Abstract
Background
Age disparity in thyroid cancer incidence and outcome in adolescents and young adults (AYAs) with thyroid cancer are underreported. We compared the molecular and clinical features of papillary thyroid cancer (PTC) in AYAs to other age groups.
Methods
1,011 patients underwent initial treatment for PTC at the University of California, San Francisco. Patients were subdivided into two age groups: 15–39 (AYA group) and 40+ years. Demographic, clinical and survival data in our cohort was also compared to SEER data. In a subset of the study cohort, the primary tumors were analyzed by genome-wide expression analysis, genotyping for common somatic mutations, and pathway specific gene expression array among the age groups.
Results
The percentage of women and lymph node metastasis rate were significantly higher in the AYA group. In the AYA group, the rate of distant metastasis was lower. The disease-free survival and median overall survival of AYAs was significantly higher. The better survival in our AYA patients was also apparent in the national SEER data. Unsupervised cluster analysis of gene expression data showed no distinct clustering in 96 PTC samples by age. The frequency and type of somatic mutations in the primary tumors were not significantly different between age groups (AYAs vs. 40+ years). Six genes (ECM1, ERBB2, UPA, PFKFB2, MEIS2 and CA2) were significantly differentially expressed between age groups.
Conclusions
The extent of disease at presentation and survival in AYA with PTC is different than in older patients. This difference may be due to several candidate genes that are differentially expressed and which may have important roles in tumor cell biology. No distinct gene expression profile exists in PTC between AYA and 40+ groups.
INTRODUCTION
The incidence of thyroid cancer, the most common endocrine malignancy, has doubled over the last 30 years, probably because of the increased detection of small, low risk papillary thyroid cancer (PTC).1, 2 Among adolescents and young adults (AYA) between the ages of 15–39 years, thyroid cancer is the fifth most common cancer and most cases are due to PTC. However, thyroid cancer remains underreported in this age group.3 In general, AYA participation in clinical trials seems to be in `no-man's land' between pediatric and adult oncologist and endocrinologists.4 Although differentiated thyroid cancer of follicular cell origin in adolescents and young adults often presents as more advanced disease with a higher prevalence of nodal and distant metastases, these younger patients have an excellent prognosis.5, 6
The disparity in cancer incidence and outcome in AYAs may be due to age-related differences in tumor biology.3 In 2006 the U.S. National Cancer Institute initiated a Progress Review Group on AYA oncology to investigate a potential biologic basis of age-related differences in outcome for adolescents and young adults with cancer. We sought to compare the clinical and molecular features as well as therapeutic outcome of AYAs with PTC with those for older age groups.
METHODS
Patients and Comparison Data from the SEER Program
We retrospectively analyzed the records of 1,011 patients who underwent initial treatment for PTC (conventional or follicular variant of papillary) at the University of California San Francisco (UCSF) Medical Center, between January 1983 and December 2003. The selection criterion for the study population was a follow-up of at least 5 years. The specific variables studied were age, gender, TNM-stage, type of surgical resection, I-131 treatment, clinical disease-free survival, and overall survival. Patients were subdivided into two age-groups: 15-39 (adolescent and young adult group, AYA) and ≥ 40 years old. We compared demographic, clinical data and overall survival outcomes for the AYA and 40+ age groups with data in the U.S. Surveillance, Epidemiology and End Results (SEER) Program from 1973–2006 (n= 46,680). The SEER program of the National Cancer Institute (NCI) currently represents approximately 26% of all cancer cases in the US population. This study was approved by the UCSF Committee on Human Research.
Genotyping for somatic mutations
Available primary PTC tumor samples from 254 patients (109 AYAs, 145 40+) were genotyped for common activating mutations that occur in PTC: BRAF V600E point mutation, RET/PTC1, RET/PTC3 and NTRK1 rearrangements, and hotspot point mutations in KRAS and NRAS. Point mutations in BRAFV600E, KRAS and NRAS were detected by PCR and direct sequencing. RET/PTC1, RET/PTC3 and NTRK1 were detected by nested PCR. The primers sets used to detect point mutations and rearrangements are listed in Table 1. We compared the number and type of mutations in the AYA age group with the 40+ age group.
Table 1.
