could be a promising approach for the development of proteasomal degradation targeting drugs for tumor therapies. Stabilization of the β-TrCP-target Pdcd4 provides an attractive tool for the identification of novel β-TrCP-inhibitors, which might be further developed for use in anti-tumor therapies.
Therefore, we set out to identify novel stabilizers of the tumor suppressor Pdcd4, which interfere specifically with β-TrCP-mediated degradation of Pdcd4.
Materials and Methods
Materials
All chemicals were purchased from Sigma-Aldrich unless noted otherwise. Rapamycin and TPA (12-O-tetradecanolyphorbol-13-acetate) were from LC Laboratories. Anti-Pdcd4, anti-phospho-S6, anti-β-TrCP and anti-β-catenin antibody were obtained from Cell Signaling Technology. Anti-luciferase antibody came from Promega, anti-nucleolin and anti-IκB antibody from Santa Cruz Biotechnology, anti-HIF-1α and anti-p21 antibody were from BD Biosciences. IRDyes 680LT and 800CM secondary antibodies were purchased from Li-COR Biosciences GmbH and horseradish peroxidase (HRP)-coupled secondary antibodies came from GE Healthcare. Blasticidin and recombinant p70S6K1 were from Invitrogen. Protease and phosphatase inhibitor mix were obtained from Roche. pRK7-S6K expression plasmids were kindly provided by J. Blenis. pcDNA3-β-TrCP1 was kindly provided by M. Pagano. AP-1 and NF-κB reporter plasmids were previously described [21], [22]. pGL3-Pdcd4(39–91)luc was previously described [23]. pRL-TK expression vector for renilla luciferase was from Promega.
Cloning of Pdcd4 Constructs and Generation of Stable Cell Lines
For the generation of phosphorylation insensitive Pdcd4 constructs, serines 67, 71 and 76 were mutated to alanines in pcDNA3.1(+)-Pdcd4 plasmid [24] using the QuikChange kit (Agilent Technologies, Waldbronn, Germany) according to the manufacturer’s protocol. Reporter constructs for Pdcd4(39–91mut)luc were generated as previously described for Pdcd4(39–91)luc [23]. Briefly, a fragment of Pdcd4 (encoding amino acids 39–91) was amplified from the mutated vector. HindIII and NarI restriction sides were added to the Pdcd4-specific amplicons. The resulting fragment was fused to the luciferase expression cassette of the pGL3-control vector. The resulting Pdcd4(mut39–91)luc vector was used for transient transfections. For generating stable cell lines, EcoRI and BamHI restriction sides were introduced into the Pdcd4(mut39–91)luc pGL3-vector by PCR amplification and the resulting construct was inserted in a modified pFB-neo plasmid where the neomycin cassette has been replaced by a blasticidin resistance cassette. Stable cell lines were created by retroviral gene transfer as described before [23]. All sequences were confirmed by sequence analysis.
Cell Culture
All cell lines (HEK, MCF7, HeLa, RKO) came from LGC Standard GmbH and were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U mL–1 penicillin, 100 µg mL–1 streptomycin and 2 mM L-glutamine. HeLa cells were maintained in MEM supplemented with 10% FBS, 100 U mL–1 penicillin, 100 µg mL–1 streptomycin and 2 mM L-glutamine. Stable HEK293 Pdcd4-luc cells were maintained in regular growth medium supplemented with 3 µg mL–1 blasticidin. Cells were cultivated in a humidified atmosphere with 5% CO2 at 37°C. Medium and supplements came from PAA and FBS was purchased from Biochrom.
Plant Material, Extraction, and Isolation
Samples of Eriophyllum lanatum var. grandiflorum (A. Gray) Jeps. were collected on a bank below a coniferous forest 27 miles east of Crescent City, CA in July 1997. The collection and identification were done by William Hess, Morton Arboretum Herbarium, Lisle, IL. A voucher specimen (collection number 0GDK760) is maintained at the Smithsonian Institution. The dried plant material (562 g) was ground and extracted by immersion in CH2Cl2-MeOH (1∶1) for 15 h in a Soxhlet apparatus [25]. The solvent was removed and the plant material was immersed for 15 h in 100% MeOH. The combined extracts were reduced to dryness in vacuo to give 36.8 g of crude extract. A portion of this extract (1.54 g) was subjected to a solvent-solvent partitioning scheme [26] that concentrated the Pdcd4 stabilizing activity in the ethyl acetate soluble fraction (288 mg). Size exclusion chromatography of this material on Sephadex LH-20 (2×75 cm) eluted with CH2Cl2-MeOH (1∶1) provided five major fractions (A–E). Fraction C (112 mg) was further purified by reversed-phase HPLC (Rainin; Dynamax C18, size 21.4×250 mm; 60 µm) eluting with a gradient of 10–100% acetonitrile in 0.05% aqueous TFA over 40 min at a flow rate of 8 mL min–1. Final separation by reversed-phase HPLC (Rainin; Dynamax C18, size 4.6×250 mm; 60 µm) eluting with a gradient of 20–40% acetonitrile in 0.05% aqueous TFA for 40 min at a flow rate of 1 mL/min provided 12.1 mg of erioflorin (0.8% yield).
Assignment of the 1H-NMR Data of Erioflorin
1H-NMR (600 MHz, CDCl3) δ 1.32 (1H, dd, J = 1.6, 12.6 Hz, H-9b), 1.44 (3H, s, H-14), 1.73 (1H, ddd, J = 2.0, 8.5, 12.4 Hz, H-2b), 1.80 (3H, d, J = 0.75, H-15), 1.90 (3H, s, H-4′), 2.45 (1H, dt, J = 3.7, 12.4 Hz, H-2a), 2.79 (1H, dd, J = 3.7, 8.3 Hz, H-1), 2.81 (1H, dd, J = 3.7, 12.6 Hz, H-9a), 2.87 (1H, m, H-7), 4.48 (1H, dd, J = 2.0, 3.7 Hz, H-3), 5.16 (1H, m, H-8), 5.31 (1H, dq, J = 1.0, 9.1 Hz, H-5), 5.59 (1H, brs, H-3′), 5.75 (1H, d, J = 1.5 Hz, H-13a), 6.09 (1H, brs, H-3′), 6.35 (1H, d, J = 1.5 Hz, H-13b ), 6.65 (1H, dd, J = 1.6, 9.1 Hz, H-6); HREIMS m/z 349.1635 [M + H]+ (Calcd for C19H25O6, 349.1646).
