Abstract
Background
Pelvic radiation has been commonly used to treat gastrointestinal, genitourinary, or hematopoietic malignancies. Conventional THA in these patients reportedly have high rates of fixation failure. Although secure short-term fixation reportedly occurs with trabecular metal implants following pelvic radiation, it is unclear whether the fixation is durable.
Questions/purposes
We determined the survival of trabecular metal acetabular components in patients having THA following pelvic radiation and assessed function and radiographic loosening.
Methods
We retrospectively reviewed 29 patients with prior pelvic radiation who had 34 arthroplasties using trabecular metal acetabular components from 1998 and 2005. The mean pelvic radiation dose was 6300 cGy. We collected the following data: patient demographics, surgery and implant information, clinical and radiographic followup, and tumor and radiotherapy related details. We obtained Harris hip scores (HHS) on all patients. Ten patients died of disease prior to 5 years and two patients were excluded, leaving 17 patients (22 hips) with a minimum of 5 years of clinical (mean, 78 months; median, 71; range, 57–116) and radiographic (mean, 73; median, 65; range, 51–116) followup.
Results
All implants were in place in the surviving patients. The mean HHS improved from 36 preoperatively to 80 at latest followup. There were no reoperations for any reason, and we observed no implant loosening or migration at final followup in surviving or deceased patients.
Conclusions
Tantalum trabecular metal acetabular components restored function and provided durable reconstruction in patients undergoing THA following prior pelvic radiation. We observed no clinical or radiographic failures at a minimum 5-year followup.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Pelvic radiation has commonly been utilized for the treatment of genitourinary, gastrointestinal, and hematopoietic malignancies, as well as metastatic lesions affecting the pelvis. The pelvic bones sustain radiation injury, varying in severity from radiation osteitis, stress fractures, and osteonecrosis to acute pathologic fractures in a dose dependent manner, with a threshold dose of approximately 3000 cGy [3, 5, 9, 14]. Radiotherapy has also reportedly caused accelerated degenerative arthritis, leading to substantial pain and disability [7, 19]. Degenerative joint disease in the presence of prior pelvic radiation has been a difficult problem to treat; traditional THA components have shown rates of early implant loosening of between 44% and 52% at 2 to 6 years using both cemented and uncemented components [4, 10, 15] (Table 1).
Table 1.
Data from literature
| Study | Acetabular fixation | Number of patients | Mean followup (months) (range) | Mean radiation dose (cGy) | Mean time between radiation and THA (months) (range) | Acetabular loosening (%) | Revision THA (%) | Mean final HHS (range) | Note |
|---|---|---|---|---|---|---|---|---|---|
| Massin et al. [15] | Cemented | 42 | 69 (6–240) | 5500 | 73 | 52 | 38 | NR | Predominantly gynecologic malignancies |
| Cemented into acetabular ring | 22 | 40 (6–132) | 19 | 10 | NR | ||||
| Jacobs et al. [10] | Noncemented | 9 | 37 (17–78) | 5551 | 77 | 44 | 22 | 82 (62–98) | Predominantly gynecologic malignancies |
| Cho et al. [4] | Noncemented | 18 | 58 (20–139) | 5250 | 68 (7–130) | 50 | 33 | NR | All cervical cancer |
| Cemented into acetabular ring | 4 | 34 (20–41) | 50 | 25 | NR | ||||
| Kim et al. [12] | Noncemented | 58 | 57.6 (24–90) | 7065 | 67 | 0 | 5% | 90 (42–100) | All prostate cancer |
| Current study | Noncemented | 22 | 78 (57–116) | 6300 | 90 (2–800) | 0 | 0 | 80 (60–100) | 8 females/14 males; variety of malignancies |
HHS = Harris hip score; NR = not reported.
A recent study by Kim et al. [12] reported 93% implant survival with uncemented THA following pelvic irradiation for prostate cancer in the short-term. In 2006, our group reported 100% survival in 12 THAs at 2 to 4 years followup with the use of porous tantalum acetabular implants following pelvic radiation therapy [20]. The high coefficient of friction and apparently high ingrowth rates in vivo associated with porous tantalum sockets have made it a potentially important implant material in irradiated bone [1, 2, 16, 17] or complex revision situations [6, 8, 21]. However, if is unclear whether the high rate of survival initially reported would be maintained.