PCR primers for BRAF, KRAS and NRAS hotspot mutations, and RET/PTC1, RET/PTC3 and NTRK1 chromosomal rearrangments
| Gene | Codon | Primer Sequences (5'–3'; a, forward: b, reverse) |
|---|---|---|
| HRAS | 12/13 | a 5'-ATGACGGAATATAAGCTGGT-3' |
| b 5'-CTCTATAGTGGGGTCGTATT-3' | ||
| HRAS | 61 | a 5'-AGGTGGTCATTGATGGGGAG-3' |
| b 5'-AGGAAGCCCTCCCCGGTGCG-3' | ||
| KRAS | 12/13 | a 5'-GGCCTGCTGAAAATGACTGAA-3' |
| b 5'-GGTCCTGCACCAGTAATATGC-3' | ||
| KRAS | 61 | a 5'-CAGGATTCCTACAGGAAGCAAGTAG-3' |
| b 5'-CACAAAGAAAGCCCTCCCCA-3' | ||
| NRAS | 12/13 | a 5'-ATGACTGAGTACAAACTGGT-3' |
| b 5'-CTCTATGGTGGGATCATATT-3' | ||
| NRAS | 61 | a 5'-TCTTACAGAAAACAAGTGGT-3' |
| b 5'-AGCGGATAACAATTTCACACAGGC CAA AAA TTTAATCAGTGGA-3' | ||
| BRAF | 600 | a 5'-TGTAAAACGACGGCCAGTCATAATGCTTGCTCTGA TAG GA-3' |
| b 5'-AGCGGATAACAATTTCACACAGGCCAA AAATTTAATCAGTGGA-3' | ||
| RET/PTC1 | a 5'-GCT GGA GAC CTA CAA ACT GA-3' | |
| b 5'-GTT GCC TTG ACC ACT TTT C-3' | ||
| Nested primer | a 5'-ACA AAC TGA AGT GCA AGG CA-3' | |
| b 5'-GCC TTG ACC ACT ACT TTT CCA AA-3' | ||
| RET/PTC3 | a 5'-AAG CAA ACC TGC CAG TGG-3' | |
| b 5'-CTT TCA GCA TCT TCA CGG-3' | ||
| Nested primer | a 5'-CCT GCC AGT GGT TAT CAA GC-3' | |
| b 5'-GGC CAC CGT GGT GTA CCC TG-3' | ||
| NTRK1 | a 5'-TGAGCAGATTAGACTGATGG-3' | |
| b 5'-GGAAGAGGCAGGCAAAGAC-3' | ||
| Nested primer | a 5'-GCTGCCGAAGAAAAGTACTC-3' | |
| b 5'-TTTCGTCCTTCTTCTCCACC-3' |
Analysis of pathway specific array genes
In our previous studies, we had found 39 genes in the pathway specific array analyses (genes involved in cell cycle, angiogenesis, and extracellular matrix and cell adhesion) to be significantly differentially expressed in thyroid cancer.7, 8,9 The expression levels of these genes were compared between the AYA group (n = 16) and the 40+ group (n = 22) in available samples. We also compared the expression levels of genes that we previously found to be associated with extent of disease in thyroid cancer (EGFR, ECM1, EFNB2, MCM7) between AYA (n = 106) and 40+ (n = 151) patients.7,8,9 Gene expression levels were determined by real-time quantitative TaqMan (Applied Biosystems, Foster City) RT-PCR in individual samples as previously reported.7,8,9 Differences in threshold cycles (delta-Ct) were used to compare gene expression levels between groups.
Genome-wide gene expression analysis
We used the Affymetrix GeneChip (U133 plus 2.0) array (~54,675 probe set including 38,500 well characterized human genes) to identify differences in gene expression between the two age groups in 96 available PTC samples (AYA group, n=46; 40+ group, n=50). RNA samples were hybridized using the Human Genome U133 plus 2.0 Chip (Affymetrix Inc, Santa Clara, CA) according to the manufacturer's instructions. The criteria used for differential gene expression between age groups were: (1) two-fold higher or lower gene expression levels; and (2) p value < 0.05 by t-test. We also used gene set enrichment analysis (GSEA) to determine any possible differences in biological pathways between the two age groups.10, 11
Data analysis
The data is presented as proportion or mean ± standard deviation (SD). Differences between the AYA group and the 40+ group for categorical data were analyzed using χ2 test and for continuous or nonparametric data using t-test or Wilcoxon Rank test. The method of Kaplan-Meier was used to estimate disease-free and overall survival rates, possible prognostic factors in the age groups were compared using the log rank test. All analyses were performed using SAS (version 9.1.3, SAS Institute, Cary, NC). A p value < 0.05 was used as the criterion for statistical significance.