Luciferase Assays
HEK293 cells stably expressing either Pdcd4(39–91)luc or Pdcd4(mut39–91)luc were seeded in a 96-well plate (1×104/well) and allowed to attach for 18 h before treatment. After incubations, cells were harvested in firefly luciferase lysis buffer (25 mM Tris, 2 mM DTT, 1% Triton X 100, 10% glycerol, pH 7.8) and frozen at –20°C for at least 2 h. After lysis at room temperature, luminescence was measured using firefly luciferase substrate solution (20 mM tricine, 2.67 mM 4MgCO3*Mg(OH)2*5H2O, 1.07 mM MgSO4*7H2O, 100 µM EDTA, 33.3 mM dithiothreitol (DTT), 530 µM ATP, 0.213 mg mL–1 coenzym A, 470 mM D-luciferin) on a Mithras LB 940 (Berthold, Bad Wildbad, Germany).
p70S6K expression plasmids were transiently co-transfected with pGL3-Pdcd4(39–91)luc or pGL3-Pdcd4(mut39–91)luc and a renilla luciferase reporter plasmid into HEK293 cells (1×104) in 96-well plates using the calcium phosphate precipitation method [27]. Briefly, plasmids were incubated for 15 min at room temperature in the presence of 125 mM CaCl2 and HBS buffer (25 mM HEPES, 140 mM NaCl, 0.75 mM Na2HPO4, 5 mM KCl, pH 7.1) and added drop-wise to cells. Eight hours later medium was changed and incubations were continued for another 16-h period before cells were treated as indicated. Cells were lysed in passive lysis buffer (Promega, Mannheim, Germany). Firefly luciferase activity was determined as described above and renilla luciferase activity was measured with renilla substrate solution (0.1 M NaCl, 25 mM Tris-HCl pH 7.5, 1 mM CaCl2, 1 µM coelenterazine).
AP-1 and NF-κB reporter plasmids were transfected and analysis was performed as described above.
Western Analysis
For western analysis, HEK293 cells (5×105/6 cm-dish) were treated as indicated, harvested by centrifugation in ice cold PBS and lysed in lysis buffer (50 mM Tris-HCl pH 6.8, 5 mM EDTA, 6.65 M urea, 1% SDS, 10% glycerol, 1 mM phenylmethylsulfonylfluoride (PMSF), 1 x protease and phosphatase inhibitor mix, 1 mM Na3VO4, 1 mM DTT). 80 µg protein were separated via SDS-PAGE, transferred onto nitrocellulose membranes and analyzed using specific antibodies with appropriate secondary antibodies. They were visualized using either the Odyssey infrared imaging system (Li-COR Biosciences GmbH) or enhanced chemiluminescence detection.
Immunoprecipitation Assays
For immunoprecipitation assays, HEK293 cells (1×106/10 cm-dish) were transiently transfected with β-TrCP1 expression plasmid or with a plasmid expressing HA-tagged ubiquitin as described above. One day after transfection, cells were treated as indicated. After treatment, cells were lysed on ice for 30 min in immunoprecipitation (IP) buffer (50 mM Tris-HCl pH 7.4, 300 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM PMSF, 1 x protease and phosphatase inhibitor mix, 1 mM Na3VO4). 1 mg protein was incubated with either 5 µL anti-Pdcd4- or 5 µL anti-luciferase-antibody in 300 µL IP buffer for 6 h. Then, 20 µL 50% slurry of Protein A Sepharose (Sigma-Aldrich) were added and incubated overnight. Sepharose was precipitated by centrifugation and washed three times with IP buffer. Proteins were eluted by addition of 2 x loading buffer and incubation at 95°C for 5 min. Immunoprecipitated proteins were separated via SDS-PAGE and visualized using western analysis with the indicated antibodies. Whole cell extracts served as loading control and analysis was performed as described above.
In Vitro-Transcription/Translation Assay
Pdcd4 and β-TrCP1 proteins were generated by in vitro-transcription/translation from pcDNA3.1(+)-Pdcd4 and pcDNA3-β-TrCP1, respectively, using the TNT Coupled Reticulocyte Lysate System from Promega according to manufacturer’s protocol. Interaction reactions were performed for 90 min at 30°C in a volume of 15 µL containing 50 mM Tris-HCl pH 7.6, 5 mM MgCl2, 2 mM ATP, 4 µL Pdcd4, 3 µL β-TrCP1 and 160 nM recombinant p70S6K1 as indicated. Immunoprecipitation and western analyses were performed as described above.
Viability Assay
HEK293 cells (1×104/well) were seeded in a 96-well plate and allowed to attach for 18 h. Cells were treated as indicated and viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay from Promega according to the manufacturer’s protocol.
Proliferation Assay
RKO, HeLa and MCF7 cells (1×104/well) were seeded in 96-well plates one day prior to the experiment. Cells were treated as indicated and analyzed in an IncuCyte® Live-Cell Imaging System (Essen Bioscience) by microscopic determination of monolayer confluency.
Cell Cycle Analysis
RKO, HeLa and MCF7 cells (1×105/3 cm-dish) were treated as indicated. Cells were harvested by centrifugation in PBS, lysed in PBS+ (PBS including 1.1 g L-1 glucose and 0.5 mM EDTA) and centrifuged again. Pellets were incubated in PBS+ + RNase (PBS+ including 50 µg mL–1 RNase) for 15 min. DNA was stained by incubation for 15 min with 10% NP-40 and 10 µg propidium iodide. Stained cells were analyzed using a LSR Fortessa flow cytometer (BD Bioscience). Cell cycle phase distribution was calculated with FlowJo software.
Scratch Wound Assay
RKO cells (5×105/well) were seeded in 24-well plates. Upon reaching 100% confluency scratches were administered using a 10 µL pipet tip. After removal of medium and floating cells, cells were treated as indicated and pictures were taken using a Zeiss microscope Axiovert 200 M. Wound closure was calculated as scratch width at 24 h relative to initial wound size.
Statistical Analysis
Each experiment was performed at least three times. Representative blots are shown. Data are presented as means ± SEM. Significance analysis was performed using Student’s t-test.
Results and Discussion
Erioflorin Stabilizes Pdcd4 from TPA-Induced Degradation
We have previously shown that TPA induces the phosphorylation-dependent proteasomal degradation of Pdcd4 [5] and further introduced a luciferase-based assay to identify compounds stabilizing Pdcd4 from TPA-induced degradation [23], [28], [29]. Briefly, a vector spanning the domain containing both the p70S6K1-phosphorylation and the β-TrCP-recognition motifs (aa 39–91) fused to luciferase (Pdcd4(39–91)luc) served as a sensitive tool to analyze TPA-induced Pdcd4 degradation. As natural products provide a rich source for the development of novel therapeutics, which is supported by the fact that 50% of all small molecule drugs introduced to the market between 1981 and 2008 (>60% for cancer therapeutics) were either natural products or derived from natural products [30], we carried out a high-throughput screen of 135,678 natural product extracts using this approach. We identified an extract from the woolly sunflower Eriophyllum lanatum (Asteraceae) to increase the luciferase signal relative to TPA-treated controls. Sequential fractionation of the extract by solvent partitioning, size-exclusion chromatography, and C18 HPLC provided erioflorin as the active agent. Erioflorin is a sesquiterpene lactone possessing a tricyclic germacranolide skeleton (Figure 1A). The structure was established by spectroscopic analyses and comparison of its spectral data with values reported in the literature. A complete assignment of the 1H NMR data for erioflorin was made as only a partial assignment has been published to date [31]. Our data corresponded closely to those reported for heliangine [32], a structurally related germacranolide sesquiterpene that only differs from erioflorin by the composition of the ester side-chain at C-8. The 13C NMR spectral data we recorded for erioflorin were fully consistent with previously reported values [33]. The relative configuration of erioflorin was confirmed by extensive ROESY correlation data.
