Abstract
Background
Return to sport is a key patient demand after hip arthroplasty and some patients are even involved in high-impact sports. Although polyethylene wear is related to the number of cycles and the importance of the load, it is unclear whether high-impact sport per se influences THA durability.
Questions/purposes
Therefore, we compared (1) function between the patients involved in high-impact sports and the patients with lower activities as measured by the Harris hip score (HHS) and the Hip Osteoarthritis Outcome Score (HOOS); (2) linear wear rates; and (3) survivorships considering revision for mechanical failure with radiographic signs of aseptic loosening as the end point.
Methods
We retrospectively identified 70 patients who engaged in high-impact sports and 140 with low activity levels from among 843 THAs from a prospectively collected database performed between September 1, 1995, and December 31, 2000. Patients were evaluated at a minimum followup of 10 years (mean, 11 years; range, 10–15 years) by two independent observers. We obtained a HHS and HOOS at each followup.
Results
The mean HOOS was higher in the high-impact group for three of the five subscales of the HOOS. Mean linear wear was higher in the high-impact group than in the low-activities group. We also found a higher number of revisions in the high-activity group.
Conclusions
Our observations confirm concern about the risk of THA mechanical failures related to high-impact sport, and patient and surgeons alike should be aware of these risks of mechanical failures.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
THA has been described as the procedure of the century and is probably the most effective modern orthopaedic procedure [21]. The population undergoing THA has changed during the last 30 years and many patients are now younger, more active, and with longer life expectancies at the time of surgery [37]. As the clinical success of total joint arthroplasty has been documented and publicized, patients’ expectations regarding the procedure have increased; returning to sport is now a major patient expectation after hip arthroplasty [34] and some patients are even involved in high-impact sports such as jogging, soccer, or martial arts practice [15, 25, 33]. Several studies have shown a high percentage of participation in athletic activities after THA [15]. Huch et al. [16] reported that 36% of patients were involved in athletic activities at the time of surgery and 52% 5 years after the surgery [6]. According to Wright and Bartel [38], return to athletic activity is the third concern after pain relief and ROM improvement among candidates for THA. One of the frequent questions of the patient before surgery is the potential ability to practice sport after surgery [34]. The surgeon naturally would wonder whether participation in sports has an adverse effect on THA durability [19, 30]. A consensus survey based on individual surgeon opinion suggested some sports were generally recommended and others not [24]. Many surgeons do not recommend high-impact sports because they may cause fracture [2] and because of excessive wear. Patients involved in such sports have high activity levels [32] with higher load magnitudes and generally higher numbers of activity cycles. Following the increasing number of patients involved in high-impact sport after THA, it is important to confirm these recommendations with studies similar to those after TKAs [27]. Based on the studies showing higher failure rates with higher activity levels and on expert opinion, we presumed involvement in high-impact sport would reduce the durability of the implant after THA.
Therefore, we compared patients involved in high-impact sports and those with lower activities to determine differences in: (1) function; (2) dislocation rate; (3) linear wear; and (4) survivorship considering revision for mechanical failure or radiographic signs of aseptic loosening as the end point. Finally we determined independent risk factors for failure.
Patients and Methods
From a prospectively collected database we retrospectively identified 70 patients who engaged in high-impact sports and 140 with low activity levels from among 843 patients who have had 843 THAs performed between September 1, 1995, and December 31, 2000. Patients were evaluated at a minimum followup of 10 years (mean, 11 years; range, 10–15 years) for Harris hip score (HHS) [14] and Hip Osteoarthritis Outcome Score (HOOS) [28, 29] radiographic analysis (wear rate) and aseptic loosening or need for revision. The indication for the procedure was primary or secondary arthritis of the hip. All patients received a cementless stem and a cementless cup and the bearing surface at that time was a ceramic head and a conventional polyethylene. The inclusion criteria were primary THA, age from 18 to 75 years at the time of surgery, patients with Charnley Grade A or B level [4], unilateral THA, primary arthritis, osteonecrosis or developmental dysplasia Stage 1, and a minimum followup greater than 10 years. We excluded patients with tumor, trauma and posttraumatic arthritis, congenital dislocation or developmental dysplasia Stage > 1, history of surgery or infection on the index hip, and patients with early revision resulting from an early mechanical failure or an infection. Institutional review board approval was obtained.