RESULTS
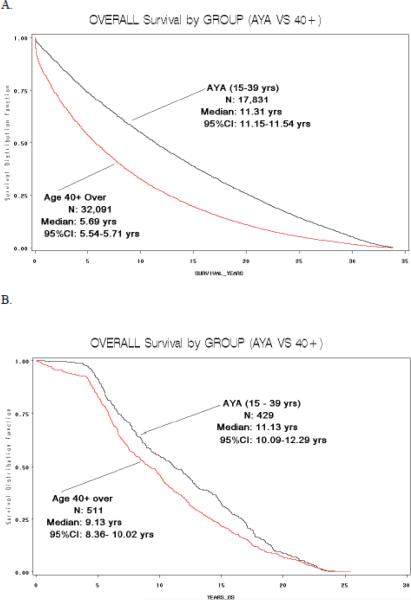
Of the 1,011 patients who underwent initial treatment for PTC at our institution during the 20-year study period, 455 were in the AYA group (15–39 years-old) and 556 were 40+ (Table 2). There were significantly more women in the AYA group than in the 40+ group; 83% vs. 69%, respectively (p< 0.001). As anticipated, the AYA group also had a higher proportion of patients with TNM Stage I disease (94% in AYA vs. 56% in 40+) because age is included in the TNM staging system (p< 0.001). The type of surgical procedure and dose of I-131 therapy were comparable in both groups. The mean tumor size was larger in the 40+ group (2.77 cm in 40+ vs. 2.19 cm in AYA group), albeit not statistically significant (p=0.075). The rate of lymph node metastases, however, was significantly higher in the AYA group (31% in AYA vs. 22% in 40+) (p =0.008). In addition, the rate of distant metastases was lower in the AYA group (4% in AYA vs. 6.5% in 40+ group) (p=0.001). Median disease-free and overall survival were significantly higher in the AYA group (p=0.013 and 0.0013, respectively) (Figure 1).
Table 2.
Characteristics and Outcomes of Patients Who Underwent Surgery for Papillary Thyroid Cancer, by Age Group
| Characteristic | Age 15–39 years (N=455) | Age ≥40 years (N=556) | P value (15–39 vs 40+) |
|---|---|---|---|
|
| |||
| Gender | |||
| Female | 378 (83.1%) | 386 (69.4%) | < 0.001 |
| Male | 77 (16.9%) | 170 (30.6%) | |
| TNM Stage | |||
| I | 298 (94%) | 234 (56%) | < 0.001 |
| II | 19 (6%) | 69 (16.5%) | |
| III | 91 (21.8%) | ||
| IV | 24 (5.7%) | ||
| Mean tumor size (± STD) in cm | 2.19 (1.53) | 2.77 (2.42) | 0.075 |
| Rate of lymph node metastasis (in %) | 31 | 22 | 0.008 |
| Rate of distant metastasis (in %) | 4 | 6.5 | 0.08 |
| Recurrence | |||
| None or disease-free | 293 (64.5%) | 317 (57.1%) | 0.017 |
| Recurrence | 32 (7.1%) | 34 (6.1%) | 0.523 |
| Death | 11 (2.4%) | 121 (21.8%) | <0.001 |
| Unknown | 118 (26%) | 83 (15.0%) | <0.001 |
| Surgery | |||
| Lobectomy | 31 (6.8%) | 93 (16.7%) | <0.001 |
| Subtotal or near total thyroidectomy | 75 (16.5%) | 91 (16.4%) | 0.966 |
| Total thyroidectomy | 338 (74.3%) | 352 (63.3%) | <0.001 |
| Disease-free Survival | |||
| Mean (±STD) in weeks | 597.68 (15.2) | 531.11 (15.0) | 0.0126 |
| Median (95%CI) | 568 (493.1–623.1) | 497.57 (417.1–525.7) | |
| Overall Survival | |||
| Mean (±STD) in weeks | 615.30 (14.1) | 532.08 (13.1) | 0.0013 |
| Median (95%CI) | 560.29 (525.3–641.01 | 476.29 (436.3–522.4) | |
Figure 1.
