Abstract
Background
Elevated blood pressure (BP) is associated with increased cardiovascular risks manifested by ischemic heart disease and stroke. Studies of cardiothoracic surgeons and neurosurgeons suggest surgery induces a hemodynamic stress malresponse. However, it is unclear whether these occur in orthopaedic surgeons.
Questions/Purposes
We measured the BP of surgeons during hallux valgus surgery, TKA, and THA with the: (1) trainee assisting the trainer, (2) the trainer assisting the trainee, (3) the trainee operating independently, and (4) compared the intraoperative changes in BP and heart rate of orthopaedic surgeons with those of a clinic day and during an exercise tolerance test.
Methods
We used an ambulatory BP monitor to measure the BP and heart rate of three consultants and their respective trainees during hallux valgus surgery, TKA, or THA. We noted if there were any differences in the stress response of the lead surgeon in comparison to when the same individual was assisting a trainee, and vice versa. Additionally, we recorded the trainee’s BP and heart rate when they were operating independently. The intraoperative changes in BP and heart rate of orthopaedic surgeons were compared with those measured during a clinic day and during an exercise tolerance test.
Results
When the trainer was leading the operation, their mean arterial pressure gradually increased to 105 (range, 102–109) until implant placement. However, when the trainee was operating and the trainer assisting, the trainer’s BP peaked (mean, 101; range, 95–111) at the beginning of the procedure and slowly declined as it progressed. The trainee’s BP remained elevated throughout. The highest peaks for trainees were noted during independent operating. All of the surgeons had higher average BP readings (mean, 100; range, 95–108) and heart rate (mean, 86; range, 57–117) on days when they did surgery compared with baseline.
Conclusions
The elective operations studied induced a hypertensive response. The response was more marked in trainees than in trainers, particularly if the trainee was operating independently.
Introduction
Cardiovascular disease is now the leading cause of morbidity and mortality worldwide [18]. It has been well documented that elevated BP is associated with increased risks of cardiovascular disease manifested by angina, myocardial infarction [4], transient cerebral ischemic attacks, and strokes [6, 8, 9, 17]. Hypertension also is related to increased risks of chronic kidney disease and end-stage renal disease [7].
Blood pressure (BP) is a measurement of the force exerted on the walls of the arteries during the cardiac cycle. The average BP in a single cardiac cycle is known as mean arterial pressure (MAP) and is calculated as 2/3 diastolic + 1/3 systolic pressure. These values are continuously changing depending on activity, temperature, diet, mental and physical states, and pharmaceuticals [3]. According to the British Hypertension Society [16], BP lower than 120/80 mm Hg is normal, but readings greater than 140/90 mm Hg are considered high. In terms of MAP, a value of 93 (70–110) is considered normal, whereas values consistently more than 110 are considered hypertensive. Heart rate is the number of heartbeats per minute, which varies based on the body’s need to absorb oxygen and excrete carbon dioxide. Normal values are 60 to 100 beats per minute, whereas values greater than 100 beats per minute are considered tachycardic.
BP and heart rate are routinely measured patient observations during hospital admissions. These values help document the current state of health of the human body and specifically the hemodynamic changes that are occurring in response to stress [1]. The body responds to stress by stimulating the sympathetic nervous system and secreting catecholamines and glucocorticoids (adrenaline, noradrenaline, and cortisol) [10]. The secretion of adrenaline and noradrenaline is linked to cardiovascular disorders such as hypertension, myocardial infarction, and stroke, whereas the secretion of cortisol is linked to cardiovascular disease [12]. When a patient undergoes surgery, the hemodynamic effects of stress on the human body are closely monitored and controlled by the anesthesia team.
Although there are guidelines for intraoperative cardiovascular evaluation of patients [5], less is known about the effect on the surgeon. Sharma et al. [14] monitored physiologic parameters using an oscillometric monitor during neurosurgery and compared the intraoperative values recorded with an exercise-related stress response. In the lead surgeons they reported an intraoperative hemodynamic stress malresponse that was substantially greater than during vigorous exercise. They observed that 28% of the BP readings recorded during surgery were greater than 180/110, and concluded that the long-term health effects of this should be studied.