Figure 1. Erioflorin stabilizes Pdcd4(39–91)luc from TPA-induced degradation.
(A) Structure of erioflorin (Mwt = 348.4). (B) Stably Pdcd4(39–91)luc expressing HEK293 cells were treated for 8 h with TPA (10 nM) with increasing concentrations of erioflorin (0.0625–10 µM). Pdcd4 stabilizing activity was determined relative to Δ(RLUcontrol–RLUTPA-only). (C) Stably Pdcd4(mut39–91)luc expressing HEK293 cells were treated as in (B). Luciferase activity is given relative to TPA-treated controls. All data are presented as means ± SEM (n≥3, *p<0.05, **p<0.01).
Dose-response studies revealed that erioflorin significantly rescued Pdcd4 from 8 h TPA-induced degradation at concentrations as low as 1.25 µM (36.7±7.5%) (Figure 1B). For a detailed calculation of the Pdcd4(39–91)luc stabilizing activity see Figure S1A and B. Maximal recovery of luciferase activity was achieved with 5 µM erioflorin (75.5±7.4%). At concentrations at or above 10 µM erioflorin luciferase activity was markedly reduced. HEK293 cells stably expressing the Pdcd4(39–91)luc construct harboring S67/71/76A mutations (Pdcd4(mut39–91)luc), i.e. an inactive phospho-degron, which prevents phosphorylation and degradation, were used to determine nonspecific effects. As no significant reduction of the luciferase signal in response to TPA-only treatment in these cells was detected, activity was calculated relative to TPA-only treated cells (Figure S1C). As shown in Figure 1C, erioflorin concentrations up to 5 µM, when co-incubated with TPA, did not affect luciferase activity, whereas concentrations at or above 10 µM significantly decreased the luciferase signal. As viability of the cells was not affected at these concentrations of erioflorin in combination with TPA (Figure S3A), the loss of luciferase signal was attributed to nonspecific inhibition of the luciferase vectors. This observation is in accordance with a previous report showing that other sesquiterpene lactones, such as parthenolide, inhibit firefly luciferase activity [34].
Based on these results, we propose that erioflorin potently stabilizes Pdcd4(39–91)luc, as an indicator of Pdcd4 protein stability, from TPA-induced degradation.
Erioflorin Stabilizes Pdcd4 not via p70S6K1 Inactivation
In line with the concept that TPA-induced degradation of Pdcd4 requires active PI3K-mTOR-p70S6K signaling [5], [11], inhibition of p70S6K [35] or upstream factors such as BCR/ABL [36] was previously shown to block Pdcd4 degradation. Thus, we next analyzed the effect of erioflorin on p70S6K activity. To this end, HEK293 cells were transiently co-transfected with expression vectors for wild type p70S6K (S6Kwt) or a constitutively active p70S6K mutant (S6Kca) in combination with either the degradable Pdcd4(39–91)luc or the stable Pdcd4(mut39–91)luc construct. The activity of the Pdcd4(mut39–91)luc construct was generally higher than the activity of the Pdcd4(39–91)luc construct when either S6Kwt or S6Kca were overexpressed (Figure S2), which verifies that the intact phospho-degron is required to respond to p70S6K. Since p70S6K1 requires activation by the upstream kinase mTOR, the mTOR inhibitor rapamycin was expected to affect the activity of S6Kwt but not of S6Kca. As anticipated, rapamycin (100 nM) increased Pdcd4(39–91)luc activity significantly more in S6Kwt than in S6Kca expressing cells. In contrast, erioflorin showed no significant difference in its ability to stabilize the Pdcd4(39–91)luc signal under S6Kwt or S6Kca co-expressing conditions (Figure 2A), which indicates that erioflorin enhances Pdcd4 stability downstream of p70S6K activity. Pdcd4(mut39–91)luc activity was neither affected by S6Kwt nor by S6Kca and consequently remained unaffected by treatment with rapamycin or erioflorin (Figure 2B).
Figure 2. Erioflorin stabilizes Pdcd4 without affecting phosphorylation events.
(A + B) HEK293 cells were transiently transfected with Pdcd4(39–91)luc (A) or Pdcd4(mut39–91)luc (B) firefly reporter vectors, in combination with expression vectors for either wildtype (S6Kwt = white bars) or constitutively active p70S6K (S6Kca = black bars) and a renilla luciferase vector one day prior to the experiment. Transfected cells were treated for 8 h with rapamycin (100 nM) or erioflorin (5 µM). Firefly normalized to renilla luciferase activity is presented relative to DMSO-treated controls. (C) HEK293 cells were treated for 8 h with TPA (10 nM) with or without erioflorin (0.625–5 µM). Whole-cell extracts were subjected to western analysis and probed with the indicated antibodies. Blots are representative of at least three independent experiments. Densitometric analysis and quantification of nucleolin-normalized Pdcd4 and phospho-S6 protein levels is shown relative to the DMSO control. (D) HEK293 cells were treated with DMSO (black diamonds), TPA (10 nM) with (white triangles) or without (gray squares) erioflorin (5 µM) for 8 h and cycloheximide (10 µM) was added for 1, 2 or 4 h. Pdcd4 protein levels were analyzed densitometrically, normalized to nucleolin and the half-life was calculated. All data are presented as means ± SEM (n≥3, *p<0.05, **p<0.01, ***p<0.001).
These observations serve as a first indication that erioflorin stabilizes Pdcd4 without interfering with p70S6K activity that is required for its phosphorylation.
Erioflorin Protects Endogenous Pdcd4 from TPA-Induced Degradation
To confirm that erioflorin not only stabilizes Pdcd4(39–91)luc from TPA-induced degradation but is also effective at the level of endogenous Pdcd4 protein, we next analyzed Pdcd4 protein expression upon TPA treatment with or without erioflorin. Figure 2C (upper panel) shows that Pdcd4 protein was markedly reduced when HEK293 cells were exposed to TPA (10 nM) for 8 h. Erioflorin rescued Pdcd4 from TPA-induced degradation at low micromolar concentrations. Quantitative analysis revealed that Pdcd4 protein was reduced to 49.9±7.1% of the DMSO control in response to TPA treatment. Erioflorin rescued Pdcd4 protein expression from TPA-induced loss in a concentration-dependent manner from 71.1±4.7% at 0.625 µM to 121.1±8.6% at 5 µM as compared to the DMSO control (Figure 2C, middle panel). Importantly, erioflorin rescued Pdcd4 protein levels from TPA-induced degradation in the tumor cell lines MCF7 (breast) and RKO (colon) in a similar fashion (Figure S4). Thus, erioflorin-mediated Pdcd4 stabilization appears to be a general mechanism rather than a cell line specific phenomenon. Furthermore, blocking de novo protein synthesis with cycloheximide (10 µM), revealed that TPA-restricted Pdcd4 protein half-life (1.2±0.2 h) was significantly extended by erioflorin co-treatment (1.9±0.3 h) (Figure 2D). To verify that this rescue was indeed independent of an effect on p70S6K1 activity, we analyzed phosphorylation of a prototypical p70S6K1-target, ribosomal protein S6. While S6-phosphorylation was increased in response to TPA to 269.9±58.4% of the DMSO control, erioflorin did not significantly change TPA-induced S6-phosphorylation (Figure 2C, upper and lower panel).