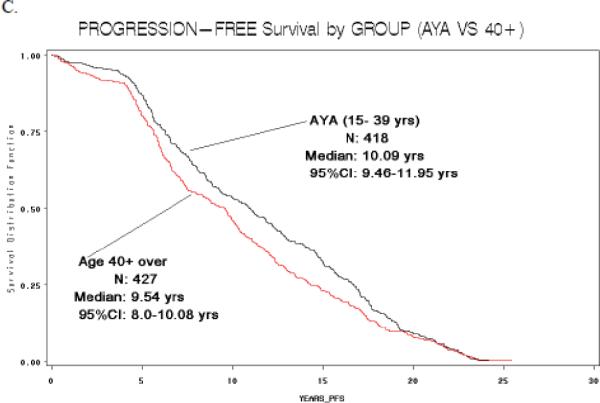
Disease-free (A) and overall survival (B) (in years) in patients with PTC, by age group, for patients treated at UCSF. (C) Overall survival (in years), by age group, for SEER data.
When we subdivided the SEER data into the two age groups (15–39 years, n=16,983; ≥ 40 years, n=29,697), we also found a significant difference in the sex distribution between the AYA and 40+ groups (p<0.001). As in our study cohort, more AYA patients were women (80% in AYA group vs. 71% in 40+ group). In the SEER data, the difference in mean tumor size was comparable to our study; 2.05 cm for the AYA group vs. 2.16 in the 40+ group but not significantly different. The number of lymph node metastases was comparable for both groups (33% vs. 34%) as well as the number of distant metastases (6% in both groups). As in our study cohort, median overall survival in the AYA group was significantly higher than in the 40+ group (588 weeks vs. 296 weeks), but disease-free survival was not available in the SEER database (Figure 1).
Genotype in AYA papillary thyroid cancer
Analysis of the most common somatic mutations showed that the number and type of somatic mutations detected in PTC in AYAs vs. 40+ groups were similar. Thirty-nine percent had no mutation in the AYA group and 44% had no mutation in the 40+ group. More patients in the AYA group had multiple somatic mutations, (18% vs. 10%, p=0.06). The prevalence of a BRAF mutation alone was not significantly different between the groups (42% in AYA group vs. 35% in 40+ group) (Table 3). Matched analysis between AYA and 40+, by extent of disease at presentation (tumor size ± 2cm, lymph node and distant metastasis status) showed no significant difference in the type and number of mutations between groups.
Table 3.
PTC genotype in AYA and 40+ group
| Group | BRAF | NRAS | KRAS | TRK | RET/PTC1 | RET/PTC3 | No Mutations | Multiple Multiple Mutations |
|---|---|---|---|---|---|---|---|---|
| AYA (n=109) | 46 | 3 | 2 | 0 | 2 | 22 | 34 | 20 |
| 40+ (n=145) | 51 | 4 | 4 | 1 | 1 | 24 | 60 | 14 |
Gene expression differences in pathway specific array genes
Of the 39 genes analyzed, three—ECM1, ERBB2, and UPA—were significantly differentially expressed between the AYA and 40+ groups. The mean delta Ct expression of ECM1 (homo sapiens extracellular matrix protein, transcript variant 1) was significantly lower for patients in the AYA group (5.47±3.41 vs. 6.69±3.09, p=0.0031). The same was true for ERBB2 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2) (71.9±60.2 vs. 95.83±39.2, p=0.01), and for UPA (urokinase plasminogen activator) (0 vs. 0.031± 0.078, p=0.025). Matched analysis between AYA and 40+, by extent of disease at presentation (tumor size ± 2cm, lymph node and distant metastasis status); showed only ECM1 was significantly differentially expressed (p=0.02).