To update our earlier report, we determined (1) revision free survival (due to any cause) for the trabecular metal acetabular implants and the overall oncologic survival (death due to any cause) for this patient population, and (2) assessed function and radiographic loosening.
Patients and Methods
We retrospectively reviewed 29 patients who received 34 THAs after prior pelvic irradiation (minimum, 3000 cGy) with a porous tantalum acetabular implant between 1998 and 2005 (Table 2); this represented all patients treated after pelvic radiation during this timeframe. We excluded patients treated with modern, high-precision, radiation delivery techniques (stereotactic or intensity modulated radiotherapy), which limited the dose to the periacetabular area. The initial cohort included 19 men (21 hips) and 10 women (13 hips) with an average age of 71 years (range, 57–89). Median interval between pelvic radiation and surgery was 56 months (mean, 90 months; range, 1–800). The patients had varying malignancies (Table 2). Most patients received their radiation elsewhere; therefore, exact treatment plans and dose maps were not available for nine patients (10 hips), although all of these patients were treated for pelvic malignancies where standard radiation treatment required doses of 4500 to 7200 cGy. Mean dose of radiation was 6300 cGy. Twelve patients died of disease at mean 45 months after surgery (range, 6–83 months), of which, 10 died before the minimum 5-year followup; these patients were only included in the cohort for overall oncologic survival and complication analysis; no deceased patients underwent reoperation prior to death. We excluded one patient who developed dementia approximately 3 years postoperatively, requiring institutionalization. The patient was ambulatory with an apparently well-functioning hip and normal radiographic followup at 60 months. Although this patient had required no reoperations 70 months postoperatively, her mental status precluded a meaningful evaluation of her outcome. A second patient was ambulatory with no revisions, but declined to forward radiographs or participate in this study and was excluded. These 12 exclusions (12 hips) left 17 patients (22 hips) for analysis (Table 2). We required a minimum 5-year followup; the mean clinical followup was 78 months (median, 71 months; range, 57–116 months) and the mean radiographic followup was 73 months (median, 65 months; range, 51–116 months). No patients were recalled specifically for this study; all data was obtained from medical records and radiographs. Our institutional review board approved this study, and we obtained informed consent from all patients included in the study.
Table 2.
Details of cases
| Patient number | Age/sex | Malignancy | Radiation dose (cGy) | Time in months | Number of screws | Adjunct fixation | HHS | |||
|---|---|---|---|---|---|---|---|---|---|---|
| RAD to THA | Clinical followup‡ | Radiographic followup | Preoperative | 5-year | ||||||
| 1 | 69/male | Prostate | 7020 | 63 | 79 | 55 | 2 | None | 19 | 60 |