Patients meeting the inclusion criteria were then allocated in each group using a specific sport and quality-of-life questionnaire. At a minimum followup of 10 years, patients were asked to fill out a questionnaire including three sections: the first section was related to the patient’s characteristics (weight, height, age, professional occupation), the second included the University of California Los Angeles score (UCLA) [40] activity score and the Weiss et al. [36] scores, and the last section included the HOOS [28, 29]. We used the UCLA score [40] to appreciate the level of activity. This score reflects only the frequency of the participation and the type of activity performed. To our knowledge, there is no technique to accurately and precisely determine the frequency and intensity of a sport activity. This questionnaire was sent to the patients with a letter from the two senior surgeons (JNA, JMA) requesting their participation. The same questionnaire was filled out by a telephone survey for the nonresponding patient (SF). Of the 843 patients screened, 70 patients reported a UCLA [40] activity score of 9 or 10 and were defined as the high-impact group. A group of patients considered as low-activity patients (UCLA [40] score from 1 to 4) was then identified from the survey. Patients of the high-impact group were then matched according to age at surgery (± 5 years), sex, BMI (± 3 kg/m2), American Society of Anesthesiology score, and followup (± 2 years) with two patients in the low-activity group by a computerized matching process. After this matching process, 210 patients were included in our study, 70 in the high-impact group and 140 in the low-activity group (Table 1). Patient demographics were described using means and SDs or medians and ranges for continuous variables and counts (percent) for categorical variables. In the high-impact group, the mean UCLA score [39] was 9.3 ± 0.4; this score was 3.4 ± 0.6 in the low-activity group.
Table 1.
Patient characteristics in the high-activity group and in the low-activity group
| Characteristic | High impact | Low activities | p value |
|---|---|---|---|
| Age (years) | 58.79 ± 9.4 | 58.57 ± 9.2 | 0.9 |
| American Society of Anesthesiologists score | 1.43 ± 0.6 | 1.51 ± 0.8 | 0.7 |
| Body mass index (kg/m²) | 25.03 ± 3.14 | 25.18 ± 3.16 | 0.8 |
| Harris hip score preoperatively | 54 ± 12 | 57 ± 14 | 0.3 |
| Sex (male) | 51.4% (36) | 51.4% (72) | 0.9 |
| Femoral head size 32 | 10 % (7) | 13 % (18) | 0.3 |
| UCLA score | 9.3 ± 0.4 | 3.37 ± 0.6 | < 0.0001 |
Mean values with standard deviation or percent with number of individuals.
All patients were operated on by the two senior authors (JMA, JNA). An anatomical cementless hydroxyapatite (HA)-coated stem was used all cases (Symbios®, Yverdon, Switzerland; FDA-approved). All the patients received a 28-mm ceramic femoral head implant. A cementless HA-coated titanium alloy acetabular cup was used in every case with a conventional UHMWPE (sterilization with gamma radiation under nitrogen).
Pre- and postoperatively we obtained a HHS to objectively determine the patient’s functional level. To evaluate the patient’s hip-related quality of life, patients were asked to fill out the HOOS [28, 29] in the questionnaire. The HOOS [28, 29] is a self-administered hip-related quality-of-life questionnaire corresponding to a validated and improved WOMAC [2]. The HOOS [28, 29] includes five dimensions scored separately: pain (nine items); symptoms (seven items); activities of daily life function (17 items); sport and recreation function (five items); and quality of life (four items). Because it is desirable to analyze and interpret the five dimensions separately, an aggregate score was not calculated. All items are scored from 0 to 4, and each of the five scores is calculated as the sum of the items included. Scores are then transformed using free calculation software available online on the web site www.koos.nu to a 0 to 100 scale with zero representing extreme knee problems and 100 representing no knee problems. HOOS [28, 29] score has not been calculated preoperatively because it was not yet available.