Genome-wide gene expression analysis
Analysis of the gene expression profile by unsupervised cluster analysis showed no distinct clustering in 96 PTC samples by age (n = 46, AYA; n = 50, 40+). The 12 most differentially expressed genes between the two age groups are shown in Table 4. Although two genes (PFKFB2, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 and MEIS2, meis homeobox 2) were more than two-fold downregulated in the AYA group, none of the genes were significantly differentially expressed. We found no differentially expressed gene sets or pathways by GSEA using a false discovery rate (FDR) of ≤ 25%. For a nominal p-value < 1%, however, 10 genes sets were differently expressed in both groups (1 upregulated in AYA, 9 downregulated in AYA group) (Table 5). Leading edge analysis of these gene sets revealed one gene, CA2, carbonic anhydrase II, which was most common in 3 out of these 10 gene sets. CA2 was downregulated in the AYA group.
Table 4.
Genes most differentially expressed between the two age groups by p-value and fold change
| Gene ID | Gene location | 40+ group | AYA group | Fold Change | P-value |
|---|---|---|---|---|---|
|
by p-value
|
|||||
| ENO2 | chr12p13 | 0.6366521 | 0.1738148 | 1.8306896 | 0.095 |
| GPR97 | chr16q13 | 0.2091818 | 0.060698 | 1.1574762 | 0.095 |
| C1orF53 | chr1q31.3 | 0.4750927 | 0.1404785 | 1.4788251 | 0.095 |
| PFKFB2 | chr1q31 | 0.7601154 | 0.2254296 | 2.071731 | 0.095 |
| THOP1 | chr19q13.3 | 0.2809511 | 0.0842729 | 1.2460743 | 0.095 |
| PPM1H | chr12q14.1-q14.2 | 0.4263619 | 0.1330219 | 1.5303188 | 0.095 |
| VDAC3 | chr8p11.2 | 0.2924406 | 0.0914445 | 1.2432137 | 0.095 |
| MANEAL | chr1p34.3 | 0.4170245 | 0.1304338 | 1.4123153 | 0.095 |
| NTHL1 | chr16p13.3 | 0.2952867 | 0.0928436 | 1.2520845 | 0.095 |
| SIPA1L3 | chr19q13.13 | 0.2441329 | 0.0767741 | 1.1996613 | 0.095 |
| LOC651466 | --- | 0.6742622 | 0.2145415 | 1.756617 | 0.095 |
| SLC36A4 | chr11q21 | 0.2974336 | 0.095671 | 1.2211342 | 0.095 |
|
by fold change
|
|||||
| PFKFB2 | chr1q31 | 0.7601154 | 0.2254296 | 2.071731 | 0.095 |
| MEIS2 | chr15q14 | 0.6290979 | 0.2367629 | 2.7578498 | 0.171 |
Table 5.
Gene set enrichment analysis
| Gene set | Description (from MSigDB) | Direction of regulation in AYA | Normalized enrichment score | Nominal p-value |
|---|---|---|---|---|
| NITROGEN_METABOLISM | Nitrogen metabolism reference pathway | Down | −1.73 | 0.002 |
| HSA00310_LYSINE_ DEGRADATION | Genes involved in lysine degradation | Down | −1.70 | 0.006 |
| MARCINIAK_CHOP_DIFF | Differentially-expressed genes in CHOP-negative mutant mouse embryo fibroblast cells after treatment with tunicamycin | Down | −1.60 | 0.006 |
| JAIN_NEMO_DIFF | Genes from XHM-ED B cells that show abnormal regulation in response to CD40L plus IL-4 | Down | −1.61 | 0.006 |
| YAGI_AML_PROG_ASSOC | Prognosis-associated probe sets in acute myeloid leukemia | Down | −1.53 | 0.008 |
| HSA00051_FRUCTOSE_AND_MANNOSE_METABOLISM | Genes involved in fructose and mannose metabolism | Down | −1.72 | 0.008 |
| AMINOACYL_TRNA_BIOSYNTHESIS | Aminoacyl tRNA biosynthesis reference pathway | Down | −1.74 | 0.008 |
| HSA00910_NITROGEN_METABOLISM | Genes in nitrogen metabolism | Down | −1.58 | 0.008 |
| HDACI_COLON_CUR2HRS_UP | Genes upregulated by curcumin at 2 hrs in SW260 colon carcinoma cells | Up | 1.61 | 0.010 |
| TSADAC_HYPOMETH_HYPERAC_OVCA_UP | Genes with basally hypomethylated promoters upregulated by the combination of TsA and decitabine in ovarian carcinoma (CP70) cells, with hyperacetylated promoters upon activation | Down | −1.62 | 0.010 |
Discussion
We found that AYA patients with PTC have a different extent of disease at presentation and better outcome. There was no significant difference in the type and number of somatic mutations that occur in PTC between the AYA and 40+ age groups. By gene expression analysis, we found several genes differentially expressed between the AYA and 40+ groups (ECM1, ERBB2, UPA, PFKFB2, MEIS2) but only ECM1 when a matched analysis was performed. These findings suggest a possible molecular basis for the difference in the extent of disease at presentation and difference in outcomes observed in the AYA group.