| 2 | 76/female | Cervical | > 4500 | 461 | 65 | 65 | 3 | None | 36 | 85 |
| 3 | 70/female | Uterine | 4530 | 87 | 93 | 63 | 4 | None | 24 | 87 |
| 4 | 70/female | Uterine | 4530 | 88 | 92 | 62 | 4 | None | 24 | 93 |
| 5 | 61/female | Colon | 5040 | 48 | 93 | 93 | 3 | None | 32 | 79 |
| 6 | 61/female | Colon | 5040 | 58 | 83 | 83 | 3 | None | 32 | 79 |
| 7 | 61/female | Ovarian | > 4500 | 61 | 112 | 112 | 2 | None | 36 | 86 |
| 8 | 61/female | Ovarian | > 4500 | 58 | 116 | 116 | 3 | None | 35 | 84 |
| 9 | 75/female | Ovarian | > 4500 | 800 | 116 | 62 | 3 | None | 20 | 81 |
| 10 | 71/male | Rectal | 5300 | 50 | 95 | 95 | 3 | None | 40 | 82 |
| 11 | 72/male | Anal | 5580 | 35 | 61 | 68 | 8 | Cup cage | 24 | 77 |
| 12 | 79/male | Prostate | 7200 | 20 | 57 | 55 | 6 | None | 39 | 79 |
| 13 | 79/male | Prostate | 7200 | 12 | 65 | 63 | 5 | None | 43 | 70 |
| 14 | 75/male | Prostate | > 4500 | 43 | 61 | 61 | 2 | None | 35 | 64 |
| 15 | 73/male | Prostate | 7200 | 44 | 64 | 88 | 0 | None | 33 | 83 |
| 16 | 80/male | Prostate | 6800 | 2 | 67 | 67 | 0 | None | 43 | 83 |
| 17 | 65/male | Prostate | 6000 | 41 | 74 | 74 | 2 | None | 45 | 81 |
| 18 | 66/male | Prostate | 6000 | 56 | 60 | 60 | 0 | None | 63 | 100 |
| 19 | 70/male | Lymphoma | > 4500 | 139 | 67 | 65 | 7 | Cup cage | 55 | 69 |
| 20 | 80/male | Myeloma | 4500 | 13 | 77 | 77 | 7 | Cup cage | NA | NA |
| 21 | 74/male* | Prostate | 6800 | 113 | 61 (67) | 61 | 3 | None | 35 | 84 |
| 22 | 77/male* | Prostate | 7380 | 4 | 64 (83) | 64 | 4 | None | 45 | 69 |
| 23 | 68/female*† | Uterine | > 4500 | 102 | 3 (6) | 3 | 3 | None | 23 | NA |
| 24 | 77/male*† | Prostate | > 4500 | 49 | 2 (38) | 0 | 3 | None | 27 | NA |
| 25 | 75/male*† | Prostate | 6400 | 125 | 35 (51) | 12 | 3 | None | 14 | NA |
| 26 | 72/male*† | Prostate | > 4500 | 80 | 28 (39) | 2 | 7 | None | 37 | NA |
| 27 | 58/female*† | Myeloma | > 4500 | 3 | 49 (58) | 13 | 6 | Augment | 22 | NA |
| 28 | 57/female*† | Myeloma | 15000 | 1 | 8 (12) | 2 | 4 | Femoral head | NA | NA |
| 29 | 76/male*† | Prostate | 7560 | 8 | 29 (30) | 26 | 4 | None | 21 | NA |
| 30 | 76/female*† | Anal | > 4500 | 89 | 21 (64) | 55 | 2 | None | 58 | NA |
| 31 | 64/female*† | Myeloma | 4000 | 134 | 70 (70) | 1 | 7 | Augment | 9 | 28 |
| 32 | 78/male*† | Prostate | > 4500 | 10 | 28 (62) | 28 | 2 | None | 26 | NA |
| 33 | 75/male*† | Prostate | > 4500 | 89 | 28 (49) | 2 | 4 | None | 20 | NA |
| 34 | 89/male*† | Prostate | > 4500 | 95 | 26 (38) | 29 | 6 | None | 29 | NA |
* Patient died.
†Patient excluded.
‡Numbers in parentheses indicate time from surgery to death.
HHS = Harris hip score; NA = not available.
Preoperative imaging demonstrated changes of radiation osteitis in 14 hips and osteonecrosis in 14 hips, with nine hips showing evidence of both conditions (Fig. 1). Amongst the patients who were available for a minimum 5-year followup, changes in radiation osteitis were seen in eight hips and osteonecrosis in nine hips, with four hips showing evidence of both conditions.
Fig. 1.
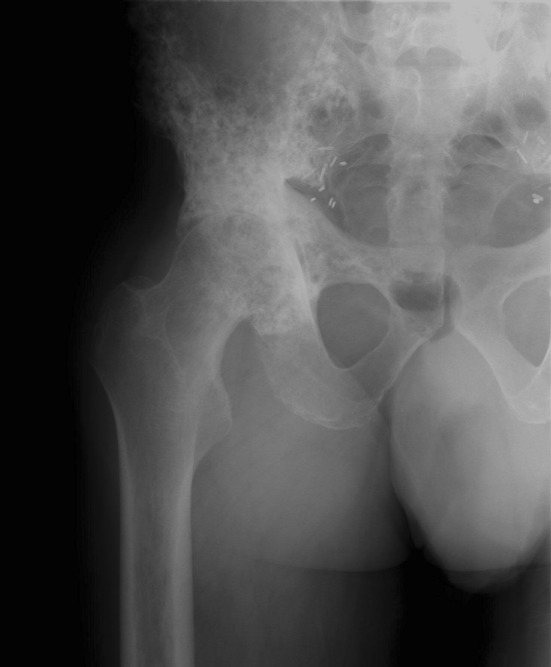
Preoperative radiograph demonstrates postradiation osteitis and arthrosis.