Taken together, we conclude that erioflorin stabilizes endogenous Pdcd4 from TPA-induced degradation not by interfering with the phosphorylation of the latter, but rather by mechanisms affecting the degradation of already phosphorylated Pdcd4.
Erioflorin Disrupts the β-TrCP1/Pdcd4-Interaction
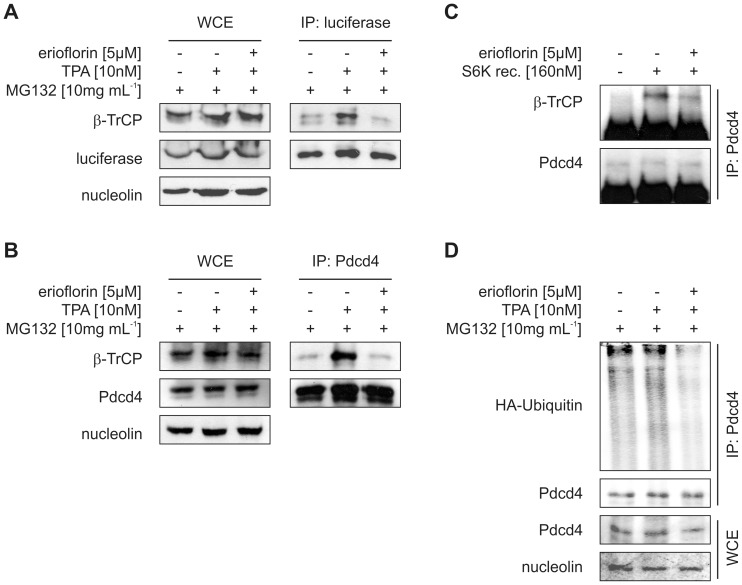
As phosphorylated Pdcd4 is recognized by the E3-ubiquitin ligase β-TrCP1, which mediates polyubiquitination and subsequent proteasomal degradation of its targets [11], we next determined the effect of erioflorin on the intracellular binding of β-TrCP1 to Pdcd4. Binding of transiently overexpressed β-TrCP1 to Pdcd4(39–91)luc increased upon treatment with TPA (8 h, 10 nM). Erioflorin (5 µM) significantly diminished the TPA-induced interaction between Pdcd4(39–91)luc and β-TrCP1 (Figure 3A, right panel). Similarly, β-TrCP1 co-immunoprecipitated with endogenous Pdcd4 in response to TPA (8 h, 10 nM), which again was significantly attenuated by 5 µM erioflorin (Figure 3B, right panel). To verify that erioflorin directly targets the interaction of Pdcd4 with β-TrCP1, we studied the effect of erioflorin on β-TrCP1-Pdcd4-binding in vitro. As anticipated, in vitro-transcribed/translated β-TrCP1 bound to in vitro-transcribed/translated Pdcd4 only in the presence of recombinant p70S6K. This binding was markedly reduced in the presence of erioflorin (Figure 3C). This observation not only supports inhibition of the interaction between β-TrCP1 and its target Pdcd4 by erioflorin, but also suggests that inhibition of additional factors within the proteasomal degradation machinery is not required for erioflorin-dependent stabilization of Pdcd4. As E3-ligases mediate ubiquitination of interacting target-proteins, we next addressed if erioflorin affects TPA-induced ubiquitination of Pdcd4. Therefore, binding of transiently expressed HA-ubiquitin to Pdcd4 was determined by immunoprecipitation. Indeed, Pdcd4 ubiquitination in TPA-treated cells (10 nM, 8 h) was reduced dramatically by co-treatment with erioflorin (5 µM) (Figure 3D).
Figure 3. Erioflorin stabilizes Pdcd4 by interfering with β-TrCP1.
(A) Stably Pdcd4(39–91)luc expressing or (B) wildtype HEK293 cells were transfected with a β-TrCP1 expression vector one day prior to the experiment. Transfected cells were treated for 8 h with DMSO or TPA (10 nM) with or without erioflorin (5 µM) in the presence of the proteasome inhibitor MG132 (10 mg mL–1). Pull-down of luciferase (A) or Pdcd4 (B) out of whole-cell extracts was performed using specific antibodies. Subsequently, immunoprecipitated (IP) proteins and whole cell extracts (WCE) were subjected to western analysis and probed with the indicated antibodies. (C) In vitro-transcribed/translated β-TrCP1 and Pdcd4 proteins were co-incubated for 90 min at 30°C with or without recombinant p70S6K1 in the absence or presence of erioflorin (5 µM). Pdcd4 protein was immunoprecipitated and β-TrCP1 binding to Pdcd4 was analyzed by western analysis. (D) HEK293 cells were transfected with a plasmid expressing HA-tagged ubiquitin. Transfected cells were treated for 8 h with DMSO or TPA (10 nM) with or without erioflorin (5 µM) in the presence of the proteasome inhibitor MG132 (10 mg mL–1). Proteins co-immunoprecipitated with endogenous Pdcd4 and whole-cell extracts were analyzed by western analysis with the indicated antibodies. All blots are representative for at least three independent experiments.
Taken together, these data indicate that erioflorin stabilizes Pdcd4 by interfering with the interaction of Pdcd4 with the E3-ligase β-TrCP1, without affecting Pdcd4 phosphorylation.