The disparity in AYA cancer incidence and outcome has received increasingly more attention since the National Cancer Institute's Progress Review Group on AYA oncology was set up to investigate potential biologic basis of age-related differences for AYAs with cancer in 2006.3 Previously, studies of age and thyroid cancer mainly addressed the fact that older age is associated with a worse prognosis.12 Age > 45 years is associated with increased mortality and differentiated thyroid cancer of follicular cell origin is the only human malignancy for which the TNM staging system includes patient age at diagnosis.13 Although differences in extent of disease at presentation, disease-free survival and cause-specific mortality in patients with differentiated thyroid cancer of follicular cell origin have been previously observed based on patient age at presentation, we specifically studied the AYA group as compared to older patients.5,6,13 An age cutoff of 40 years was chosen to divide patients into the two groups because it is the official definition of the upper end of the AYA age range according to the National Cancer Institute AYA Oncology Progress Review Group (www.cancermeetings.org/AYAO/), which has been used for virtually all AYA analyses, including SEER (http://seer.cancer.gov/csr/1975_2006/results_merged/sect_32_aya.pdf).3 In our study, the mortality rate was significantly higher in the 40+ group, and disease-free survival was significantly lower than in the AYA group. The difference in survival rate in our study was concordant with the SEER data, in which median overall survival in the AYA group was significantly higher than in the 40+ group. Our AYA patients had an even higher overall survival rate than those in the SEER database. This may be due to confounding factors (different stage, race/ethnicity distribution) or to differences in the treatment and care.
We found no differences in the type and number of somatic mutations that occur in about two-thirds of sporadic PTC. We were surprised to find that the percentages of BRAF V600E mutations were comparable in the AYA and 40+ groups, since the presence of a BRAF mutation is associated with older age, more aggressive disease, and worse prognosis.14 Given our finding it is unlikely that the clinical difference in extent of disease and outcome observed between the two age groups is related to the presence of BRAF V600E mutation. RET/PTC1 and RET/PTC3 rearrangements are the most common RET/PTC rearrangements in PTC. RET/PTC1 is common in young patients who developed PTC due to exposure to the Chernobyl meltdown.15 In a recent study of 54 patients with recurrent PTC, double mutations (BRAF and RET/PTC rearrangements) were present in significantly more older patients (>45 years).16 In contrast, a recent study of 125 patients with PTC and simultaneous occurrence of PTEN, RET/PTC and BRAF mutations showed that young age renders individuals more prone to multi-genetic events or earlier presentation due to multi-genetic events.17 This finding is consistent with our recent study in which we found patients with PTC who had 2 or more mutations presented at a younger age (mean 8 years younger than patients with PTC with single mutations or no mutations). KRAS and NRAS oncogenes encode membrane-associated guanine nucleotide binding proteins (p21ras) and are associated with aggressive cancer behavior in some studies.18 RAS mutations are identified in 80% of follicular thyroid cancers and appear to be of high diagnostic value.19 In PTC, the presence of RAS mutations is much lower, as we found in our study and was not different between the two age groups compared.