Song et al. [15] assessed what happens to the trainer’s heart rate variability while performing coronary artery bypass grafting, and a comparison was made with when the trainer was supervising a trainee. When the trainer was performing the surgery, markers of stress were highest at the beginning of the operation and slowly came down toward the end, and when assisting, the trainer was most anxious when the trainee was performing the most crucial part of the procedure. Yamaguchi et al. [19] described an alternative method of gauging stress after operations and at night, by using questionnaire scoring systems and measuring urine biopyrin levels. They found surgeons were at increased levels of stress when operating for a longer duration and when operating at night. They suggested that this may lead to impaired performance the next day. It is unclear whether these findings apply to orthopaedic surgeons.
The aim of our research was to compare BP during hallux valgus surgery, TKA, and THA with the: (1) trainee assisting the trainer (2) the trainer assisting the trainee, and (3) the trainee operating independently, and (4) compare the intraoperative changes in BP and heart rate of orthopaedic surgeons with their BP and heart rate during a clinic day and during an exercise tolerance test.
Materials and Methods
We measured the ambulatory BP and heart rate of six trauma and orthopaedic surgeons, three of whom were experienced specialist consultants and three were registrars at the same stage of training. None of the participants had preexisting medical conditions (ie, heart disease, diabetes mellitus, coexisting hypertension, hyperlipidemia, and morbid obesity). The surgeons were not on-call during the duration of the study. We studied the surgeons during a total of 21 elective surgeries consisting of hallux valgus (n = 7), TKA (n = 7), and THA (n = 7). These were routine orthopaedic cases performed by the surgical teams on a regular basis, in which the consultants and trainees were primary lead surgeon and assistant. We also monitored trainees while performing these operations without supervision, which was deemed by their consultant trainer to be appropriate for their level of training and experience.
To assess cardiovascular function and fitness, each surgeon underwent an exercise stress test using the protocol described by Bruce et al. [2]. The surgeon was on a treadmill (Johnson Fitness T8000, Johnson Health Tech Co, Ltd, Taichung, Taiwan) and the parameters changed at 3-minute intervals. The parameters consisted of speed and incline and the surgeon continued until their maximal endurance as per the protocol. We recorded the BP and heart rate measurements as data to assess the surgeon’s normal response to physical stress.
We measured systolic BP, diastolic BP, and heart rate using an ambulatory BP monitor (ABPM) (Spacelabs Medical 90217, Spacelabs Healthcare Ltd, Hertford, UK) strapped to the surgeon’s nondominant arm. The monitor used is an approved instrument by the Association for the Advancement of Medical Instrumentation’s standards and British Hypertension Society’s protocols [11]. We used the associated software (Spacelab’s ABP Application) to program and transfer data to a personal computer.
Each trainer and trainee pair wore the ABPM for a total of 3 days. There were two operating days and one control day. The control day was a day spent in the outpatient clinic. On operating days the device automatically took measurements every 15 minutes from 8 AM to 5 PM, to give the most accurate possible picture of the changing measurements during an operative procedure. Measurements then were taken hourly from 5 PM to 8 PM. The measurements were taken at least 1 hour preoperatively and all operations ended by 5 PM. On the control day, the device took hourly measurements from 8 AM to 8 PM and the workday ended by 5 PM.
All intraoperative readings were taken with the surgeon standing and the monitor was holstered around his waist under the surgical gown; the surgeon was blinded to the results. As the machine inflated, the surgeon was instructed to keep his arm still. If any error readings were recorded (ie, if the surgeon did not keep his arm still during the reading), the machine would stop and deflate. The cuff then would reinflate 2 minutes later to take another reading, providing a reliable method of data capture. If at any point the surgeon felt the ABPM to be a hindrance to the procedure, he was instructed to descrub and remove the monitor. We recorded the BP and heart rate of the lead surgeon and his assistant for the consultant and trainee while operating. Any differences in the stress response of the lead surgeon versus the same individual when assisting were recorded. In addition, we measured the same parameters when the trainee was operating independently. All of the intraoperative measurements were compared with those of their control day readings and their stress exercise test response. MAP was chosen as our outcome parameter because it gives a good overall indication of physiologic health [1]. It combined the different events of a single cardiac cycle into one value.