Erioflorin Specifically Stabilizes Targets of the E3-Ligase β-TrCP
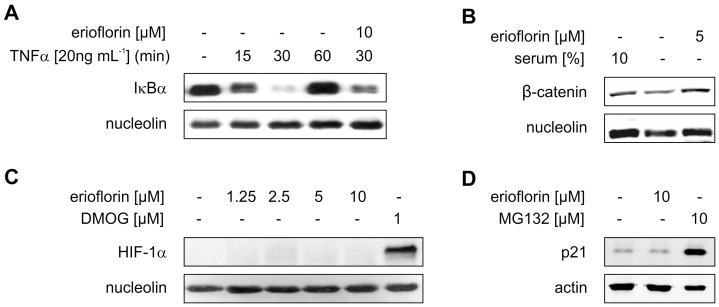
In an attempt to gain insights into the selectivity of erioflorin, we questioned whether erioflorin only interferes with the interaction between Pdcd4 and β-TrCP or if other β-TrCP targets are stabilized as well. Among the numerous proteins targeted for degradation by β-TrCP [37], IκBα is among the best characterized ones [38]. When IκBα is phosphorylated by IKKs it is recognized and bound by β-TRCP and marked for proteasomal degradation, allowing for activation of the transcription factor NF-κB [39]. In line, activation of IKKs by TNFα (20 ng mL–1) led to the rapid, but transient decrease in IκBα protein levels in HEK293 cells (Figure 4A). Reduced IκBα was observed already after 15 min and was maximal at 30 min of TNFα treatment (lane 3). Longer treatments with TNFα (60 min) allowed for a complete recovery of the protein. Erioflorin (10 µM) stabilized IκBα at 30 min of TNFα (compare lanes 1, 3 and 5). Similarly, erioflorin (8 h, 5 µM) stabilized the β-TrCP-target β-catenin, which is phosphorylated by the glycogen synthase kinase 3β (GSK3β) [40], from serum deprivation-induced degradation (Figure 4B). These results suggest that erioflorin does not interfere exclusively with the interaction of Pdcd4 and β-TrCP, instead it appears to stabilize various β-TrCP-targets. We next determined if targets of other E3-ligases are affected by erioflorin as well. Therefore, we analyzed the effect of erioflorin on the stability of HIF-1α protein, which is targeted for proteasomal degradation by the E3-ligase von Hippel-Lindau protein (pVHL) [41]. Inhibition of the prolyl-hydroxylases, which mark HIF-1α for interaction with pVHL, using 1 µM dimethyloxalylglycine (DMOG) for 8 h resulted in strong accumulation of HIF-1α protein (Figure 4C). However, erioflorin (up to 10 µM) did not stabilize HIF-1α. Along the same line, p21, a target of the closely related SCF-E3-ligase Skp2 [42], strongly accumulated when proteasomal degradation was blocked with MG132 (10 µM) for 8 h whereas it did not increase in response to erioflorin (5 µM) (Figure 4D). Thus, we propose that erioflorin stabilizes β-TrCP targets by interfering with the interaction of β-TrCP1 with them, while leaving targets of other E3-ligases unaffected. The fact that erioflorin did not stabilize targets of other E3-ligases can be taken as an indicator that it does not affect enzymes that would be expected to affect broader target spectra such as E1- or E2-enzymes. Importantly, further studies are required to establish the exact site of action of erioflorin, i.e. if it directly interacts with β-TrCP or rather with phospho-degrons on the target-proteins.
Figure 4. Erioflorin specifically inhibits E3-ligase β-TrCP1 activity.
(A) HEK293 cells were treated with TNFα (20 ng mL–1) for the indicated times with or without erioflorin (10 µM). (B) HEK293 cells were maintained under full medium conditions (10% serum) or serum deprived for 24 h following treatment with erioflorin (5 µM) for 8 h. (C) HEK293 cells were treated for 8 h with erioflorin (1.25–10 µM) or the prolyl-hydroxylase inhibitor dimethyloxallylglycine (DMOG, 1 µM). (D) HeLa cells were serum deprived for 48 h prior to treatment with erioflorin (10 µM) or the proteasome inhibitor MG132 (10 µM) for 8 h. Whole cell extracts were subjected to western analysis and probed with the indicated antibodies. Blots are representative for at least three independent experiments.
Taken together, our results imply that erioflorin specifically inhibits the interaction of the E3-ligase β-TrCP1 with various of its targets, while it does not affect the stability of proteins regulated by other E3-ligases. E3-ubiquitin ligases have been increasingly appreciated as powerful new therapeutic targets in recent years [43], [44]. MLN4924 was identified to inhibit cullin-RING E3-ubiquitin ligases (CRL) by attenuating the NEDD8-activating enzyme and thus, preventing neddylation of CRLs [45]. In addition, various compounds have been identified to inhibit the activity of or the interaction with targets of specific E3-ligases such as Skp2 [46], Mdm2 [47], Met30 [48] or Cdc4 [49]. Yet, erioflorin appears as the first compound interfering with the degradation of various β-TrCP1 targets such as Pdcd4.
Erioflorin Reduces AP-1- and NF-κB-Dependent Transcription
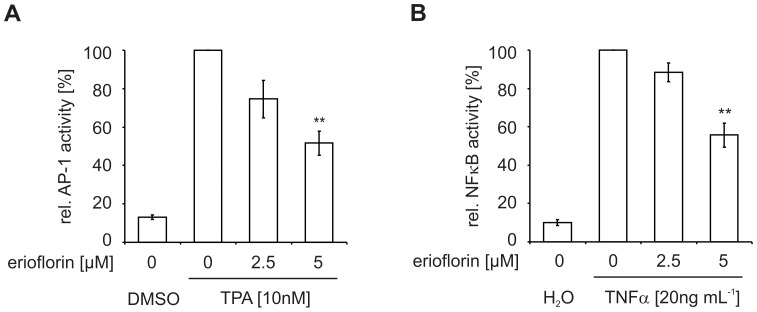
As β-TrCP was suggested to be pro-tumorigenic, despite its broad target-protein spectrum including both pro- (β-catenin) and anti-oncogenic proteins (Pdcd4, IκBs, BimEL) [37], we next aimed at determining the functional, tumor-related consequences of erioflorin. Initially, we analyzed its impact on the activity of the tumor-associated transcription factors AP-1 and NF-κB. AP-1 was chosen based on previous reports, which showed that Pdcd4 affects AP-1 activity [24], [50], while NF-κB was selected since it is the direct target of the above analyzed IκBα [39]. To this end, HEK293 cells were transiently transfected with an AP-1 reporter vector one day prior to the experiment. Treatment with TPA (10 nM, 16 h) strongly induced AP-1 activity. Erioflorin reduced TPA-induced AP-1 activity to 74.6±9.7% at 2.5 µM and to 51.7±6.2% at 5 µM (Figure 5A). This effect was not due to toxicity as TPA in combination with erioflorin (up to 20 µM) was not toxic in this setting (Figure S3A). To assess the impact of erioflorin on NF-κB activity, HEK293 cells were transiently transfected with a NF-κB reporter vector one day prior to the experiment. Treatment with TNFα (20 ng mL–1, 16 h) strongly induced NF-κB activity. While erioflorin co-treatment only slightly inhibited TNFα-induced NF-κB activity at 2.5 µM, 5 µM erioflorin sufficed to significantly reduce the activity to 55.7±6.3% of TNFα-only treated cells (Figure 5B). Again, erioflorin (up to 5 µM) in combination with TNFα did not adversely affect cell viability in these cells (Figure S3B).
Figure 5. Erioflorin inhibits AP-1- and NF-κB-trancriptional activities.
