Abstract
Background
Although numerous in vitro studies report on the tribological performance of and, separately, on the corrosion properties of cobalt-based alloys in metal-on-metal (MoM) bearings, the few studies that take into account the synergistic interaction of wear and corrosion (tribocorrosion) have used canonical tribo-test methods. We therefore developed synergistic study using a test method that more closely simulates hip bearing conditions.
Questions/purposes
(1) Is the total material loss during tribocorrosion larger than the sum of its components generated during isolated mechanical wear and isolated corrosion? (2) How is the tribocorrosive process affected by the presence of protein?
Methods
High carbon CoCrMo alloy discs (18) were subjected to corrosion and tribocorrosion tests under potentiostatic conditions in an apparatus simulating hip contact conditions. The input variables were the applied potential and the protein content of the electrolyte (NaCl solution versus bovine serum, 30 g/L protein). The output variables were mass loss resulting from wear in the absence of corrosion, mass loss resulting from corrosion in the absence of wear, and the total mass loss under tribocorrosion, from which the additional mass loss resulting from the combined action of wear and corrosion, or synergism, was determined in the presence and absence of protein.
Results
The degradation mechanisms were sensitive to the interaction of wear and corrosion. The synergistic component (64 μg) in the presence of protein amounted to 34% of total material loss (187 μg). The presence of protein led to a 23% decrease in the total mass loss and to a considerable reduction in the mean current (4 μA to 0.05 μA) under tribocorrosion.
Conclusions
Synergistic effects during tribocorrosion may account for a considerable portion of MoM degradation and are affected by proteins.
Clinical Relevance
The in vivo performance of some large-diameter MoM joints is unsatisfactory. The synergistic component resulting from tribocorrosion may have been missed in conventional preclinical wear tests.
Introduction
Metal-on-metal joints for total THA are under considerable scrutiny because of reported high failure rates and concerns over released metal ions affecting local tissues and remote organs [11, 18, 19, 40, 47, 48]. Reports of local adverse tissue reactions, likely mediated by the metallic degradation products generated during wear and corrosion, have led to a steep decline in the popularity of metal-on-metal bearings [8, 9, 22, 26, 38, 41, 47, 48]. Metals in articulating bearing surfaces are subjected to the simultaneous action of wear and corrosion, termed tribocorrosion [13–15, 27, 29]. Wear and corrosion can act synergistically to increase the amount of metal release [29, 31–33, 39, 53–57]. The concept of tribocorrosion may be traced to the late 1960s with the work of Heinecke on tribochemistry [16, 37] and has been known under various names such as tribooxidation, tribochemistry, triboelectrochemistry, and mechanically assisted corrosion [13, 15, 25]. The modern era of tribocorrosion started perhaps with the work of Drees et al. [10] and Mischler et al. [34, 35] in the early 1990s. Tribocorrosion models were used to evaluate erosion-corrosion [42, 43, 46, 52], sliding wear-corrosion [44, 54, 55], fretting-corrosion [4, 5, 7, 15], microabrasion-corrosion [45], and thin films [29]. Application of tribocorrosion to biomaterials started only in the last decade with studies on CoCrMo alloys used in orthopaedics [31, 32, 36, 53–57] and on titanium alloys used in dentistry [12, 30].
Historically, in vitro testing of metal-on-metal bearing systems has been based on conventional tribological methods wherein material loss is measured in an apparatus that simulates the motion of the hip in a simulated synovial fluid (ISO-14242 [17] and ASTM-F1714 [3]). Although valuable, this type of analysis does not provide information on the electrochemical processes occurring at the bearing surfaces and the interaction between wear and corrosion nor an analysis of the effect that extraneous potentials, eg, from galvanic effects, may have. The tribocorrosion approach addresses these limitations of conventional tribological testing by investigating wear, corrosion, and their interaction (synergism) in a single testing set-up [21, 27, 29–32]. Toward this end we have developed and built a custom tribocorrosion test rig with a pin-on-ball configuration that simulates the approximately localized contact in hip bearings [31, 32] and is therefore closer to the clinical application than the pin-on-flat configuration used previously [53–55]. It allows the systematic investigation of factors that may influence the tribocorrosion of implant metals. Concurrently, we have developed a protocol to isolate and quantify the synergy component of metal loss. The effect of proteins on wear-corrosion synergism is of primary interest because proteins are known to influence metal-on-metal (MoM) friction and wear [36, 45, 46].
We therefore investigated the synergistic component in a high carbon alloy used for metal-on-metal bearing applications and answered the following research questions: (1) Is the material degradation resulting from tribocorrosion sensitive to the presence of proteins in the biochemical environment? (2) Does the synergistic component add substantially to metal release, exceeding the simple summation of corrosion and wear conducted independently?