Radiographic postoperative evaluation consisted of AP and lateral views of the hip and pelvis and a true lateral view of the hip. The first postoperative radiograph was then used as a baseline from which subsequent radiographs were interpreted. At last followup, the radiographs were digitalized with a high-density scanner (SIERRA Advantage VIDAR Systems Corporation®, Herndon, VA) and examined by two independent observers (SP, MO). Magnification correction factors were calculated for each film based on the known diameter of the prosthetic head. Polyethylene wear was measured using IMAGIKA® software (GSI Medical, Neuilly sur Seine, France); data processing procedures were based on the dual circle method to analyze digitalized radiographs. This software has already proved its reproducibility and accuracy [12]. The examiners analyzed each radiograph twice, once on each of 2 separate days. All data processing was performed independently from one another. Intra- and interobserver variability was calculated using the root mean square (RMS) error and coefficient of variation (COV) of all data. The differences of intraobserver values were 0.128 ± 0.031 mm with a coefficient of repeatability of 0.109 mm, an RMS error of 0.098 mm, and a COV of 2.3%. The difference in interobserver values was 0.107 ± 0.027 mm with a coefficient of repeatability of 0.94 mm and an RMS error of 0.087 mm. Because not all patients had the same period of followup, the total wear was converted to a wear rate per year, and this linear wear was expressed as a mean ± SD (millimeters per year). Osteolysis or loosening was analyzed using the following criteria for the femur and the acetabular cup. The femur was analyzed according to the seven zones described by Gruen et al. [13] and the corresponding seven zones on the lateral radiograph. Progressive radiolucencies, or radiolucencies greater than 2 mm in width, were recorded as well as signs of osteolysis. The femoral component stability was evaluated by the criteria of Engh et al. [9]. The stem was considered loose if a subsidence greater than 2 mm or a modification in the axis of more than 2° was recorded. Acetabular cup stability was evaluated with the Massin et al. [24] method analyzing the distance between femoral head center and landmark along a vertical axis (the distance between the center of the cup and the teardrop line) and horizontal axis (distance between the center of the cup and the vertical line through the teardrop). A difference between the postoperative value and at last followup greater than 3° was considered as a migration. The tilt of the acetabular component (alpha angle) was defined as the angle between the cup and the teardrop line. A variation greater than 3° between initial and followup alpha angle was interpreted as a migration. Radiolucent lines in the DeLee and Charnley zone [6] were analyzed and interpreted as important depending of their size, localization, and evolution. Patient demographic data, dislocation rate, and the radiographic data were expressed as the mean and the SD (Tables 1, 2).
Table 2.
Factors influencing wear in THA
| Study | Number of hips | Number of design | Age | Male gender | Weight | Height | Activity |
|---|---|---|---|---|---|---|---|
| Charnley and Cupic [5] | 106 | 1 | – | – | No | – | No |
| Livermore et al. [22] | 385 | 3 | – | – | Yes | – | – |
| Xenos et al. [39] | 100 | 1 | Yes | No | No | No | – |
| Devane et al. [7] | 80 | 1 | Yes | No | No | – | Yes |
| Schmalzried et al. [31] | 37 | 2 | Yes | No | Yes | Yes | Yes |
| Lübbeke et al. [23] | 433 | 1 | No | No | – | – | No |
| Current study | 210 | 1 | No | Yes | No | No | Yes |
Clinical improvement between the pre- and the last followup evaluation as described by the HHS [14] was analyzed using a t-test for paired comparisons in each group. A comparison of the HOOS [28, 29] at last followup in each group was performed.
To analyze the influence of activity on survival and polyethylene wear, we defined two groups of patients: (1) patients involved in high-impact sports (UCLA 9-10); and (2) patients only involved in low activities (UCLA 1-4). Sample size was calculated based on the estimated linear wear [22] rate of 0.10 mm/year ± 0.06 for the 32-mm femoral head size and 0.08 mm/year ± 0.07 for the 28-mm femoral head size and, with an expected difference of wear rate between groups of 20%, a required level of statistical significance of α = 0.05 and a power of 1-β = 0.95; 50 subjects per group would be required to detect a difference of 0.025 mm/year. We tried to include all the patients with UCLA score > 8 from our database and including more patients with UCLA < 5 did not improve our power of calculation. The mean amount of wear was calculated for each of the two groups.