Patients were operated on by one of five surgeons. Approach was posterolateral in 12 patients (14 hips) and anterolateral in 17 patients (20 hips) at the discretion of the treating surgeon. A cemented femoral component was used in 21 patients (25 hips) and an uncemented stem in eight patients (nine hips). A multihole trabecular metal (tantalum) acetabular revision shell (Zimmer Corp, Warsaw, IN, USA) was used for cementless fixation in the acetabular cavity. This implant was FDA approved and originally designed and available for use with cemented polyethylene liners to prevent backside wear and facilitate stable screw fixation. All patients received the same revision-shell design. Although acetabular bone quality assessment was not standardized, reaming generally demonstrated areas of punctate bleeding interspersed with areas of no bleeding, consistent with geographic areas of radiation osteitis. In case of bone deficiency or discontinuity, a trabecular metal augment or a cage were used as indicated (six cases) to buttress the acetabular component (Fig. 2) [18]. We utilized a cup cage construct, as previously described in the literature [13], whenever a cage was needed. The cup used in this study was elliptical and oversized by 2 mm at the peripheral rim. Reaming the acetabulum to the labeled size for a particular cup resulted in line-to-line contact at the dome of the cup and 2-mm interference fit at the periphery. Multiple screws (mean, four; range, two to eight) in a diverging or fan screw orientation to maximize fixation through the tantalum shell. Supplemental screw holes were created as needed using a carbide burr in the already seated cup so that additional screws could be placed into areas of better bone quality to enhance fixation (Fig. 3). The polyethylene liner then was cemented (Surgical Simplex® P; Stryker, Mahwah, NJ, USA) into the acetabular shell to provide secure fixation, resulting in a locking screw effect as the screw heads were stabilized by the cement mantle [22]. We routinely used antibiotics in the cement for all the cases to improve infection prophylaxis.
Fig. 2.
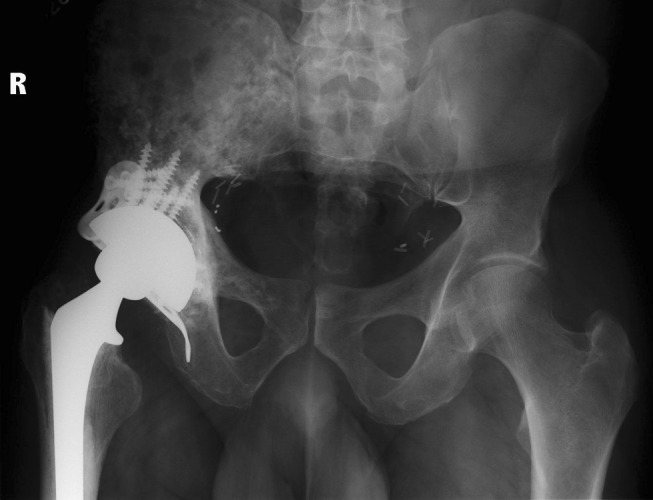
AP Pelvis radiograph taken 72 months postoperatively shows reconstruction with cup-cage construct.
Fig. 3.
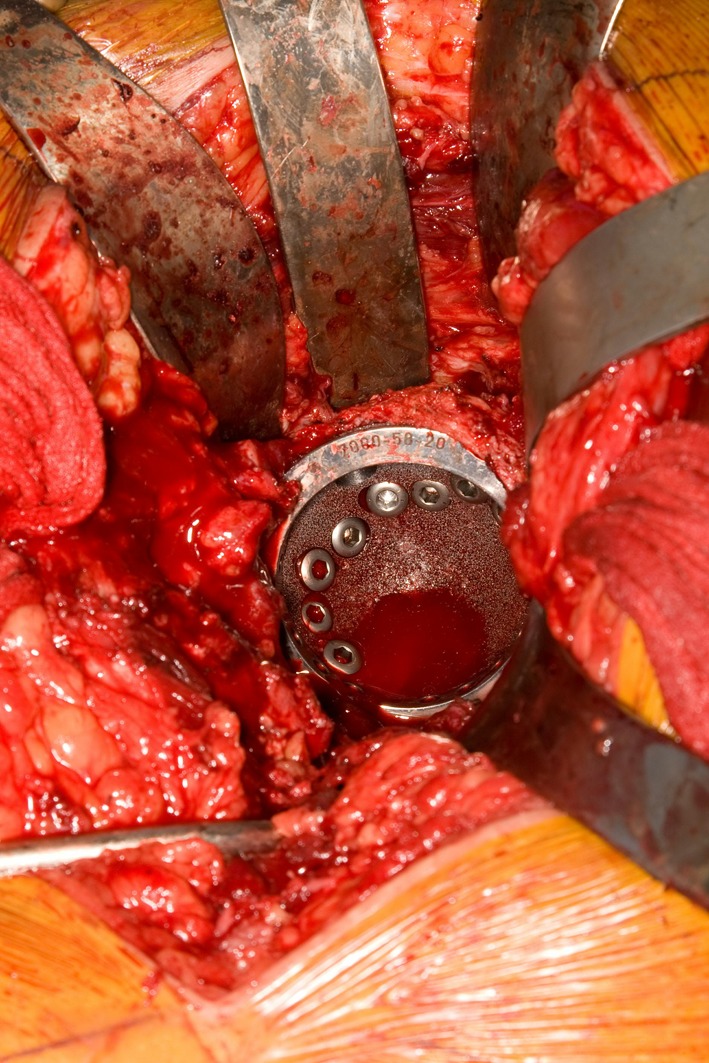
Intraoperative photograph demonstrates supplemental holes drilled through the cub to provide maximal fixation.