(A) HEK293 cells were co-transfected with an AP-1 firefly reporter and a renilla luciferase plasmid one day prior to experiments. Transfected cells were treated for 16 h with TPA (10 nM) with or without erioflorin (2.5 and 5 µM). Relative AP-1 activity was normalized to renilla luciferase and presented relative to TPA-only treated cells. (B) HEK293 cells were co-transfected with a NF-κB firefly reporter and a renilla luciferase plasmid one day prior to experiments. Transfected cells were treated for 16 h with TNFα (20 mg mL–1) with or without erioflorin (2.5 and 5 µM). Relative NF-κB activity was normalized to renilla luciferase and presented relative to TNFα-only treated cells. All data are given as means ± SEM (n≥3, **p<0.01).
These results imply that erioflorin not only stabilizes β-TrCP-target-proteins, but also inhibits transcriptional activity of downstream effectors such as AP-1 and NF-κB.
Erioflorin Shows Anti-Proliferative Potential In Vitro
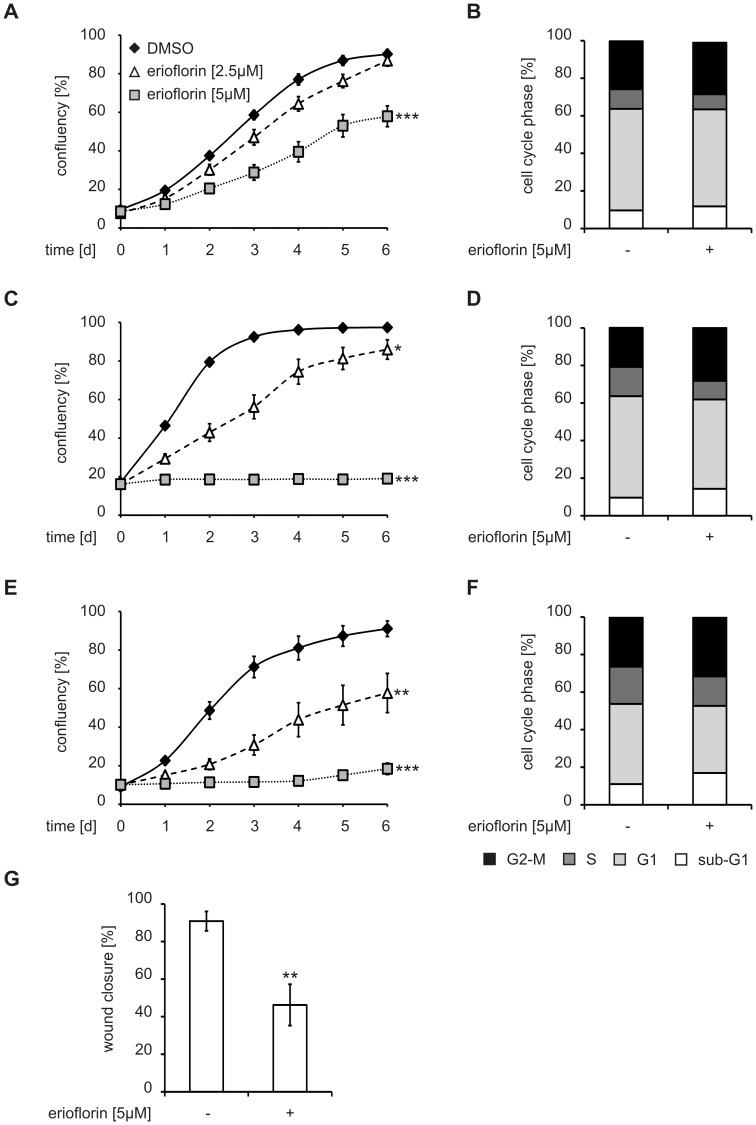
Both AP-1 and NF-κB have been extensively shown to play an important role in tumorigenesis and tumor cell proliferation. Therefore, we next addressed the influence of erioflorin on the proliferation of various cancer cell lines. Erioflorin treatment (2.5 and 5 µM) reduced proliferation of MCF7 (weakly), HeLa (moderately-strongly) and RKO cells (strongly) (Figure 6A, C, E). This is in line with a recent report demonstrating that inhibition of β-TrCP by genetic means attenuated viability and proliferation of breast cancer cells and rendered them more sensitive to various anticancer drugs [18]. Interestingly, cell cycle analysis revealed that, while MCF7 cells showed only minor changes in cell cycle distribution in response to erioflorin (5 µM), both HeLa and RKO cells displayed a pronounced increase in G2/M- and subG1-phases, whereas G1- and S-phases were reduced (Figure 6B, D, F and Table S1). These findings closely correlate with our observations on proliferation, where MCF7 cells also appeared to be least sensitive. Interestingly, while the increase in sub-G1, i.e. apoptosis, in the tumor cell lines (RKO>HeLa>MCF7) in response to erioflorin appears to contradict previous observations that the viability of HEK293 cells was not affected by erioflorin (Figure S3), this might be due to different experimental conditions and/or cell types. Specifically, the lack of toxicity in HEK293 cells fits well with the observed sequence of sensitivities, i.e. more advanced tumor cells (e.g. RKO) being more sensitive to erioflorin than non-invasive tumor cells (e.g. MCF7) or non-tumorigenic cells (e.g. HEK293). Therefore, we lastly performed scratch wound assays using the highly invasive, yet most erioflorin-sensitive colon carcinoma cell line RKO. RKO cells efficiently closed (90.9±5.2%) scratches in confluent cell layers at 24 h, whereas erioflorin (5 µM) significantly inhibited wound closure to 53.8±11.0% (Figure 6G). These observations further strengthen the notion that disrupting the interaction between β-TrCP and its targets could open a novel avenue for the development of future anti-cancer therapeutics.
Figure 6. Erioflorin inhibits cell proliferation and alters cell cycle progression.
(A, C, E) MCF7, HeLa and RKO cells were seeded at 10–20% confluency one day prior to the experiment and treated with DMSO (black diamonds) or erioflorin (2.5 (white triangles) and 5 µM (gray squares)). Cell confluency was followed for six days. (B, D, F) MCF7, HeLa and RKO cells were serum deprived for 48 h and treated with erioflorin (5 µM) for 16 h. After propidium iodide staining, distribution of the cells to the different phases of the cell cycle (subG1 (white), G1 (light gray), S (dark gray), G2/M (black)) was determined. (G) RKO colon carcinoma cells were subjected to a scratch wound assay. After administration of the scratch, medium was changed to control or erioflorin (5 µM) containing medium. Wound closure was measured after 24 h and relative wound closure is given as the ratio of the width of the scratch at 24 h and to 0 h. All data are given as means ± SEM (n≥3, *p<0.05, **p<0.01, ***p<0.001).
In summary, these data show that erioflorin suppresses tumor-associated events such as activation of AP-1- and NF-κB-dependent transcription and in addition alters cell cycle progression resulting in inhibition of proliferation in tumor cells. Interestingly, erioflorin appears to affect proliferation and cell cycle progression preferentially in more advanced tumor cell lines as compared to non-invasive lines or non-tumorigenic lines, which might be interest with respect to the development of future tumor therapeutics targeting β-TrCP.