The analysis of polyethylene wear in the two groups was performed in two parts: (1) analysis of the risk factors that were selected for evaluation before the study was started (BMI, age, sex, femoral head size, activity, followup time) using linear regression analysis or Student’s t-test; and (2) analysis of subsets of patients and risk factors that were statistically significant or clinically important in the primary hypothesis-driven risk-factor analysis (BMI, sex, femoral head size, activity) using a Pearson’s Chi square test.
The prevalence of failure was calculated for each of the two groups excluding revisions related to septic loosening. Implant survival was estimated with use of the Kaplan-Meier method. Confidence intervals (CIs) at the 95% level were determined. The end point was defined by: revision for a mechanical failure of the components (one or both), or a fracture occurring during the athletic activities, or a mechanical failure defined as a migration of the components (one or both). Survivorships in the two groups were compared first in a univariate model according to a log-rank method. Then an extended Cox model with use of a generalized estimating equation theory was used to compare survival by risk factor in the high- and low-activity groups and to perform a multivariate analysis of survival of implants. Odd ratios were reported with their 95% CIs and considered as significant if excluding the value 1. Analysis was performed using SPSS software (Version 12; SPSS Inc, Chicago, IL, USA). All calculations assumed two-tailed tests.
Results
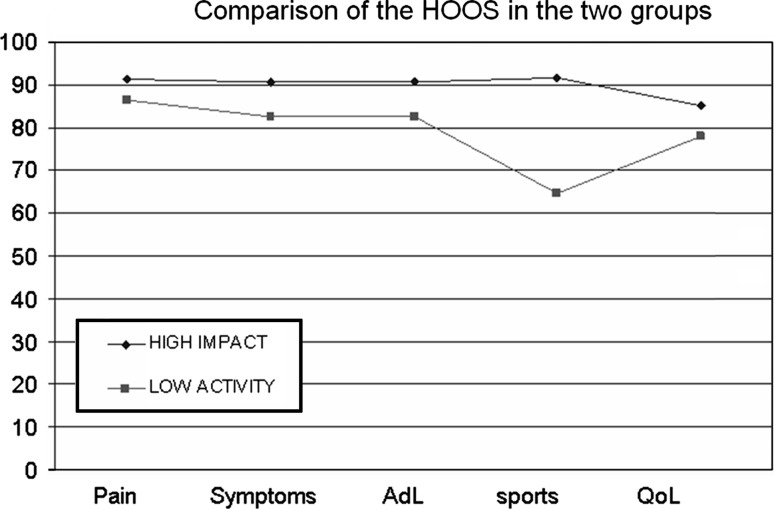
The mean HHS [14] improved from 54 ± 12.8 preoperatively to 88.29 ± 12.5 points (range, 53–98) postoperatively in the high-impact group and from 55 ± 14.5 to 69.38 ± 19.4 points (range, 23–98) in the low-activity group. The mean HHS [14] improvement was greater (p < 0.001) in the high-impact group with a mean improvement of 34 points in the high-impact group and 14 points in the low-activity group. The mean HOOS [28, 29] was higher in the high-impact group for the symptoms, activities of daily living, and sport subscales and comparable between the two groups for the pain and quality-of-life subscales (Fig. 1).
Fig. 1.
The mean postoperative HOOS scores were higher in the high-impact group for the symptoms, activities of daily living, and sport subscales and comparable between the two groups for the pain and quality-of-life subscales. AdL = activities of daily living; Sports = sports and recreational function; QoL = quality of life.
We observed no difference (p = 0.5) in dislocation rate between the two groups. Four patients experienced hip dislocation (1.9%), one in the high-impact group (1.4%) and three in the low-activity group (2.14%).
Practicing a high-impact sport and being a male increased wear rate (both p < 0.001). At 10 to 15 years followup, the mean polyethylene wear rate was 1.62 ± 0.69 mm (range, 3.29–0.54 mm) and the linear wear was 0.14 ± 0.06 mm/year (range, 0.29–0.05 mm/year) in the high-impact group. In the low-activity group, the mean wear was 0.74 ± 0.52 (range, 2.76–0.06) and the linear wear was 0.06 ± 0.04 (range, 0.2–0.06).