Materials and Methods
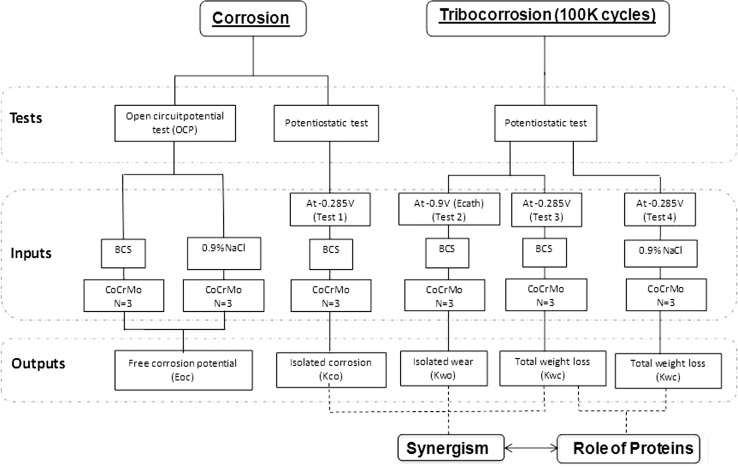
In this study, a series of corrosion and tribocorrosion tests were planned to understand the role of synergistic interaction of wear and corrosion in the case of MoM hips. As a result of its prevalence in MoM implants, a wrought high-carbon CoCrMo alloy was chosen for this investigation. Two types of solution were used, solution representing the joint (bovine calf serum, protein content 30 g/L) and solution without protein (0.9% NaCl solution). The study design (Fig. 1) has two main components: (1) understanding the synergism; and (2) analysis of the role of protein. A corrosion test (T1) and three tribocorrosion tests (T2, T3, and T4) were conducted in two media under potentiostatic conditions (Table 1).
Fig. 1.
A schematic diagram is shown of the experiment design of the current study for corrosion and tribocorrosion tests.
Table 1.
Experimental details
| Aim | Tests | Solution | Cycles | Corrosion conditions |
|---|---|---|---|---|
| Synergism | T1 (isolated corrosion, Kco) | BCS | 100 K sec | Potentiostatic test (no movement) at −0.285 V (close to free corrosion potential Eoc) |
| T2 (isolated wear, Kwo) | BCS | 100 K | Potentiostatic test (tribocorrosion) at (−0.9 V) (cathodic potential) | |
| T3 (total weight loss, Kwc) | BCS | 100 K | Potentiostatic test (tribocorrosion) at −0.285 V (close to free corrosion potential Eoc) | |
| Effect of protein | T4 (total weight loss, Kwc) | 0.9% NaCl solution | 100 K | Potentiostatic test (tribocorrosion) at −0.285 V (close to free corrosion potential Eoc) |
BCS = bovine calf serum.
The specific elemental composition and supplier details of CoCrMo alloy are listed (Table 2). The alloy was received as a 29-mm diameter rod from ATI Allvac (Monroe, WI, USA). Using electrical discharge machining (VW Broaching Service, Chicago, IL, USA), 18 disks of 12-mm diameter with a thickness of 7 mm were manufactured and polished to a mirror surface finish of Ra approximately 10 nm using standard metallographic equipment in the laboratory. Six disks were used for corrosion tests and 12 disks for tribocorrosion tests (Fig. 1). Initially, to identify parameters for the tribocorrosion tests, free corrosion potential (Eoc) of the metal was measured as per ASTM requirements [1] (Table 3). Using a potentiostat (Gamry Instruments, Warminster, PA, USA), the working electrode was the CoCrMo alloy sample with a known area (0.38 cm2) exposed to the same medium as used during tribocorrosive testing. The reference electrode was a standard calomel electrode and all potentials in this study are referenced to this electrode. As the counter electrode, a graphite rod was used. Before testing, the system was given time to stabilize at a temperature of 37°C.
Table 2.
Source, Rockwell hardness, and elemental composition of the high-carbon wrought CoCrMo alloy used in this study (manufacturer’s data)
| Manufacturer | Original rod diameter (mm) | Rockwell C hardness | Chemical composition (%wt) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Co | Cr | Mo | Si | Mn | Al | |||
| Allvac TJA-1537(Monroe, WI, USA) | 29 | 44 | 0.241 | 64.6 | 27.63 | 5.70 | 0.66 | 0.70 | < 0.02 |
C = carbon; Co = cobalt; Cr = chromium; Mo = molybdenum; Si = silicon; Mn = manganese; Al = aluminum.
Table 3.
Electrochemical parameters
| Solutions | Free corrosion potential (Eoc [V] E versus SCE) |
|---|---|
| BCS | −0.325 ± 0.09 |
| 0.9% NaCl solution | −0.288 ± 0.11 |
BCS = bovine calf serum.