Results
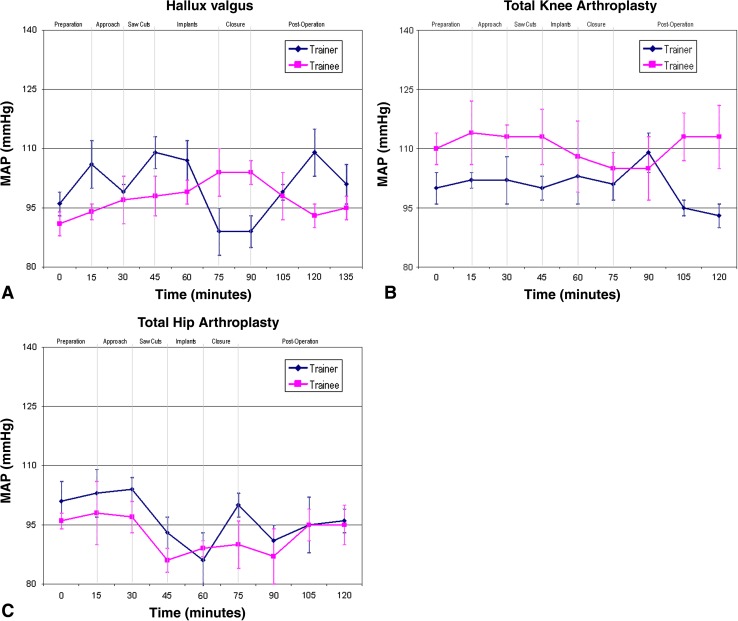
When the trainer was the primary surgeon for the respective operation, the MAP of the surgeon and his assistant gradually increased during the procedure (Fig. 1). For the trainer, this trend continued until the respective implants were being inserted. For the trainee the MAP continued to increase until wound closure, after which the BP generally returned to baseline.
Fig. 1A–C.
The MAPs are shown for when (A) the consultant trainer is the lead surgeon and the trainee is assisting during hallux valgus surgery, (B) the consultant trainer is the lead surgeon and the trainee is assisting during TKA, and (C) the consultant trainer is the lead surgeon and the trainee is assisting during THA.
When the trainee was the lead surgeon, consultant trainers repeatedly recorded peaks in their BP during preparation of the surgical site (Fig. 2). This settled once the procedure was underway. The trainee’s BP also was elevated, but it was fairly constant. However, at crucial parts of the procedure, the MAP of the trainer and trainee peaked with greater fluctuation for the trainer.
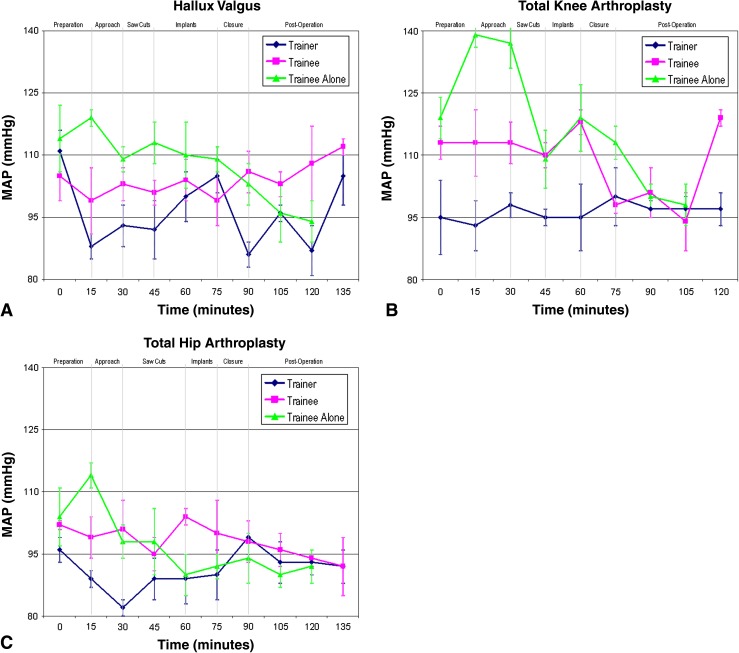
Fig. 2A–C.