We found three genes involved in extracellular matrix and cell adhesion, and angiogenesis (ECM1, ERBB2, UPA) were significantly differently expressed between the two age groups. When a matched analysis was performed to account for possible confounding factors due to differences in the extent of disease at presentation between the two groups, only ECM1 remained significantly differentially expressed. The function of ECM1, an extracellular matrix protein, is not completely characterized in carcinogenesis. In a study of serial analysis of gene expression in both benign and malignant thyroid tumors, ECM1 expression was higher in malignant tissue samples.20 Also, the level of ECM1 expression was higher in older patients with differentiated thyroid cancer of follicular cell origin. The fact that aggressive tumors are capable of growth without interactions between the cell surface and extracellular matrix for survival and cell cycle progression suggests that the decreased expression of ECM1 in more aggressive differentiated thyroid cancers may reflect cell anchorage-independent tumor growth.8,9 In a study of 32 patients with PTC, ERBB-2 overexpression was associated with a significantly higher risk of recurrent disease and mortality.21 Similarly, we found lower levels of ERBB-2 in AYA patients with PTC who had a lower risk of recurrent disease. UPA is a serine protease that converts plasminogen into plasmin. Plasmin can activate metalloproteases, which can breakdown the extracellular matrix and basement membrane resulting in tumor dissemination.22 UPA has been associated with larger tumor size and metastasis, and may be a prognostic marker in PTC.23,24 These findings taken together suggest that the three genes differentially expressed in AYA PTC may have an important role in tumor cell biology, and may account for the difference in extent of disease at presentation and outcome of AYA patients with PTC.
Our genome-wide expression analysis revealed two genes with a two-fold higher expression in the 40+ group (PFKFB2 and MEIS2) but no significantly differentially expressed genes or clustering by age group. To our knowledge, none of the genome-wide expression studies in PTC have specifically examined the effects of age. The protein encoded by PFKFB2 is involved in both the synthesis and degradation of fructose-2,6-bisphosphate, a regulatory molecule that controls glycolysis in eukaryotes. The gene involves metabolic processes that typically transform small molecules, but also include macromolecular processes such as DNA repair and replication, and protein synthesis and degradation. The MEIS 2 gene encodes a homeobox protein belonging to the TALE ('three amino acid loop extension') family of homeodomain-containing proteins. TALE homeobox proteins are highly conserved transcription regulators, and several members have been shown to be essential contributors to developmental programs. By less stringent criteria, GSEA revealed the CA2 gene to be downregulated in the AYA group. The CA2 gene has been described to be downregulated in neoplastic colorectal mucosa compared to normal colorectal mucosa.25 CA2 protein expression may be useful in the differential diagnosis of autoimmune pancreatitis and pancreatic cancer.26
In summary, we found that AYA patients with PTC have different extent of disease at presentation and better outcome. Gene expression analysis suggests several candidate genes (ECM1, ERBB2, UPA, PFKFB2, MEIS2, CA2), which may account for the difference in extent of disease at presentation and outcome in AYA patients with PTC. We believe future functional genomic studies focusing on the role of the candidate genes identified in our study, and using other genomic approaches such as genome-wide epigenetic, microRNA and proteomic analyses will shed further light on whether a biologic basis exists to account for the disparity in AYA cancer incidence and outcome. Such studies could possibly provide new screening, diagnostic and therapeutic options in the evaluation and treatment of cancers common in AYAs population which have been under investigated.
Acknowledgments
Funding: This research was supported, in part, by a grant from the Lance Armstrong Foundation through the LIVESTRONG™ Young Adult Alliance.