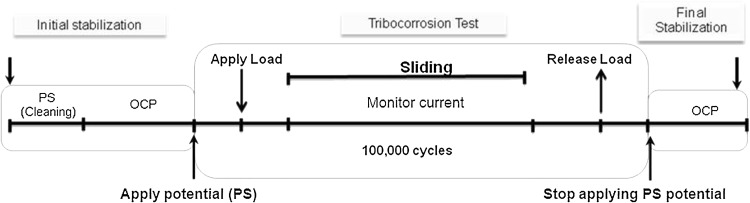
The tribocorrosion tests were conducted in a ball-on-flat configuration that had been constructed to mimic the tribological conditions in MoM joints (Fig. 2). Twelve tribological pairs consisted of a flat CoCrMo cylindrical disk of 12-mm diameter (surface roughness, Ra: 9.4 ± 2.6 nm, exposed area 1.0 cm2) that articulated against a ceramic ball of 28-mm diameter with ± 15° of rotation at 1 Hz. Ceramic balls (alumina [Al2O3], commercially known as BIOLOX® forte) were supplied by CeramTec Inc, Plochingen, Germany. The surface hardness is 17 ± 1 GPa. The surface roughness is in the range of Ra = 0.024 ± 0.011 μm. A force of 16 N, which corresponds to a mean Hertzian pressure of 10 MPa at the end of the test, was chosen as the contact load. The tribological pair was fully immersed in testing medium. The protein-containing medium was identical to lubricant typically used for wear testing and contained 30 g/L protein from diluted bovine calf serum. In the case of the protein-free medium, only the basic 0.9% NaCl solution of this lubricant was used (Table 4). A standard protocol for potentiostatic testing was applied that consisted of an initial stabilization period, a sliding stage, and a final stabilization period (Fig. 3) [29–31]. In this study, the tribocorrosion tests were conducted in potentiostatic mode at a potential of −0.285 V versus SCE. The current flow could also have been estimated from potentiodynamic tests under tribocorrosive exposure per ASTM Standard G119-09 [2]. However, as a result of the changing potential, the corrosion kinetics in the vicinity of the exposed surface would continuously vary, preventing meaningful weight loss measurements over time and/or cycles, an important consideration for biomedical applications. Furthermore, the potentiodynamic procedure destroys the sample surface, preventing any meaningful study of the damage mechanisms.
Fig. 2.
Details are shown of the tribocorrosion test system including both the mechanical electro-chemical data collection methods.
Table 4.
Composition of solutions
| Solution | Distilled water (mL) | NaCl (g/L) | EDTA (g/L) | Tris (g/L) | Protein (g/L) |
|---|---|---|---|---|---|
| BCS solution | 1000 | 9 | 0.2 | 27 | 30 |
| NaCl solution | 1000 | 9 | – | – | – |
BCS = bovine calf serum.
Fig. 3.
A standard tribocorrosion test protocol was used in the current study. PS = potentiostatic test; OCP = open circuit potential test.
The evolutions of the current as well as the friction coefficient as a function of sliding time were monitored. This protocol was repeated three times (n = 3) with new samples for every condition, namely (1) isolated corrosion; (2) isolated wear; (3) tribocorrosion with protein; and (4) tribocorrosion without protein.
The isolated corrosion test (T1) was conducted for 100,000 seconds (corresponding to 100,000 cycles) and at potentiostatic condition close to free corrosion potential Eoc (Table 3). A potential of –0.285 V was selected for this test because it was slightly above both Eoc values of CoCr in bovine calf serum and NaCl solution. The weight loss resulting from corrosion (Kc) was estimated using Faraday’s Law [29, 30, 42, 43]:
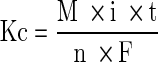 |
1 |
and
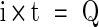 |
2 |
where M is the atomic mass of the material or equivalent weight in g/mol, i is the total current in A/cm2, t is the total exposure time in seconds, n is the number of electrons involved in the corrosion process, which can be 2 and 3 (for simplicity, we assumed n = 2), F is Faraday’s constant (96500 C/mol−1), and Q is the charge passed through the working electrode in coulombs.
Subsequently, an isolated wear test (T2) and a tribocorrosion test (T3) were conducted at potentiostatic conditions. Potentials being cathodic (−0.9 V) and close to free corrosion potential (−0.285 V) were chosen for T2 and T3, respectively. Again, the selected potentials were based on the free corrosion potential values from the open circuit potential tests in bovine calf serum (BCS) and 0.9% NaCl solution (Table 3). For the best approximation of isolated wear (Kwo), the tribocorrosion test, T2, was conducted at cathodic potentials. The effect on the exposed surface resulting from the chemical processes under cathodic conditions and subsequent variation in the chemical nature of proteins could not be neglected. However, this is beyond the scope of this study. The following test, T3, was conducted close to the free potential (Eoc), possibly representing physiological conditions at the MoM joint. To our knowledge, no published data are available on the electrochemical potential for physiological conditions. The weight loss resulting from tribocorrosion was estimated from the observed wear scar volume using white light interferometry (New View 6000; Zygo Corp, Middlefield, CT, USA). Based on these measurements, the synergistic component (S) was estimated:
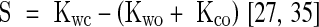 |
3 |
where Kwc is the measured total weight loss, Kwo the loss resulting from isolated wear, and Kco the loss resulting from isolated corrosion.
Last, a tribocorrosion test (T4) close to free corrosion potential at -0.285 V was conducted using testing media without protein (Table 1). Also here, total weight loss was estimated using white light interferometry.
We determined differences in weight loss parameters (total weight loss [Kwc]), isolated wear (Kwo), isolated corrosion (Kco), and synergistic component (S) in bovine calf serum using the one-way analysis of variance followed by post hoc Scheffe tests (SPSS Inc, Chicago, IL, USA). The role of protein is determined by analyzing the differences in total weight loss (Kwc) between BCS and 0.9% NaCl solution with independent t-tests (SPSS Inc).