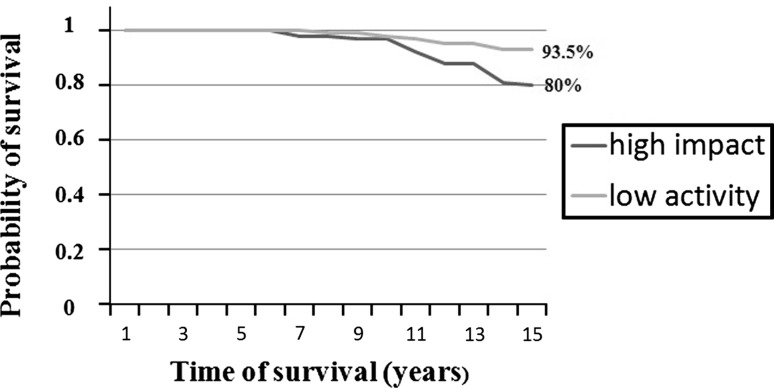
Survivorship was worse (p < 0.001) in the high-impact group than in the low-activities group (Fig. 2). At last followup, 23 patients had been revised for mechanical failure or had implants that had radiographically migrated. Fourteen patients in the high-impact group reached the end point for the following reasons: two revisions for acetabular loosening, four revisions for femoral aseptic loosening, six with radiographic acetabular loosening, and two with radiographic loosening of both components. In the low-activity group, nine patients reached the end point for the following reasons: one revision for acetabular loosening, four with radiographic femoral loosening, and four with radiographic acetabular loosening. One patient in the high-activity group (a skiing instructor) revised for aseptic loosening also had a femoral fracture during downhill skiing 2 years after acetabular revision. At 15 years followup, the survivorship in the high-impact sport group was 80% (range, 74%–86%) and 93.5% (range, 88.2%–97.6%) in the low-activity group.
Fig. 2.
Results of the Kaplan-Meier analysis showing a worse survivorship in the high-activity group at 15 years followup.
Practicing a high-impact sport was a risk factor for failure with an odds ratio of 3.64 (95% CI, 1.49–8.9). Patients in the high-impact sport group had more mechanical failures (p = 0.001) than patients involved in low activity.
Discussion
One of the frequent questions of the patient before THA is the potential ability to practice sport after surgery and the potential risk related to sport practice [35]. We presumed long-term participation in high-impact sports would reduce function and durability of the implant after THA. Therefore, we compared patients involved in high-impact sports and those with lower activities to determine differences in: (1) function; (2) dislocation rate; (3) linear wear; and (4) survivorship considering revision for mechanical failure or radiographic signs of aseptic loosening as the end point; we then determined independent risk factors for failure.
Readers should understand the major limitations of our study. First, the duration of the followup is too limited to clearly show the potential long-term adverse effect of the sport practice. The followup of the two groups of patients should then be continued; one study [8] reported a correlation between the number of revisions at 20 years and polyethylene wear with a linear wear rate of 0.2 mm/year or greater. However, our study is relatively large and does have a relatively long minimum followup of 10 years. Second, we lacked data indicating the duration and the intensity of the patient’s sportive activity. The only way to appreciate patient duration and intensity of athletic activity is pedometric analysis, but it reflects only a short period of patient activity (on pedometer) and is only available for prospective study. To minimize the effect of this limitation, we used the UCLA activity score [40], which includes the type of sport and also the frequency of practice particularly for the values 9 and 10. These values correspond to patients involved; frequently, in high-impact sport, much of these are nonrecommended activities after THA, but some of them are recommended sports like hiking.
The clinical outcomes and functional improvement were greater in the high-impact sport group compared with the low-activity group at 10 years. Sechriest et al. [32] found a UCLA score [40] positively correlated with postoperative HHS [14]. Lübbeke et al. [23] recently noticed that patients with the highest activity (evaluated by UCLA score) had the best outcome and highest satisfaction after surgery. These findings are also consistent with those reporting THA in the younger and active patient [10] or in the younger patient with a resurfacing hip [1]. Our observations demonstrated high functional score and high hip-related quality-of-life scores can also be obtained for older patients involved in high-impact sport.