Conclusion
This is the first report characterizing a natural product, small molecule compound that tumor suppressor Pdcd4 by interrupting its interaction with the E3-ligase β-TrCP. As erioflorin selectively stabilizes a number of β-TrCP targets leaving targets of other E3-ligases unaffected, it appears to functionally work as a β-TrCP inhibitor. We further provide evidence that erioflorin holds anti-tumorigenic potential as it inhibits AP-1 and NF-κB transcriptional activity, and interferes with cell cycle progression and proliferation of tumor cells. Based on the distinct set of proteins targeted by β-TrCP1 for degradation, we predict that erioflorin will generate less adverse side effects as compared to general proteasome inhibitors currently used in tumor therapy.
Supporting Information
Conversion of relative Pdcd4(39–91)luciferase light units to relative Pdcd4(39–91) stabilizing activity. (A) Stably Pdcd4(39–91)luc expressing HEK293 cells were treated for 8 h with TPA (10 nM) with or without rapamycin (100 nM). Relative light units (RLU) of the Pdcd4(39–91)luciferase fusion protein were normalized to DMSO. (B) Experiment from (A). Pdcd4(39–91) stabilizing activity of rapamycin was determined relative to Δ(RLUcontrol–RLUTPA-only). (C) Stably Pdcd4(mut39–91)luc expressing HEK293 cells were treated as in (A). Luciferase activity is given relative to TPA-treated controls.
(DOC)
Expression of Pdcd4(39–91)luc and Pdcd4(mut39–91)luc in the presence of overexpressed p70S6K. HEK293 cells were transiently transfected with Pdcd4(39–91)luc (left bars) or Pdcd4(mut39–91)luc (right bars) firefly reporter vectors, in combination with expression vectors for either wildtype (S6Kwt = white bars) or constitutively active p70S6K (S6Kca = black bars) and a renilla luciferase vector one day prior to the experiment. Firefly luciferase levels were normalized to renilla luciferase and presented relative to Pdcd4(39–91)luc/S6Kwt (relative light units = RLU).
(DOC)
Erioflorin does not influence cell viability in combination with TPA or TNFα. (A) HEK293 cells were treated for 16 h with TPA (10 nM) with or without erioflorin (2.5 to 20 µM). Cell viability was analyzed using the CellTiter glow assay (Promega) according to manufacturer’s protocol and is given relative to DMSO-treated controls. (B) HEK293 cells were treated for 16 h with TNFα (20 ng mL–1) or with or without erioflorin (2.5 and 5 µM). Cell viability was analyzed as described in A.
(DOC)
Erioflorin stabilizes Pdcd4 in breast and colon carcinoma cells. (A) MCF7 and (B) RKO cells were treated for 8 h with TPA (10 nM) with or without erioflorin (10 µM). Whole-cell extracts were subjected to Western analysis and probed with the indicated antibodies.
(DOCX)
Cell cycle distribution in response to erioflorin.
(DOC)
Acknowledgments
We thank M. Talamini and A. Baker for excellent technical assistance, D. Newman and T. McCloud for sample collection and extraction.
Funding Statement
This work was supported by the DFG (BR999 and GRK1172) and the LOEWE Schwerpunkt OSF (III L 4-518/55.004 (2009)) funded by the Hessian Ministry of Higher Education, Research and Arts. This project was funded in part with Federal funds from the Frederick National Laboratory for Cancer Research, National Cancer Institute, National Institutes of Health (NIH), under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. This research was also supported in part by the Intramural Research Program of NIH, Frederick National Laboratory for Cancer Research, National Cancer Institute, Center for Cancer Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, et al. (2003) The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 23: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang HS, Knies JL, Stark C, Colburn NH (2003) Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene 22: 3712–3720. [DOI] [PubMed] [Google Scholar]
- 3. Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, et al. (2007) Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene 26: 4550–4562. [DOI] [PubMed] [Google Scholar]
- 4. Jansen AP, Camalier CE, Colburn NH (2005) Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res 65: 6034–6041. [DOI] [PubMed] [Google Scholar]
- 5. Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, et al. (2008) Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res 68: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 6. Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, et al. (2007) Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 110: 1697–1707. [DOI] [PubMed] [Google Scholar]
- 7. Wei NA, Liu SS, Leung TH, Tam KF, Liao XY, et al. (2009) Loss of Programmed cell death 4 (Pdcd4) associates with the progression of ovarian cancer. Mol Cancer 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jansen AP, Camalier CE, Stark C, Colburn NH (2004) Characterization of programmed cell death 4 in multiple human cancers reveals a novel enhancer of drug sensitivity. Mol Cancer Ther 3: 103–110. [PubMed] [Google Scholar]
- 9. Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, et al. (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11: 141–147. [DOI] [PubMed] [Google Scholar]
- 10. Allgayer H (2010) Pdcd4, a colon cancer prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol 73: 185–191. [DOI] [PubMed] [Google Scholar]
- 11. Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, et al. (2006) S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314: 467–471. [DOI] [PubMed] [Google Scholar]
- 12. Schmid T, Bajer MM, Blees JS, Eifler LK, Milke L, et al. (2011) Inflammation-induced loss of Pdcd4 is mediated by phosphorylation-dependent degradation. Carcinogenesis 32: 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2: 489–501. [DOI] [PubMed] [Google Scholar]
- 14. Dancey J (2010) mTOR signaling and drug development in cancer. Nat Rev Clin Oncol 7: 209–219. [DOI] [PubMed] [Google Scholar]
- 15. Cardozo T, Pagano M (2004) The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol 5: 739–751. [DOI] [PubMed] [Google Scholar]
- 16. Frescas D, Pagano M (2008) Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8: 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ougolkov A, Zhang B, Yamashita K, Bilim V, Mai M, et al. (2004) Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. J Natl Cancer Inst 96: 1161–1170. [DOI] [PubMed] [Google Scholar]
- 18. Tang W, Li Y, Yu D, Thomas-Tikhonenko A, Spiegelman VS, et al. (2005) Targeting beta-transducin repeat-containing protein E3 ubiquitin ligase augments the effects of antitumor drugs on breast cancer cells. Cancer Res 65: 1904–1908. [DOI] [PubMed] [Google Scholar]
- 19. Adams J, Kauffman M (2004) Development of the proteasome inhibitor Velcade (Bortezomib). Cancer Invest 22: 304–311. [DOI] [PubMed] [Google Scholar]
- 20. Patel S, Player MR (2008) Small-molecule inhibitors of the p53-HDM2 interaction for the treatment of cancer. Expert Opin Investig Drugs 17: 1865–1882. [DOI] [PubMed] [Google Scholar]