Results
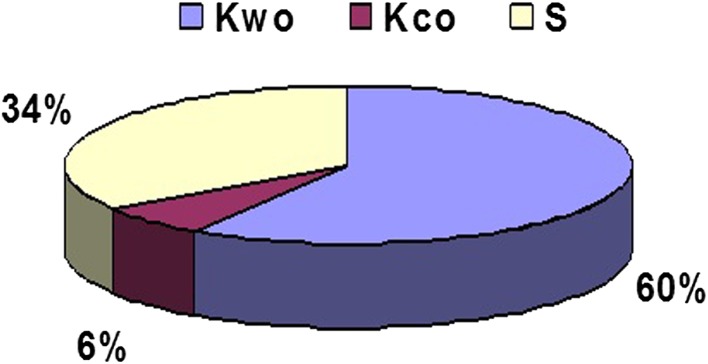
Tribocorrosive wear under potentiostatic condition at free corrosion potential (Kwc) was higher (p < 0.001) than isolated wear (Kwo) or isolated corrosion (Kco). Also, the sum of Kwo and Kco was with 123 μg considerably lower (p < 0.001) than Kwc, which amounted to 187 μg (Table 5). This demonstrates tribocorrosive wear is not a mere summation of isolated wear and isolated corrosion but has a synergistic component. Its percentage of contribution to total weight loss is estimated (Fig. 4). The synergistic component (S) is 34%, whereas wear is 60% and isolated corrosion is 6%.
Table 5.
Weight loss distribution data in micrograms (μg) from tribocorrosion test with protein contact testing media (BCS)
| Solution | Isolated corrosion (Kco) at −0.285 V (E versus SCE) | Isolated wear (Kwo) at −0.9 V (E versus SCE) | Total weight loss (Kwc) at −0.285 V (E versus SCE) | Synergistic component (S) |
|---|---|---|---|---|
| BCS | 12.00 ± 1.15 | 110.83 ± 25.51 | 187.03 ± 16.57 | 64.17 ± 20.51 |
BCS = bovine calf serum.
Fig. 4.
The percentage of contributions to total weight loss from isolated wear (Kwo), isolated corrosion (Kco), and the synergistic component (S) for the CoCrMo alloy in bovine calf serum (BCS) is shown.
Tribocorrosive wear was affected by the presence or absence of proteins. Total weight loss was higher (p < 0.001) in 0.9% NaCl solution compared with the BCS solution (Fig. 5). The evolution of current during testing with protein containing and protein-free media (Tests T3 and T4) is comparatively analyzed for five cycles (Fig. 6). The presence of protein led to a 23% decrease in the total mass loss and to a considerable reduction in the mean current (4 μA to 0.05 μA) under tribocorrosion. Interestingly, it follows a specific pattern of variation as a function of oscillatory cycles and proves sensitive to the sliding motion of the counterbody. Also, the surrounding biochemical environment has a major impact. The current values were lower (p < 0.001) in BCS solution compared with 0.9% NaCl solution indicating less corrosion in the protein-containing medium. The prevention of current decay in the case of BCS suggests the involvement of proteins in the electrochemical reactions during tribocorrosive wear.
Fig. 5.
Total weight loss (Kwc) from the tribocorrosion test in the presence of bovine calf serum (BCS) and 0.9% NaCl solutions is shown.
Fig. 6.
The evolution of current during the tribocorrosion test is shown for five cycles in the presence of bovine calf serum (BCS) and 0.9% NaCl solutions. A specific pattern is observed as a function of oscillatory cycles along with an influence of protein under tribocorrosion. Current values for the NaCl solution are much higher than for the solution containing protein content solution.
Discussion
High carbon CoCrMo alloys are extensively used in MoM hip bearings so that understanding the tribological processes that control material loss for these alloys is of particular importance. Because metal surfaces are subject to electrochemical processes that lead to interaction between wear and corrosion, an approach that goes beyond the material loss measurements of conventional methods is necessary. In our study of a high carbon-wrought CoCrMo alloy, we therefore took a tribocorrosion approach in which wear, corrosion, and their interaction (synergism) are studied in a single testing set-up and in which variations in friction and corrosion can be monitored in real time. Using this methodology, we investigated the effect of proteins on wear-corrosion synergism because proteins are known to influence MoM friction and wear [36, 45, 46]. We endeavored to answer the following research questions: (1) Is the material degradation resulting from tribocorrosion sensitive to the presence of proteins in the biochemical environment? (2) Does the synergistic component add substantially to metal release, exceeding the simple summation of corrosion and wear conducted independently?
The study has several limitations. First, we used a ceramic ball instead of a metal ball to articulate against the metal component. This was necessary to electrically decouple the metal disk from the countersurface so that reliable estimates of material degradation resulting from corrosion could be made. Using a metal ball would have made its surface electrochemically active such that the surface area of the working electrode would be difficult to control. Second, we used flat, nonconforming samples rather than dished conforming ones. This led to high contact pressures initially and an increase in wear rate throughout the running-in period; however, at the same time, it allowed us to control for identical surface properties such as roughness and sample geometry (flatness) using standard laboratory equipment. Third, proteins may have reacted differently at cathodic potential than at the physiological, free corrosion potential. This is neglected in our synergy model and might have affected the percentage of the synergistic contribution. However, the general trend of the data should be unchanged.