All patients received standard perioperative antibiotics (three doses of cefazolin or two doses of vancomycin) and pharmacologic thromboembolic prophylaxis with low molecular weight heparin or warfarin depending on surgeon preference. The patients received the standard postoperative rehabilitation protocol for THA without any modification. All patients were mobilized on day one following surgery and were allowed to bear weight as tolerated. The patients received physical therapy for walking assistance with a walker and stair climbing. After discharge the patients were allowed activity as tolerated and advised to follow precautions against dislocation which included limitation of hip flexion to less than 90°, avoidance of adduction, and avoidance of excessive external rotation.
The postoperative followup was at 3 and 6 months, 1 year, and then annually for all patients with AP pelvis and cross table lateral radiographs obtained at each followup. Clinical exam at each visit consisted of inspection of the wound, gait, range of motion, and Trendelenburg tests. We obtained Harris hip scores (HHS) on all patients.
Three observers (SBJ, PSR, and DGL) independently reviewed the pre- and postoperative radiographs independently by (SBJ, PSR, and DGL) for all the patients, including the ones who died. While all available radiographs were reviewed, final followup radiographs were compared with the initial postoperative and 2- and 3-year followup radiographs for detailed assessment of loosening. The observers used the criteria laid out by Yoder et al. [23] to assess acetabular loosening with any lucency entirely around the acetabular component greater than or equal to 2 mm or a position change greater than 4 mm involving the center of rotation of the hip as measured by the cup height and cup horizontal distance or greater than 4° change in the angulation of the cup.
We performed survivorship analysis using the Kaplan-Meier log curve analysis for overall patient survival. The primary endpoints for our study were death or revision due to any reason, and the secondary endpoint was radiological evidence of loosening of acetabular component. We performed statistical calculations using Microsoft® Excel version 2003 SP3 (Microsoft®, Redmond, WA, USA) using descriptive statistics and paired t-tests as indicated.
Results
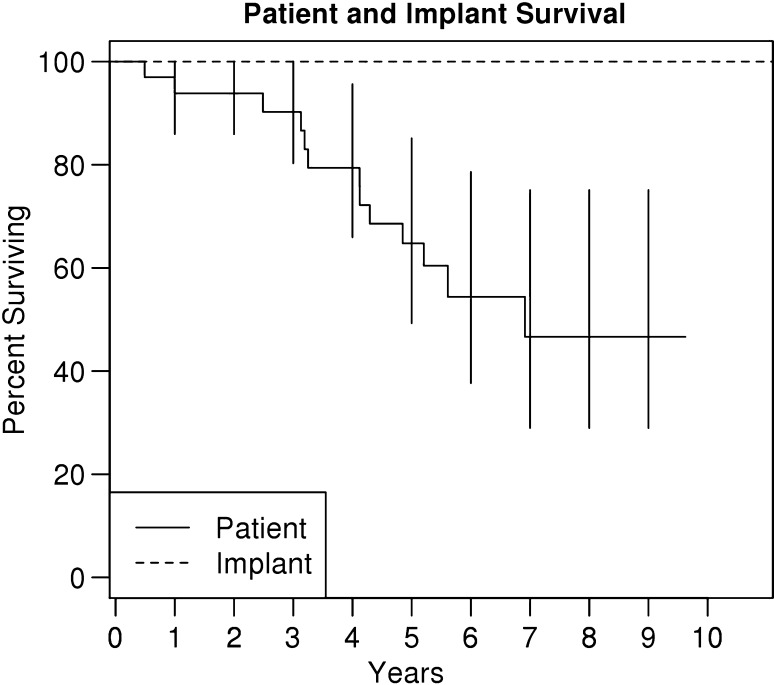
No patients, alive or deceased, required a revision for any reason. Oncologic survival of the cohort at 10 years was 50% as estimated by the Kaplan-Meier analysis (Fig. 4).