The MAPs are shown for when (A) the registrar trainee is the lead surgeon and the consultant trainer is assisting during hallux valgus surgery (the trainee operating independently is charted for comparison), (B) the registrar trainee is the lead surgeon and the consultant trainer is assisting during TKA (the trainee operating independently is charted for comparison), and (C) the registrar trainee is the lead surgeon and the consultant trainer is assisting during THA (the trainee operating independently is charted for comparison).
The highest MAP readings recorded were when the trainee was operating independently without the trainer in the operating room. This was considerably higher than their respective baselines and when operating with the trainer present. The MAP readings for all of the trainees surpassed 110, the value used for the definition of hypertension [16], with one trainee reaching a peak MAP of almost 140.
For all of the surgeons, the readings were higher on the operating days than on the clinic or control day (Table 1). The values were higher in four of the six, but actually decreased in two trainees. The lowest heart rates were observed during the nonoperative clinic day (Table 2). On operating days, the HR increased to varying degrees for all of the surgeons. However, they were not as high as when the body was under exercise stress, during which times heart rate readings increased for all of the subjects by as much as 50%. The BP and heart rate were normalized by 2 hours after the conclusion of the surgery.
Table 1.
MAP for subjects throughout the duration of the study
| Subject | Clinic/control day | Surgery day | After protocol of Bruce et al. [2] |
|---|---|---|---|
| Hallux valgus trainer | 94 | 99 | 114 |
| Hallux valgus trainee | 99 | 103 | 91 |
| TKA trainer | 98 | 101 | 104 |
| TKA trainee | 102 | 108 | 99 |
| THA trainer | 93 | 96 | 99 |
| THA trainee | 93 | 95 | 102 |
MAP = mean arterial pressure.
Table 2.
Heart rate of all subjects throughout the duration of the study
| Subject | Clinic/control day | Surgery day | After protocol of Bruce et al. [2] |
|---|---|---|---|
| Hallux valgus trainer | 78 (67–98) | 86 (69–102) | 125 |
| Hallux valgus trainee | 89 (71–102) | 92 (71–108) | 163 |
| TKA trainer | 84 (78–105) | 99 (75–117) | 153 |
| TKA trainee | 79 (67–106) | 84 (61–117) | 158 |
| THA trainer | 68 (58–74) | 68 (57–84) | 110 |
| THA trainee | 86 (75–98) | 89 (79–104) | 105 |
Discussion
A consistently elevated BP is associated with increased cardiovascular risk leading to ischemic heart disease, stroke, and renal failure [6–9, 17]. A MAP regularly greater than 110 is considered hypertensive [16]. The anesthetist routinely monitors the physiologic parameters of the patient during surgery, but less is known about the effect on the surgeon. There have been studies in the cardiothoracic and neurosurgical literature, but this is the first study that we are aware of regarding the effect on BP in orthopaedic surgeons during elective procedures. In addition, we wanted to assess if there was a difference in the effect on the surgeon, whether a trainer or a trainee. We therefore measured the BP during hallux valgus surgery, TKAs, and THAs with the: (1) trainee assisting the trainer, (2) the trainer assisting the trainee, (3) the trainee operating independently, and (4) compared the intraoperative changes in BP and heart rate of orthopaedic surgeons with their BP and heart rate on a clinic day and during an exercise tolerance test.
We acknowledge the limitations of our study. First, the study is small, with only six participants. However, as this is a pilot study, we believe the trends merit investigation as part of a larger study. Second, we did not perform an a priori power analysis because there are insufficient published data to establish a clinically relevant change in MAP during surgery. In a study [13] regarding hazard ratios of stroke and variability in BP, a clinically relevant increase of 8 mm Hg was identified. Presuming a standard deviation of 10 from our data, we calculated with an alpha value of 0.05 and a test power of 0.80, that a future a study would require a minimum of 13 subjects in each group to study the benefits of any potential intervention.
Third, we performed no statistical analysis on the data. We believed the numbers studied were too small to perform any useful analysis, but the study provides a direction for future research. Fourth, consultants and registrars did not have identical levels of experience. The consultant trainers all had between 4 and 10 years of independent subspecialist practice, whereas the registrar trainees were the equivalent of 2nd year residents. We were unable to avoid slight variations in surgeon experience, and do not think that this detracts from the trends consistently noted.