Our data suggest the material loss from a metal surface concurrently undergoing wear and corrosion exceeds the sum of the material loss from isolated wear and the material loss from isolated corrosion. That is, we observed a synergistic interaction between wear and corrosion of metal surfaces. The estimated synergistic component (S) is subjected to the tested experimental conditions. However, this finding clearly demonstrates that other than the individual components resulting from wear and corrosion, the synergistic interaction between both phenomena has a major influence on the total degradation process. Identification of the resulting mechanistic transitions will require further investigation.
Furthermore, the tribocorrosion rate was reduced in the presence of proteins. Cobalt-chromium alloys are spontaneously passivating in physiological environments, forming a thin chromium-oxide film on the surface. The passivating oxide film can be transiently removed during mechanical articulation thereby exposing the bare metallic surface to the solution. Depending on the electrochemical interactions with the surrounding medium, the oxide film might be reformed, which is called repassivation. Generally, the kinetics of this process depend on the alloy composition and the environment [20]. Repassivated oxides may have a different structure and properties than the original oxide films, thus making them more or less prone to wear. If proteins are present in the surrounding medium, they might become involved in the electrochemical reactions and influence in the oxide formation, potentially forming metalloorganic composites [49]. An attempt will be made for in-depth surface chemical analysis to generate a clearer understanding of the influence of protein on the surface.
The success or failure of a metallic implant undergoing tribocorrosion may depend on (1) the ability of the oxide film to resist wear; (2) the speed of the reformation of oxide films after wear (repassivation rate); and (3) the susceptibility of the repassivated surface to subsequent wear and corrosion. The role of protein in this process is evident and influencing the electrochemical kinetics under sliding conditions (Fig. 6). The low value of current and the difference in the current evolution during each tribological cycle in BCS compared with NaCl solution suggests the surface chemistry has substantially changed. Repassivation and depassivation kinetics are closely related to the tribological events as can be seen in the individual motion cycles. Moreover, proteins play a role in the current decay (see marked zone in Fig. 6). In previous studies, the improvements in the corrosion kinetics in the presence of proteins were noticeable during electrochemical impedance testing [30, 31]. Hence, such corrosion-inhibiting characteristics could be a reason for the reduction in the total weight loss (Kwc) using BCS compared with 0.9% NaCl solution (Fig. 4). Mischler et al. [34, 35] also highlighted the role of proteins regarding the degradation mechanism.
In our own studies, we attributed a positive effect to tribolayers in MoM bearings, which were believed to be generated from proteins as a result of tribochemical reactions [50, 51]. The formation and presence of a tribolayer appear to mitigate total weight loss (Kwc) (Fig. 5). Electrochemically, proteins can influence the corrosion behavior of alloys by adhering to metal surfaces [23, 24, 36]. Once the tribological process starts, the nature of the proteins can be changed turning them into lubricous material [28]. From this it becomes clear that in situations in which corrosion and wear act in synergy, a complex series of reactions at the metal/aqueous interface may evolve.
Previous studies demonstrated that protein had a major effect on the friction developed between the joint surfaces. This is believed to be the result of the formation of solid-like films (tribolayers) resulting from the tribochemical reaction of proteins with the bearing surfaces. However, studies showed such a lubricating effect of protein would be beneficial in terms of wear but can either increase or decrease the friction between the contacting surfaces [38]. Furthermore, although the corrosion-inhibiting characteristics of the carbonaceous film could be advantageous for electrochemical protection of the surface, this may influence the rate of passive/oxide film formation just after mechanical rubbing. Hence, the role of protein on the synergistic interaction between wear and corrosion needs particular attention.
Our findings demonstrate corrosion studies in the absence of articulating motion or tribological studies in the absence of electrochemical testing will not provide a complete picture of the degradation mechanisms and process occurring in MoM bearings. Tribocorrosion testing has the ability to characterize the interplay between wear and corrosion [27, 30–32, 53–57]. Our data suggest the synergistic component of material loss exceeds that of either isolated wear or corrosion. Both processes will be controlled to a certain extent by the properties of the oxide layer on the surface of the material and the interaction of the environment with that surface [6, 23, 24]. Furthermore, these observations are in agreement with earlier reports that complex synergistic interactions with proteins might assist in the formation of tribochemical reaction layers on the articulating surfaces [50, 51], which improve the MoM implant performance [28]. A better understanding of the dominant tribocorrosive interactions is required to further improve MoM wear in vivo.
Acknowledgments
We thank Dr Laurent (Rush University Medical Centre, Chicago, IL, USA) for valuable discussion and suggestions for manuscript editing. We also thank Howard Fries, ATI Allvac, Monroe, NC, USA, for providing CoCr rods; Dr Thomas Pandorf of Ceramtech, Plochingen, Germany, for providing ceramic heads; and Dr Judy Yuan for assistance with statistical analysis of the data.