Fig. 4.
Implant survival (reoperation for any reasons; dashed) and patient (solid) survival by the method of Kaplan-Meier error bars represent 95% CI: 22 of 29 patients at 5 years and 15 of 29 at 10 years.
The mean preoperative HHS was 36 (range, 19–63), while the mean 5-year HHS was 80 (range, 60–100) (p < 0.001). The hips were fully functional at the time of latest followup for the patients who were lost to followup or who had died.
We observed no implant migration or progressive lucent lines in any of the hips at final followup. Two hips had stable nonprogressive lucencies in Zone 1, and one hip had a Zone 2 stable nonprogressive radiolucency, while one hip had evidence of lucency in both Zones 1 and 2, which was stable and nonprogressive at final followup. Three additional hips had Zone 1 lucencies which were not present at final followup.
One patient developed an intraoperative proximal femur fracture treated with cerclage fixation. Early postoperative complications included one urinary tract infection, one case of lower lobe pneumonia, and one case of cellulitis involving the distal operative extremity; all were successfully managed with antibiotic therapy. Three patients developed postoperative blood loss anemia that required transfusion. One patient developed multiple subsegmental pulmonary emboli without hemodynamic compromise or untoward outcomes, despite prophylactic anticoagulation without any evidence of deep vein thrombosis. No patient developed prosthetic joint infection. There were no late postoperative complications at final followup.
Discussion
THA following pelvic radiation presents challenges in terms of durability of fixation (Table 1). Most studies have shown high rates of fixation failure with conventional implants [4, 10, 15]. The reported acetabular loosening rates of 44% to 52% at 2 to 6 years [10, 15] highlighted the difficulty in obtaining durable acetabular fixation in this patient population. There has been a need for secure long-term fixation in this cohort, with 50% of the patients alive at the 10-year mark (Fig. 4). Our study aimed to determine if the 100% short-term implant survival experienced by our group in our initial report [20] with the use of porous tantalum acetabular components persisted at the 5- to 10-year followup interval and assessed function and radiographic loosening.
Our study had several limitations. First, the sample size of 29 patients (34 hips) was modest, and we were able to include only 17 patients (22 hips) for the survivorship analysis at a minimum 5-year followup because of patient demise. However, the number of patients having large doses of radiation to the pelvis is not large, and will likely decrease with contemporary radiation therapy. Second, since many patients lived a great distance from our center, followup was often by correspondence with local physicians, and some patients who were clinically doing well failed to provide radiographs at their final followup. For this reason, mean radiographic followup was 73 months, while mean clinical followup was 78 months for surviving patients. Third, we had no direct comparison group of alternative implants for this relatively rare condition. Finally, since we had no reoperations or autopsy retrievals, we were unable to confirm true osseointegration of the implants.