When the trainee was assisting the trainer, hemodynamic changes were observed for all surgeons regardless of their grade and experience. The MAP and heart rate increased when compared with their baseline. The systolic variations were greater than the diastolic, and MAP readings showed peaks and troughs relating closely to the more technically challenging and critical points during the procedure. For the trainer specifically, the trend of increasing MAP started preoperatively, and continued until the respective implants were placed. The MAP readings settled thereafter. We believe this reflects a normal or physiologic response to performing an elective orthopaedic procedure, and that all surgery increases BP. However, we did not see the dramatic elevations that were noted by Sharma et al. [14]. This may reflect a difference in the nature of the surgery being performed in the two studies and the fact that all of our operations were elective (Table 3).
Table 3.
Comparative studies on cardiovascular effects of surgery on surgeons
| Study | Specialty | Subjects and cases | Methodology | Aims | Stress measuring instrument | Authors’ conclusions | Limitations |
|---|---|---|---|---|---|---|---|
| Sharma et al. [14] | Neurosurgery | 5 consultant-trainer surgeons; 11 cases total: 10 aneurysm clippings and 1 schwannoma | Prospective study | 1. To compare intraoperative pulse and BP recordings with rest and exercise values in neurosurgeons | 1. Oscillometric monitor | 1. Neurosurgery can induce a significant hemodynamic malresponse in the consultant-trainer that exceeds those induced by vigorous physical activity | 1. Small sample size 2 Did not report any effect of independent operating 3. Single separate operation should be excluded |
| Song et al. [15] | Cardiothoracic surgery | 1 consultant-trainer surgeon; 50 cases total: coronary artery bypass grafting | Prospective study | 1. To determine HR variability of the consultant-trainer as a measure of mental strain 2. To determine whether there were differences in the HR variability when the consultant-trainer performed coronary artery bypass grafting versus when he assisted registrar-trainees |
1. Holter electrocardiogram | 1. When leading, the consultant-trainer’s HR variability was the highest at the beginning of the operation and slowly trended down toward the end 2. When assisting, the consultant-trainer’s HR variability was the highest when the registrar-trainee was performing the most crucial part of the procedure |
1. No control – study assessed HR variability of a single consultant-trainer 2. Only HR variability measured as assessment of cardiovascular system 3. Did not report any effect of independent operating |
| Current study | Orthopaedic surgery | 3 consultant-trainer surgeons and 3 registrar-trainee surgeons; 21 elective cases total: 7 hallux valgus, 7 TKAs, and 7 THAs | Prospective study | 1. To quantify the intraoperative variations in BP of orthopaedic surgeons 2. To assess the variations in BP of the lead consultant-trainer in comparison to when the same individual is assisting a registrar-trainee 3. To assess the variations in BP of the lead registrar-trainee in comparison to when the same individual is assisting a consultant-trainer 4. To assess the effect on BP when the registrar-trainee was operating independently |
1. Ambulatory BP monitor | 1. All surgeons had higher BP and HR readings on operating days compared with baseline 2. When the consultant-trainer was leading, the BP gradually increased until implant placement 3. When the registrar-trainee was leading and the consultant-trainer assisting, the consultant-trainer’s BP peaked at the beginning of the procedure and slowly declined as it progressed; the registrar-trainee’s BP remained elevated throughout. 4. The highest peaks for registrar-trainees were noted during independent operating. |
1. Small sample size 2. Study was not powered 3. No statistical analysis 4. Difference in years of training for consultant-trainer surgeons |
BP = blood pressure; HR = heart rate.
When the trainer was assisting the trainee, MAP readings for consultant trainers were consistently elevated at the start of surgery and steadily decreased during the procedure. A possible explanation for this is the trainer’s anxiety in anticipation of his registrar trainee performing the surgery. Other than the surgical site preparation stage, trainer MAP readings were generally lower when assisting than when performing the surgery themselves. Song et al. [15] found that markers of stress in the trainer were most evident when the trainee was performing a critical step in the operation; this was less evident in our study. The BP of the trainees’ was consistently slightly higher throughout the operation when they were performing in the presence of their trainer than when they were assisting him. Therefore, for the trainer and the trainee, increases in BP are more likely if performing rather than assisting during the operation. This study is the first to assess the physiologic parameters of trainees during surgery and in turn the effect of supervising and assisting. Furthermore, it is the first study to show the trends were reproduced when the operations were repeated in subsequent cases and in different subspecialty areas of practice.