Footnotes
One or more of the authors has or may receive payments or benefits, in any 1 year, an amount in excess of $10,000 (JJJ) from a commercial entity related to this work (Zimmer, Inc, Warsaw, IN, USA). The institution of the authors has received, in any 1 year, funding from Zimmer, Inc (Prinicipal Investigator [PI] JJJ), the National Institutes of Health (Bethesda, MD, USA; NIH Grant AR39310) (PI JJJ) and the Crown Family Chair (JJJ), NIH RC2 grant (1RC2AR058993, 2009) (PI JJJ, MAW), Zimmer, Inc (PI JJJ, MAW), and Medtronic Sofamor Danek (Memphis, TN, USA; PI JJJ, MAW).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.ASTM G61-86. Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. West Conshohocken, PA, USA: ASTM International; 2009; DOI: 10.1520/G0061-86R09. www.astm.org/Standards/G61.htm.
- 2.ASTM F1714-96. Standard Guide for Gravimetric Wear Assessment of Prosthetic Hip Designs in Simulator Devices. West Conshohocken, PA, USA: ASTM International; 2008; DOI: 10.1520/F1714-96R08. www.astm.org/Standards/F1714.htm.
- 3.ASTM G119-09. Standard Guide for Determining Synergism Between Wear and Corrosion. West Conshohocken, PA, USA: ASTM International; 2009; DOI:10.1520/G0119-09. http://www.astm.org/Standards/G119.htm.
- 4.Barril S, Debaud N, Mischler S, Landolt D. A tribo-electrochemical apparatus for in vitro investigation of fretting–corrosion of metallic implant materials. Wear. 2002;252:744–754. doi: 10.1016/S0043-1648(02)00027-3. [DOI] [Google Scholar]
- 5.Barril S, Mischler S, Landolt D. Influence of fretting regimes on the tribocorrosion behaviour of Ti6Al4 V in 0.9 wt% sodium chloride solution. Wear. 2004;256:963–972. doi: 10.1016/j.wear.2003.11.003. [DOI] [Google Scholar]
- 6.Bidiville A, Favero M, Stadelmann P, Mischler S. Effect of surface chemistry on the mechanical response of metals in sliding tribocorrosion systems. Wear. 2007;263:207–217. doi: 10.1016/j.wear.2007.01.066. [DOI] [Google Scholar]
- 7.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 8.Cohen D. Revision rates for metal on metal hip joints are double that of other materials. BMJ. 2011;343:d5977. doi: 10.1136/bmj.d5977. [DOI] [PubMed] [Google Scholar]
- 9.Smet K, Campbell PA, Gill HS. Metal-on-metal hip resurfacing: a consensus from the Advanced Hip Resurfacing Course, Ghent, June 2009. J Bone Joint Surg Br. 2010;92:335–336. doi: 10.1302/0301-620X.92B3.23946. [DOI] [PubMed] [Google Scholar]
- 10.Drees D, Celis JP, Dekempeneer E, Meneve J. The electrochemical and wear behaviour of amorphous diamond-like carbon coatings and multilayered coatings in aqueous environments. Surf Coat Technol. 1996;86–87:575–580. doi: 10.1016/S0257-8972(96)03054-X. [DOI] [Google Scholar]
- 11.Ebramzadeh E, Campbell PA, Takamura KM, Lu Z, Sangiorgio SN, Kalma JJ, De Smet KA, Amstutz HC. Failure modes of 433 metal-on-metal hip implants: how, why, and wear. Orthop Clin North Am. 2011;42:241–250, ix. [DOI] [PubMed]
- 12.Fernandes AC, Vaz F, Ariza E, Rocha LA, Ribeiro ARL, Viera AC, Riviere JP, Pichon L. Tribocorrosion behaviour of plasma nitrided and plasma nitrided plus oxidised Ti6A14 V alloy. Surf Coat Technol. 2006;200:6218–6224. doi: 10.1016/j.surfcoat.2005.11.069. [DOI] [Google Scholar]
- 13.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27:1533–1544. doi: 10.1002/jbm.820271210. [DOI] [PubMed] [Google Scholar]
- 14.Hallab NJ, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–436. doi: 10.1302/0301-620X.83B3.9674. [DOI] [PubMed] [Google Scholar]
- 15.Hallab NJ, Messina C, Skipor A, Jacobs JJ. Differences in the fretting corrosion of metal-metal and ceramic-metal modular junctions of total hip replacements. J Orthop Res. 2004;22:250–259. doi: 10.1016/S0736-0266(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 16.Heinicke G. Tribochemistry. Munich, Germany: Hanser; 1984. [Google Scholar]
- 17.ISO 14242. Implants for Surgery–Wear of Total Hip-joint Prostheses–Part 1: Loading and Displacement Parameters for Wear-testing Machines and Corresponding Environmental Conditions for Test. Geneva, Switzerland: International Organization for Standardization; 2012.