Our series is consistent with literature showing increased polyethylene wear in patients with higher activities. Many factors seem to influence polyethylene wear; some of them may be confusing factors correlated to the degree of activity of the patient such as age, weight, and height of the patient (Table 2). The observations show participation in high-impact sports and being a male increased the wear rate. Why males have a higher rate of wear remains unclear [32]. Sex-specific differences have been mentioned to explain these differences including body anatomy such as weight distribution, gait pattern, or body composition as well as physiological considerations such as the lubricating properties of synovial fluid. Patient activity remains, however, the most important patient-related factor influencing prosthetic wear [31] depending on the applied load and the number of cycles the load is applied across the articulating surfaces [38]. Recent literature reported improved function and higher midterm survivorship for highly active patients using metal-on-metal bearing surfaces [26]. Better function and higher survivorship have also been reported after hip resurfacing in highly active men but with limited followup [1] and unanswered questions related to the potential adverse effect of resurfacing and metal-on-metal bearing surfaces such as corrosion, pseudotumor, or cytotoxicity [18]. Ceramic-on-ceramic bearing surfaces showed limited wear [17], but fractures and squeaking may adversely balance the potential benefit in terms of wear in highly active patients. One interesting solution to limit the osteolysis without having each potential drawback of the hard-on-hard bearing surfaces may be the use of highly crosslinked polyethylene showing lower wear rates at 5 and now 10 years [3].
We found a lower survivorship in the high-impact group with more mechanical failures. Our findings were consistent with those in the literature (Table 3). Flugsrud et al. [11] reported a comparable revision rate from the Norwegian database with a 17% revision rate after 13 years for patients involved in intermediate to intense sport activity during leisure and 13% for patients involved in lower activities. Lübbeke et al. [23] first used the UCLA scale to differentiate patient activity groups for clinical and radiographic femoral osteolysis showing a greater amount of osteolysis around the femoral component in the high-activity group without any difference in terms of wear rate between the moderate- and the high-activity groups. This is in accordance with the experts’ recommendations concerning the practice of high-impact activities at least when using conventional polyethylene [20]. Most of the very active patients may however not follow their surgeon’s recommendations, as suggested by Seyler et al. [33]. On the other hand, nonimpact sports reportedly promote bone growth [8], reduce obesity, and improve psychological health [15] and are therefore important after total joint arthroplasties.
Table 3.
Number of revision and THA radiological outcomes of high-activity patients
| Study | Number of hips | Mean age | Mean followup | Couple | Mechanical failure | Femoral osteolysis | Linear PE wear |
|---|---|---|---|---|---|---|---|
| Migaud et al. [26] | 39 | 41 years | 13 years | Metal/metal | 0/39 | – | – |
| 39 | Ceramic/PE | 11/39 | |||||
| Lübbeke et al. [23] | 58 | 67.7 years | 10 years | Ceramic/PE | – | 14/58 | – |
| Bedigrew et al. [1] | 52 | 50 years | 2 years | Metal/metal | 0/52 | 0/52 | – |
| Flugsrud et al. [11] | 188 | 63 years | 16 years | All types | 32/188 | – | – |
| Calvert et al. [3] | 60 | 59 years | 4 years | Ceramic/chromium linked | 0 | – | 0.02 mm/year |
| 59 | – | – | Ceramic/PE | 0 | – | 0.11 mm/year | |
| Current study | 70 | 58.3 years | 11 years | Ceramic/PE | 14/70 | 11/70 | 0.145 mm/year |
PE = polyethylene.
Our findings suggest patients with cementless THA, practicing a high-impact sport such as football, skiing, tennis, or martial arts, have better function than patients involved in low activities. However, involvement in athletic activities increased the wear rate and adversely affected implant survivorship. Our observations therefore confirm experts’ concerns about the potential risk related to the high-impact sport and both patients and surgeons should be aware of these risks. The potential adverse effect related to the hard-on-hard bearing surfaces and the potential benefit related to the highly crosslinked polyethylene should be evaluated in prospective comparative studies including active patients to enhance the surgeon’s bearing surface choices because sport is now a reality after THA.