We observed no clinical or radiographic failures in the 17 surviving patients undergoing 22 arthroplasties following pelvic radiation at a minimum 5-year followup (Table 2). Kim et al. [12] demonstrated no loosening rates at a minimum 2-year followup; however, their study included only males with prostate cancer. Our study involved a diverse population (Table 2), thus improving the generalizability of the study. The uniform survivorship of the implants in this study was in contrast to most prior reports [4, 10, 15]. This may have been secondary to the combination of the bone ingrowth and biomechanical properties of porous tantalum we used. This implant provided a high coefficient of friction and allowed the use of multiple screws in an array across the dome, posterior column, and base of the ischium in a 180° arc, providing rigid initial fixation. The technique of cementing polyethylene liners into acetabular shells was first described for use during revision of well-fixed acetabular components, which has had a reliable mid-term survival [11, 22]. The multihole tantalum revision shell design provided for the use of a cemented liner combined with multiple screws placed into the shell. The cement also provided a locking effect to the screw heads and unified the polyethylene liner with the porous tantalum shell, preventing backside wear. This strategy was designed to maximize initial fixation and prevent motion during the early postoperative period, allowing bony ingrowth to occur from the scattered islands of viable bone remaining in the periacetabular region. Supplemental fixation with an antiprotrusion cup-cage construct, tantalum augments, or both were used to achieve rigid fixation for six cases in which the surgeon judged the bony stock to be particularly poor. We presumed that the extensive fixation (multiple screws and augments as necessary) and high coefficient of friction of tantalum allowed for successful bony ingrowth of these implants. Apart from the early complications, none of the patients developed late complications that threatened implant survival.
Malignancies and their treatment with chemotherapy, radiation, or surgery can lead to morbidity and disability. This cohort is far more debilitated from their condition compared to patients presenting with primary degenerative joint disease for THA. Patients in this study had a widely variable delay (mean, 90 months; median, 56 months; range, 1–800 months) between therapeutic radiation therapy and THA for disabling symptoms with a mean preoperative HHS of 36 (range, 9–63). These data were consistent with prior studies (Table 1). Kim et al. [12] reported a mean preoperative HHS of 47 (range, 8–81), and Jacobs et al. [10] reported a mean preoperative HHS of 41 (range, 31–55). Massin et al. [15] did not use the HHS system to assess function, which prevented direct comparison; however, they concluded that this cohort suffered rapid functional decline at varying periods after radiation, and was very different from patients with primary osteoarthritis. We observed an improvement in function, as measured by HHS, at a 5-year followup for all cases. The final HHS scores were also not as high as seen after primary THA at similar followup, highlighting the challenge presented by this patient population. However, the function compare favorably with other reports in the literature (Table 1).
Tantalum trabecular metal acetabular components provided durable reconstruction in patients undergoing THA following prior pelvic radiation. There were no clinical or radiographic failures at the minimum 5-year followup, and we observed substantial improvements in functional scores. These findings build on those previously reported with this technique to establish the durability of this reconstruction [20].
Footnotes
One of the authors (DGL) has reported receiving royalties from a company (Zimmer, Warsaw, IN, USA) related to the implant or technique described in this manuscript.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bobyn JD, Poggie RA, Krygier JJ, Lewallen DG, Hanssen AD, Lewis RJ, Unger AS, O’Keefe TJ, Christie MJ, Nasser S, Wood JE, Stulberg SD, Tanzer M. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint Surg Am. 2004;86(Suppl. 2):123–129. doi: 10.2106/00004623-200412002-00017. [DOI] [PubMed] [Google Scholar]
- 2.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81:907–914. doi: 10.1302/0301-620X.81B5.9283. [DOI] [PubMed] [Google Scholar]
- 3.Bragg DG, Shidnia H, Chu FC, Higinbotham NL. The clinical and radiographic aspects of radiation osteitis. Radiology. 1970;97:103–111. doi: 10.1148/97.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Cho MR, Kwun KW, Lee DH, Kim SY, Kim JD. Latent period best predicts acetabular cup failure after total hip arthroplasties in radiated hips. Clin Orthop Relat Res. 2005;438:165–170. doi: 10.1097/01.blo.0000167671.10820.29. [DOI] [PubMed] [Google Scholar]