- 21. Li JJ, Westergaard C, Ghosh P, Colburn NH (1997) Inhibitors of both nuclear factor-kappaB and activator protein-1 activation block the neoplastic transformation response. Cancer Res 57: 3569–3576. [PubMed] [Google Scholar]
- 22. von Knethen A, Callsen D, Brune B (1999) Superoxide attenuates macrophage apoptosis by NF-kappa B and AP-1 activation that promotes cyclooxygenase-2 expression. J Immunol 163: 2858–2866. [PubMed] [Google Scholar]
- 23. Blees JS, Schmid T, Thomas CL, Baker AR, Benson L, et al. (2010) Development of a high-throughput cell-based reporter assay to identify stabilizers of tumor suppressor Pdcd4. J Biomol Screen 15: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, et al. (2001) A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene 20: 669–676. [DOI] [PubMed] [Google Scholar]
- 25.McCloud TG (2010) High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules 4526–4563. [DOI] [PMC free article] [PubMed]
- 26. Meragelman KM, McKee TC, Boyd MR (2000) Siamenol, a new carbazole alkaloid from Murraya siamensis. J Nat Prod 63: 427–428. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- 28. Zhao LX, Huang SX, Tang SK, Jiang CL, Duan Y, et al. (2011) Actinopolysporins A-C and tubercidin as a Pdcd4 stabilizer from the halophilic actinomycete Actinopolyspora erythraea YIM 90600. J Nat Prod 74: 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grkovic T, Blees JS, Colburn NH, Schmid T, Thomas CL, et al. (2011) Cryptocaryols A-H, alpha-pyrone-containing 1,3-polyols from Cryptocarya sp. implicated in stabilizing the tumor suppressor Pdcd4. J Nat Prod 74: 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109: 3012–3043. [DOI] [PubMed] [Google Scholar]
- 31. Torrance SJ, Geissman TA, Chedekel MR (1969) Sesquiterpene lactones. The constituents of Eriophyllum confertiflorum. Phytochemistry 8: 2381–2392. [Google Scholar]
- 32. Bohlmann F, Gupta RK, Jakupovic J, King RM, Robinson H (1981) Eudesmanolides and heliangolides from Calea rotundifolia. Phytochemistry 20: 1635–1637. [Google Scholar]
- 33. Morimoto H, Oshio H (1981) Isolation of deacetylviguiestenin and erioflorin from Helianthus tuberosus. J Nat Prod 44: 748–749. [Google Scholar]
- 34. Lindenmeyer MT, Garcia-Pineres AJ, Castro V, Merfort I (2004) Sesquiterpene lactones inhibit luciferase but not beta-galactosidase activity in vitro and ex vivo. Anal Biochem 328: 147–154. [DOI] [PubMed] [Google Scholar]
- 35. Akar U, Ozpolat B, Mehta K, Lopez-Berestein G, Zhang D, et al. (2010) Targeting p70S6K prevented lung metastasis in a breast cancer xenograft model. Mol Cancer Ther 9: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 36. Carayol N, Katsoulidis E, Sassano A, Altman JK, Druker BJ, et al. (2008) Suppression of programmed cell death 4 (PDCD4) protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J Biol Chem 283: 8601–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skaar JR, D'Angiolella V, Pagan JK, Pagano M (2009) SnapShot: F Box Proteins II. Cell 137: 1358, 1358 e1351. [DOI] [PubMed]
- 38. Tan P, Fuchs SY, Chen A, Wu K, Gomez C, et al. (1999) Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Mol Cell 3: 527–533. [DOI] [PubMed] [Google Scholar]
- 39. Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18: 2195–2224. [DOI] [PubMed] [Google Scholar]
- 40. Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997) beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, et al. (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2: 423–427. [DOI] [PubMed] [Google Scholar]
- 42. Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, et al. (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 278: 25752–25757. [DOI] [PubMed] [Google Scholar]
- 43. Jia L, Sun Y (2011) SCF E3 ubiquitin ligases as anticancer targets. Curr Cancer Drug Targets 11: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nalepa G, Rolfe M, Harper JW (2006) Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov 5: 596–613. [DOI] [PubMed] [Google Scholar]
- 45. Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, et al. (2009) An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458: 732–736. [DOI] [PubMed] [Google Scholar]
- 46. Chen Q, Xie W, Kuhn DJ, Voorhees PM, Lopez-Girona A, et al. (2008) Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood 111: 4690–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, et al. (2004) In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848. [DOI] [PubMed] [Google Scholar]
- 48. Aghajan M, Jonai N, Flick K, Fu F, Luo M, et al. (2010) Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat Biotechnol 28: 738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Orlicky S, Tang X, Neduva V, Elowe N, Brown ED, et al. (2010) An allosteric inhibitor of substrate recognition by the SCF(Cdc4) ubiquitin ligase. Nat Biotechnol 28: 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, et al. (2006) Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol 26: 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conversion of relative Pdcd4(39–91)luciferase light units to relative Pdcd4(39–91) stabilizing activity. (A) Stably Pdcd4(39–91)luc expressing HEK293 cells were treated for 8 h with TPA (10 nM) with or without rapamycin (100 nM). Relative light units (RLU) of the Pdcd4(39–91)luciferase fusion protein were normalized to DMSO. (B) Experiment from (A). Pdcd4(39–91) stabilizing activity of rapamycin was determined relative to Δ(RLUcontrol–RLUTPA-only). (C) Stably Pdcd4(mut39–91)luc expressing HEK293 cells were treated as in (A). Luciferase activity is given relative to TPA-treated controls.
(DOC)
Expression of Pdcd4(39–91)luc and Pdcd4(mut39–91)luc in the presence of overexpressed p70S6K. HEK293 cells were transiently transfected with Pdcd4(39–91)luc (left bars) or Pdcd4(mut39–91)luc (right bars) firefly reporter vectors, in combination with expression vectors for either wildtype (S6Kwt = white bars) or constitutively active p70S6K (S6Kca = black bars) and a renilla luciferase vector one day prior to the experiment. Firefly luciferase levels were normalized to renilla luciferase and presented relative to Pdcd4(39–91)luc/S6Kwt (relative light units = RLU).
(DOC)
Erioflorin does not influence cell viability in combination with TPA or TNFα. (A) HEK293 cells were treated for 16 h with TPA (10 nM) with or without erioflorin (2.5 to 20 µM). Cell viability was analyzed using the CellTiter glow assay (Promega) according to manufacturer’s protocol and is given relative to DMSO-treated controls. (B) HEK293 cells were treated for 16 h with TNFα (20 ng mL–1) or with or without erioflorin (2.5 and 5 µM). Cell viability was analyzed as described in A.
(DOC)
Erioflorin stabilizes Pdcd4 in breast and colon carcinoma cells. (A) MCF7 and (B) RKO cells were treated for 8 h with TPA (10 nM) with or without erioflorin (10 µM). Whole-cell extracts were subjected to Western analysis and probed with the indicated antibodies.
(DOCX)
Cell cycle distribution in response to erioflorin.
(DOC)