Acknowledgments
We thank Pr Jean Manuel Aubaniac for his surgical involvement.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bedigrew KM, Ruh EL, Zhang Q, Clohisy JC, Barrack RL, Nunley RM. 2011 Marshall Urist Young Investigator Award: when to release patients to high-impact activities after hip resurfacing. Clin Orthop Relat Res. 2012;470:299–306. doi: 10.1007/s11999-011-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 3.Calvert GT, Devane PA, Fielden J, Adams K, Horne J. Double-blind, prospective, randomized controlled trial comparing highly cross-linked and conventional polyethylene in primary total hip arthroplasty. J Arthroplasty. 2009;24:505–510. doi: 10.1016/j.arth.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. J Bone Joint Surg Br. 1972;54:61–76. [PubMed] [Google Scholar]
- 5.Charnley J, Cupic Z. The nine and ten year results of the low-friction arthroplasty of the hip. Clin Orthop Relat Res. 1973;95:9–25. [PubMed] [Google Scholar]
- 6.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 7.Devane PA, Home JG, Martin K, Coldham G, Krause B. Three dimensional polyethylene wear of a press-fit titanium prosthesis: factors influencing generation of polyethylene debris. J Arthroplasty. 1997;12:256–266. doi: 10.1016/S0883-5403(97)90021-8. [DOI] [PubMed] [Google Scholar]
- 8.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 9.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 10.Flecher X, Pearce O, Parratte S, Aubaniac JM, Argenson JN. Custom cementless stem improves hip function in young patients at 15-year followup. Clin Orthop Relat Res. 2010;468:747–755. doi: 10.1007/s11999-009-1045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flugsrud GB, Nordsletten L, Espehaug B, Havelin LI, Meyer HE. The effect of middle-age body weight and physical activity on the risk of early revision hip arthroplasty: a cohort study of 1535 individuals. Acta Orthop Scand. 2007;78:99–107. doi: 10.1080/17453670610013493. [DOI] [PubMed] [Google Scholar]
- 12.Girard J, Touraine D, Soenen M, Massin P, Laffargue P, Migaud H. Measurement of head penetration on digitalized radiographs: reproducibility and accuracy. Rev Chir Orthop. 2005;91:137–142. doi: 10.1016/s0035-1040(05)84291-6. [DOI] [PubMed] [Google Scholar]
- 13.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 14.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 15.Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29:377–388. doi: 10.1177/03635465010290032301. [DOI] [PubMed] [Google Scholar]
- 16.Huch K, Müller KA, Stürmer T, Brenner H, Puhl W, Günther KP. Sports activities 5 years after total knee or hip arthroplasty: the Ulm Osteoarthritis Study. Ann Rheum Dis. 2005;64:1715–1720. doi: 10.1136/ard.2004.033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huet R, Sakona A, Kurtz SM. Strength and reliability of alumina ceramic femoral heads: review of design, testing, and retrieval analysis. J Mech Behav Biomed Mater. 2011;4:476–483. doi: 10.1016/j.jmbbm.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Jarrett CA, Ranawat AS, Bruzzone M, Blum YC, Rodriguez JA, Ranawat CS. The squeaking hip: a phenomenon of ceramic-on-ceramic total hip arthroplasty. J Bone Joint Surg Am. 2009;91:1344–1349. doi: 10.2106/JBJS.F.00970. [DOI] [PubMed] [Google Scholar]
- 19.Kilgus DJ, Moreland JR, Finerman GA, Funahashi TT, Tipton JS. Catastrophic wear of tibial polyethylene inserts. Clin Orthop Relat Res. 1991;273:223–231. [PubMed] [Google Scholar]
- 20.Klein GR, Levine BR, Hozack WJ, Strauss EJ, D’Antonio JA, Macaulay W. Return to athletic activity after total hip arthroplasty : consensus guidelines based on a survey of the Hip Society and American Association of Hip and Knee Surgeons. J Arthroplasty. 2007;22:171–175. doi: 10.1016/j.arth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 22.Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72:518–522. [PubMed] [Google Scholar]
- 23.Lübbeke A, Garavaglia G, Barea C, Stern R, Peter R, Hoffmeyer P. Influence of patient activity on femoral osteolysis at five and ten years following hybrid total hip replacement. J Bone Joint Surg Br. 2011;93:456–463. doi: 10.1302/0301-620X.93B4.25868. [DOI] [PubMed] [Google Scholar]