References
- 1.Davies L, Welch H. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Grodski S, Brown T, Sidhu S, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144:1038–43. doi: 10.1016/j.surg.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nature Reviews Cancer. 2008;8:288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari F, Montello M, Budd T, et al. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer. 2008;50:1101–1104. doi: 10.1002/pbc.21459. [DOI] [PubMed] [Google Scholar]
- 5.Hod N, Hagag P, Baumer M, et al. Differentiated thyroid carcinoma in children and young adults: evaluation of response to treatment. Clin Nucl Med. 2005;30:387–390. doi: 10.1097/01.rlu.0000162602.48653.54. [DOI] [PubMed] [Google Scholar]
- 6.Miccoli P, Minuto M, Ugolini C, et al. Papillary thyroid cancer: pathological parameters as prognostic factors in different classes of age. Otolaryngology. 2008;138:200–203. doi: 10.1016/j.otohns.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Kebebew E, Peng M, Reiff E, et al. Diagnostic and prognostic value of angiogenesis-modulating genes in malignant thyroid neoplasms. Surgery. 2005;138:1102–1109. doi: 10.1016/j.surg.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Kebebew E, Peng M, Reiff E, et al. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann Surg. 2005;242:353–361. doi: 10.1097/01.sla.0000179623.87329.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kebebew E, Peng M, Reiff E, et al. Diagnostic and extent of disease multigene assay for malignant thyroid neoplasms. Cancer. 2006;106:2592–2597. doi: 10.1002/cncr.21922. [DOI] [PubMed] [Google Scholar]
- 10.Subramaniana A, Tamayoa P, Moothaa VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. PNAS. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genetics. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 12.Kumar Das D. Age of patients with papillary thyroid carcinoma: is it a key factor in the development of variants? Gerontology. 2005;51:149–154. doi: 10.1159/000083985. [DOI] [PubMed] [Google Scholar]
- 13.Haymart M. Understanding the relationship between age and thyroid cancer. The Oncologist. 2009;14:216–221. doi: 10.1634/theoncologist.2008-0194. [DOI] [PubMed] [Google Scholar]
- 14.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–471. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klugbauer S, Lengfelder E, Demidchik EP, et al. High prevalence of RET rearrangement in thyroid tumors of children from Belarus after the Chernobyl reactor accident. Oncogene. 1995;11:2459–67. [PubMed] [Google Scholar]
- 16.Henderson Y, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15:485–491. doi: 10.1158/1078-0432.CCR-08-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YL, Wang JC, Wu Y, et al. Incidentally simultaneous occurrence of RET/PTC, H4-PTEN and BRAF mutation in papillary thyroid carcinoma. Cancer Letters. 2008;263:44–52. doi: 10.1016/j.canlet.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Roston G, Zhao H, Camp L, et al. Ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21:3226–3235. doi: 10.1200/JCO.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov Y, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine needle aspiration diagnosis of thyroid nodules. J Clin Endocrin Metab Epub ahead. 2009 doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 20.Pauws E, Veenboer GJ, Smit JW, et al. Genes differently expressed in thyroid carcinoma identified by comparison of SAGE expression profiles. Faseb J. 2004;18:560–561. doi: 10.1096/fj.03-0101fje. [DOI] [PubMed] [Google Scholar]
- 21.Freudenberg LS, Shue S, Gorges R, et al. Prognostic value of c-erbB-2 expression in papillary thyroid carcinoma. Nuklearmedizin. 2005;44:179–84. [PubMed] [Google Scholar]
- 22.Chu QD, Hurd TC, Harvey S, et al. Overexpression of urinary plasminogen activator (UPA) protein and mRNA in thyroid carcinogenesis. Diagn Mol Pathol. 2004;13:241–246. doi: 10.1097/01.pdm.0000137100.26010.64. [DOI] [PubMed] [Google Scholar]
- 23.Ulisse S, Baldini E, Toller M, et al. Differential expression of the components of the plasminogen activating system in human thyroid tumour derived cell lines and papillary carcinomas. Eur J Cancer. 2006;42:2631–2638. doi: 10.1016/j.ejca.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Herceg GH, Herceg D, Kralik M, et al. Urokinase-type plasminogen activator and its inhibitor in thyroid neoplasms: a cytosol study. Wien Klin Wochenschr. 2006;20:601–609. doi: 10.1007/s00508-006-0703-1. [DOI] [PubMed] [Google Scholar]
- 25.Kummola L, Hämäläinen JM, Kivelä J, et al. Expression of a novel carbonic anhydrase, CA XIII, in normal and neoplastic colorectal mucosa. BMC Cancer. 2005;18:41. doi: 10.1186/1471-2407-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosoda H, Okawa-Takatsuji M, Shinmura W, et al. Potential for differential diagnosis of autoimmune pancreatitis and pancreatic cancer using carbonic anhydrase II antibody. Pancreas. 2008;37:e1–7. doi: 10.1097/MPA.0b013e318162cb3a. [DOI] [PubMed] [Google Scholar]