The highest MAP recordings were taken when trainees operated independently, suggesting that is when they are at their most stressed. The technique of Yamaguchi et al. [19] to measure urinary biopyrin levels may offer an additional, alternative marker of this. Research studies to gauge the time and number of procedures necessary under supervision before performing safe independent surgeries are needed.
An operating day produces sustained elevation in BP compared with clinic days. This response is not predictable from an exercise tolerance test. A study conducted by Oxford University aimed to establish the importance of visit-to-visit variability, maximum systolic BP, and episodic hypertension [13]. Rothwell et al. found that visit-to-visit variability in systolic BP and maximum systolic BP predicted the subsequent occurrence of stroke independent of mean systolic BP [13]. We found that all operations produced a pattern of altered BP and heart rate. Trained consultant surgeons had gradual increases in their MAP until implant placement when they were operating. Trainee registrars showed a similar trend, but their elevation in MAP was more likely to persist until wound closure. Despite peaks in trainer BP before commencing surgery, we did not find any other pattern of worsening elevations and fluctuations brought about by surgeons training their trainees. The MAP in those assisting generally was lower. The greatest increases in BP when the trainee had no supervision.
While we do not believe training an orthopaedic surgeon induces hypertension in the trainer the trainees may be inducing episodic hypertension in themselves. This is likely to settle to a more physiologic level as the experience of the surgeon increases. Further studies should establish how long this period lasts, whether it is procedure or practitioner specific and with the knowledge from the literature of clinically relevant variability, what specific treatment strategies will be effective. It is possible these findings may be mirrored in all surgical and medical specialties where trainees perform procedures on patients without senior supervision.
Acknowledgments
We thank Spacelabs Healthcare Ltd for the loan of the ambulatory blood pressure monitors used in the study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Boron WF, Boulpaep EL. Medical Physiology: A Cellular and Molecular Approach. 2. Philadelphia, PA, USA: WB Saunders; 2009. [Google Scholar]
- 2.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Rocella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed]
- 4.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. doi: 10.1001/archinte.1995.00430070053006. [DOI] [PubMed] [Google Scholar]
- 5.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society for Vascular Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–499. [DOI] [PubMed]
- 6.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Op Reimer WS, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen L, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgözoglu L, Wiklund O, Zampelas A; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe 9IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):S1–113. [DOI] [PubMed]
- 7.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 8.Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. [DOI] [PubMed]
- 9.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed]
- 10.Lundberg U. Stress hormones in health and illness: the roles of work and gender. Psychoneuroendocrinology. 2005;30:1017–1021. doi: 10.1016/j.psyneuen.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, Swiet M, Mee F. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British hypertension society. BMJ. 2000;320:1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosmond R, Björntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 14.Sharma MS, Thapa A, Chandra SP, Suri A, Singh M, Bahl VK, Sharma BS. Intraoperative pulse and blood pressure recordings of neurosurgeons: a pilot study of cardiovascular performance. Neurosurgery. 2010;66:893–899; discussion 899. [DOI] [PubMed]
- 15.Song MH, Tokuda Y, Nakayama T, Sato M, Hattori K. Intraoperative heart rate variability of a cardiac surgeon himself in coronary artery bypass grafting surgery. Interact Cardiovasc Thorac Surg. 2009;8:639–641. doi: 10.1510/icvts.2008.195941. [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, McG Thom S; British Hypertension Society. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004 BHS-IV. J Hum Hypertens. 2004;18:139–185. [DOI] [PubMed]
- 17.Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens. 1994;7:7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. The top ten causes of death. Available at: www.who.int/mediacentre/factsheets/fs310/en/index.html. 2011. Accessed March 31, 2012.
- 19.Yamaguchi K, Kanemitsu S; Kitakyushu Surgical Study Group. Surgeons’ stress from surgery and night duty: a multi-institutional study. Arch Surg. 2011;146:271–278. [DOI] [PubMed]