- 18.Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19(Suppl 3):59–65. doi: 10.1016/j.arth.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JJ, Skipor AK, Doorn PF, Campbell P, Schmalzried TP, Black J, Amstutz HC. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop Relat Res. 1996;329(Suppl):S256–S263. doi: 10.1097/00003086-199608001-00022. [DOI] [PubMed] [Google Scholar]
- 20.Jemmely P, Mischler S, Landolt D. Electrochemical modeling of passivation phenomena in tribocorrosion. Wear. 2000;237:63–76. doi: 10.1016/S0043-1648(99)00314-2. [DOI] [Google Scholar]
- 21.Jiang J, Stack MM, Neville A. Modelling the tribo-corrosion interaction in aqueous sliding conditions. Tribol Int. 2002;35:669–679. doi: 10.1016/S0301-679X(02)00058-0. [DOI] [Google Scholar]
- 22.Jiang Y, Zhang K, Die J, Shi Z, Zhao H, Wang K. A systematic review of modern metal-on-metal total hip resurfacing vs standard total hip arthroplasty in active young patients. J Arthroplasty. 2011;26:419–426. doi: 10.1016/j.arth.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Khan MA, Williams RL, Williams DF. In-vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials. 1996;17:2117–2126. doi: 10.1016/0142-9612(96)00029-4. [DOI] [PubMed] [Google Scholar]
- 24.Khan MA, Williams RL, Williams DF. The corrosion behaviour of Ti–6Al–4 V, Ti–6Al–7Nb and Ti–13Nb–13Zr in protein solutions. Biomaterials. 1999;20:631–637. doi: 10.1016/S0142-9612(98)00217-8. [DOI] [PubMed] [Google Scholar]
- 25.Kostetskaya NB. Mechanism of the deformation and fracture of friction surfaces in tribooxidative processes. Sov J Superhard Mater. 1986;8:56–62. [Google Scholar]
- 26.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 27.Landolt D, Mischler S, Stemp M. Electrochemical methods in tribocorrosion: a critical appraisal. Electrochim Acta. 2001;46:3913–3929. doi: 10.1016/S0013-4686(01)00679-X. [DOI] [Google Scholar]
- 28.Liao Y, Pourzal R, Wimmer M, Jacobs J, Fischer A, Marks L. Graphitic tribological layers in metal-on-metal hip replacements. Science. 2011;334:1687–1690. doi: 10.1126/science.1213902. [DOI] [PubMed] [Google Scholar]
- 29.Mathew MT, Ariza E, Rocha LA, Vaz F, Fernandes AC, Stack MM. Tribocorrosion behaviour of TiCxOy thin films in bio-fluids. Electrochim Acta. 2010;56:929–937. doi: 10.1016/j.electacta.2010.08.067. [DOI] [Google Scholar]
- 30.Mathew MT, Barão VA, Yuan JC, Assunção WG, Sukotjo C, Wimmer MA. What is the role of lipopolysaccharide on the tribocorrosive behavior of titanium? J Mech Behav Biomed Mater. 2012;8:71–85. doi: 10.1016/j.jmbbm.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Mathew MT, Runa MJ, Laurent M, Jacobs JJ, Rocha LA, Wimmer MA. Tribocorrosion behavior of CoCrMo alloy for hip prosthesis as a function of loads: a comparison between two testing systems. Wear. 2011;271:1210–1219. doi: 10.1016/j.wear.2011.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew MT, Uth T, Hallab NJ, Pourzal R, Fischer A, Wimmer MA. Construction of a tribocorrosion test apparatus for the hip joint: validation, test methodology and analysis. Wear. 2011;271:2651–2659. doi: 10.1016/j.wear.2011.01.085. [DOI] [Google Scholar]
- 33.Mischler S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: a comparative evaluation. Tribol Int. 2008;41:573–583. doi: 10.1016/j.triboint.2007.11.003. [DOI] [Google Scholar]
- 34.Mischler S, Ayrault S, Landolt D. Investigation of tribocorrosion of TiC coatings using electrochemical techniques. Mater Sci Forum. 1997;247:9–18. doi: 10.4028/www.scientific.net/MSF.247.9. [DOI] [Google Scholar]
- 35.Mischler S, Rosset E, Stachowiak GW, Landolt D. Effect of sulfuric-acid concentration on the rate of tribocorrosion of iron. Wear. 1993;167:101–108. doi: 10.1016/0043-1648(93)90314-C. [DOI] [Google Scholar]
- 36.Munoz AI, Mischler S. Effect of the environment on wear ranking and corrosion of biomedical CoCrMo alloys. J Mater Sci Mater Med. 2011;22:437–450. doi: 10.1007/s10856-010-4224-0. [DOI] [PubMed] [Google Scholar]
- 37.Neukirchner J. Tribocorrosion—occurrence, causes, prevention (Part 2) Schmierungstechnik. 1973;4:370–374. [Google Scholar]
- 38.Scholes SC, Unsworth A. The effects of proteins on the friction and lubrication of artificial joints. Proc Inst Mech Eng H. 2006;220:687–693. doi: 10.1243/09544119JEIM21. [DOI] [PubMed] [Google Scholar]