- 24.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. J Arthroplasty. 1989;4:245–251. doi: 10.1016/S0883-5403(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 25.McGrory BJ, Stuart MJ, Sim FH. Participation in sports after hip and knee arthroplasty: review of literature and survey of surgeon preferences. Mayo Clin Proc. 1995;70:342–348. doi: 10.4065/70.4.342. [DOI] [PubMed] [Google Scholar]
- 26.Migaud H, Putman S, Krantz N, Vasseur L, Girard J. Cementless metal-on-metal versus ceramic-on-polyethylene hip arthroplasty in patients less than fifty years of age: a comparative study with twelve to fourteen-year follow-up. J Bone Joint Surg Am. 2011;93:137–142. doi: 10.2106/JBJS.J.01720. [DOI] [PubMed] [Google Scholar]
- 27.Mont MA, Marker DR, Seyler TM, Gordon N, Hungerford DS, Jones LC. Knee arthroplasties have similar results in high- and low-activity patients. Clin Orthop Relat Res. 2007;460:165–173. doi: 10.1097/BLO.0b013e318042b5e7. [DOI] [PubMed] [Google Scholar]
- 28.Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and Osteoarthritis Outcome Score (HOOS)—validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;30:4–10. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ornetti P, Parratte S, Gossec L, Tavernier C, Argenson J-N, Roos EM, Guillemin F, Maillefert JF. Cross-cultural adaptation and validation of the French version of the Hip disability and Osteoarthritis Outcome Score (HOOS) in hip osteoarthritis patients. Osteoarthritis Cartilage. 2010;18:522–529. doi: 10.1016/j.joca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Ritter MA, Meding JB. Total hip arthroplasty. Can the patient play sports again? Orthopedics. 1987;10:1447–1452. doi: 10.3928/0147-7447-19871001-15. [DOI] [PubMed] [Google Scholar]
- 31.Schmalzried TP, Shepherd EF, Dorey FJ, Jackson WO, Rosa M, Fa’vae F, McKellop HA, McClung CD, Martell J, Moreland JR, Amstutz HC. The John Charnley Award. Wear is a function of use, not time. Clin Orthop Relat Res. 2000;381:36–46. doi: 10.1097/00003086-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Sechriest VF, II, Kyle RF, Marek DJ, Spates JD, Saleh KJ, Kuskowski M. Activity level in young patients with primary total hip arthroplasty: a 5-year minimum follow-up. J Arthroplasty. 2007;22:39–47. doi: 10.1016/j.arth.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 33.Seyler TM, Mont MA, Ragland PS, Kachwala MM, Delanois RE. Sports activity after total hip and knee arthroplasty: specific recommendations concerning tennis. Sports Med. 2006;36:571–583. doi: 10.2165/00007256-200636070-00003. [DOI] [PubMed] [Google Scholar]
- 34.Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74:978–982. doi: 10.4065/74.10.978. [DOI] [PubMed] [Google Scholar]
- 35.Visuri T, Honkanen R. Total hip replacement: its influence on spontaneous recreation exercise habits. Arch Phys Med Rehabil. 1980;61:325–328. [PubMed] [Google Scholar]
- 36.Weiss JM, Noble PC, Conditt MA, Kohl HW, Roberts S, Cook KF, Gordon MJ, Mathis KB. What functional activities are important to patients with knee replacements? Clin Orthop Relat Res. 2002;404:172–188. doi: 10.1097/00003086-200211000-00030. [DOI] [PubMed] [Google Scholar]
- 37.Wright JG, Young NL. The patient-specific index: asking patients what they want. J Bone Joint Surg Am. 1997;79:974–983. doi: 10.2106/00004623-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Wright TM, Bartel DL. The problem of surface damage in polyethylene total knee components. Clin Orthop Relat Res. 1986;205:67–74. [PubMed] [Google Scholar]
- 39.Xenos JS, Hopkinson WJ, Callaghan JJ, Heekin RD, Savory CG. Osteolysis around an uncemented cobalt chrome total hip arthroplasty. Clin Orthop Relat Res. 1995;317:29–36. [PubMed] [Google Scholar]
- 40.Zahiri CA, Scmalzried TP, Szuszczewcz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty. 1998;13:890–895. doi: 10.1016/S0883-5403(98)90195-4. [DOI] [PubMed] [Google Scholar]