- 5.Dalinka MK, Edeiken J, Finkelstein JB. Complications of radiation therapy: adult bone. Semin Roentgenol. 1974;9:29–40. doi: 10.1016/0037-198X(74)90007-8. [DOI] [PubMed] [Google Scholar]
- 6.Davies JH, Laflamme GY, Delisle J, Fernandes J. Trabecular metal used for major bone loss in acetabular hip revision. J Arthroplasty. 2011;26:1245–1250. doi: 10.1016/j.arth.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Deleeuw HW, Pottenger LA. Osteonecrosis of the acetabulum following radiation therapy. a report of two cases. J Bone Joint Surg Am. 1988;70:293–299. [PubMed] [Google Scholar]
- 8.Fernandez-Fairen M, Murcia A, Blanco A, Merono A, Murcia A, Jr, Ballester J. Revision of failed total hip arthroplasty acetabular cups to porous tantalum components: a 5-year follow-up study. J Arthroplasty. 2010;25:865–872. doi: 10.1016/j.arth.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Howland WJ, Loeffler RK, Starchman DE, Johnson RG. Postirradiation atrophic changes of bone and related complications. Radiology. 1975;117:677–685. doi: 10.1148/117.3.677. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JJ, Kull LR, Frey GA, Gitelis S, Sheinkop MB, Kramer TS, Rosenberg AG. Early failure of acetabular components inserted without cement after previous pelvic irradiation. J Bone Joint Surg Am. 1995;77:1829–1835. doi: 10.2106/00004623-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Khanuja HS, Aggarwal A, Hungerford MW, Hungerford DS, Jones LC, Mont MA. Cementing polyethylene liners into non-modular acetabular components in revision total hip arthroplasty. J Orthop Surg (Hong Kong). 2010;18:184–188. doi: 10.1177/230949901001800210. [DOI] [PubMed] [Google Scholar]
- 12.Kim KI, Klein GR, Sleeper J, Dicker AP, Rothman RH, Parvizi J. Uncemented total hip arthroplasty in patients with a history of pelvic irradiation for prostate cancer. J Bone Joint Surg Am. 2007;89:798–805. doi: 10.2106/JBJS.F.00183. [DOI] [PubMed] [Google Scholar]
- 13.Kosashvili Y, Backstein D, Safir O, Lakstein D, Gross AE. Acetabular revision using an anti-protrusion (ilio-ischial) cage and trabecular metal acetabular component for severe acetabular bone loss associated with pelvic discontinuity. J Bone Joint Surg Br. 2009;91:870–876. doi: 10.1302/0301-620X.91B7.22181. [DOI] [PubMed] [Google Scholar]
- 14.Kwon JW, Huh SJ, Yoon YC, Choi SH, Jung JY, Oh D, Choe BK. Pelvic bone complications after radiation therapy of uterine cervical cancer: evaluation with MRI. AJR Am J Roentgenol. 2008;191:987–994. doi: 10.2214/AJR.07.3634. [DOI] [PubMed] [Google Scholar]
- 15.Massin P, Duparc J. Total hip replacement in irradiated hips. a retrospective study of 71 cases. J Bone Joint Surg Am. 1995;77:847–852. [PubMed] [Google Scholar]
- 16.Meneghini RM, Ford KS, McCollough CH, Hanssen AD, Lewallen DG. Bone remodeling around porous metal cementless acetabular components. J Arthroplasty. 2010;25:741–747. doi: 10.1016/j.arth.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Meneghini RM, Meyer C, Buckley CA, Hanssen AD, Lewallen DG. Mechanical stability of novel highly porous metal acetabular components in revision total hip arthroplasty. J Arthroplasty. 2010;25:337–341. doi: 10.1016/j.arth.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Nehme A, Lewallen DG, Hanssen AD. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin Orthop Relat Res. 2004;429:201–208. doi: 10.1097/01.blo.0000150133.88271.80. [DOI] [PubMed] [Google Scholar]
- 19.Phillips TW, Rao DR. Destructive arthropathy of the hip following pelvic irradiation: report of four cases. Can J Surg. 1989;32:353–357. [PubMed] [Google Scholar]
- 20.Rose PS, Halasy M, Trousdale RT, Hanssen AD, Sim FH, Berry DJ, Lewallen DG. Preliminary results of tantalum acetabular components for THA after pelvic radiation. Clin Orthop Relat Res. 2006;453:195–198. doi: 10.1097/01.blo.0000238854.16121.a3. [DOI] [PubMed] [Google Scholar]
- 21.Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21(Suppl. 2):87–90. doi: 10.1016/j.arth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Springer BD, Hanssen AD, Lewallen DG. Cementation of an acetabular liner into a well-fixed acetabular shell during revision total hip arthroplasty. J Arthroplasty. 2003;18(Suppl. 1):126–130. doi: 10.1016/S0883-5403(03)00287-0. [DOI] [PubMed] [Google Scholar]
- 23.Yoder SA, Brand RA, Pedersen DR, O’Gorman TW. Total hip acetabular component position affects component loosening rates. Clin Orthop Relat Res. 1988;228:79–87. [PubMed] [Google Scholar]