- 39.Scholes SC, Unsworth A, Hall RM. The effects of material combination and lubricant on the friction of total hip prostheses. Wear. 2000;241:209–213. doi: 10.1016/S0043-1648(00)00377-X. [DOI] [Google Scholar]
- 40.Shetty VD, Villar RN. Development and problems of metal-on-metal hip arthroplasty. Proc Inst Mech Eng H. 2006;220:371–377. doi: 10.1243/095441105X63264. [DOI] [PubMed] [Google Scholar]
- 41.Silva M, Heisel C, Schmalzried TP. Metal-on-metal total hip replacement. Clin Orthop Relat Res. 2005;430:53–61. doi: 10.1097/01.blo.0000149995.84350.d7. [DOI] [PubMed] [Google Scholar]
- 42.Stack MM, Abd El-Badia TM. On the construction of erosion-corrosion maps for WC/Co-Cr-based coatings in aqueous conditions. Wear. 2006;261:1181–1190. doi: 10.1016/j.wear.2006.03.038. [DOI] [Google Scholar]
- 43.Stack MM, ChaconNava JG, Stott FH. Synergism between effects of velocity, temperature, and alloy corrosion resistance in laboratory simulated fluidised bed environments. Mater Sci Technol. 1995;11:1180–1185. [Google Scholar]
- 44.Stack MM, Chi K. Mapping sliding wear of steels in aqueous conditions. Wear. 2003;255:456–465. doi: 10.1016/S0043-1648(03)00203-5. [DOI] [Google Scholar]
- 45.Stack MM, Jawan H, Mathew MT. On the construction of micro-abrasion maps for a steel/polymer couple in corrosive environments. Tribol Int. 2005;38:848–856. doi: 10.1016/j.triboint.2005.02.013. [DOI] [Google Scholar]
- 46.Stack MM, Stott FT. An approach to modeling erosion-corrosion of alloys using erosion-corrosion maps. Corros Sci. 1993;35:1027–1034. doi: 10.1016/0010-938X(93)90321-7. [DOI] [Google Scholar]
- 47.Tompkins GS, Jacobs JJ, Kull LR, Rosenberg AG, Galante JO. Primary total hip arthroplasty with a porous-coated acetabular component. Seven-to-ten-year results. J Bone Joint Surg Am. 1997;79:169–176. doi: 10.2106/00004623-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457–476. doi: 10.1302/0301-620X.82B3.9310. [DOI] [PubMed] [Google Scholar]
- 49.Williams RL, Brown SA, Merritt K. Electrochemical studies on the influence of proteins on the corrosion of implant alloys. Biomaterials. 1988;9:181–186. doi: 10.1016/0142-9612(88)90119-6. [DOI] [PubMed] [Google Scholar]
- 50.Wimmer MA, Fischer A, Büscher R, Pourzal R, Sprecher C, Hauert R, Jacobs JJ. Wear mechanisms in metal-on-metal bearings: the importance of tribochemical reaction layers. J Orthop Res. 2010;28:436–443. doi: 10.1002/jor.21020. [DOI] [PubMed] [Google Scholar]
- 51.Wimmer MA, Sprecher C, Hauert R, Täger G, Fischer A. Tribochemical reaction on metal-on-metal hip joint bearings: a comparison between in-vitro and in-vivo results. Wear. 2003;255:1007–1014. doi: 10.1016/S0043-1648(03)00127-3. [DOI] [Google Scholar]
- 52.Wood RJK, Hutton SP. The synergistic effect of erosion and corrosion—trends in published results. Wear. 1990;140:387–394. doi: 10.1016/0043-1648(90)90098-U. [DOI] [Google Scholar]
- 53.Yan Y, Neville A, Dowson D. Biotribocorrosion an appraisal of the time dependence of wear and corrosion interactions: I. The role of corrosion. J Phys D Appl Phys. 2006;39:3200. doi: 10.1088/0022-3727/39/15/S10. [DOI] [Google Scholar]
- 54.Yan Y, Neville A, Dowson D. Understanding the role of corrosion in the degradation of metal-on-metal implants. Proc Inst Mech Eng H. 2006;220:173–181. doi: 10.1243/095441105X63246. [DOI] [PubMed] [Google Scholar]
- 55.Yan Y, Neville A, Dowson D. Tribo-corrosion properties of cobalt-based medical implant alloys in simulated biological environments. Wear. 2007;263:1105–1111. doi: 10.1016/j.wear.2007.01.114. [DOI] [Google Scholar]
- 56.Yan Y, Neville A, Dowson D, Williams S. Tribocorrosion in implants—assessing high carbon and low carbon Co–Cr–Mo alloys by in situ electrochemical measurements. Tribol Int. 2006;39:1509–1517. doi: 10.1016/j.triboint.2006.01.016. [DOI] [Google Scholar]
- 57.Yan Y, Neville A, Dowson D, Williams S, Fisher J. Electrochemical instrumentation of a hip simulator: a new tool for assessing the role of corrosion in metal-on-metal hip joints. Proc Inst Mech Eng H. 2010;224:267–273. doi: 10.1243/09544119JEIM771. [DOI] [PubMed] [Google Scholar]